Abstract
Melanoma is a destructive skin disease with few therapeutic options in the developed stage and therefore there is a critical need for reliable biomarkers for early diagnosis. In this context, microRNAs could play an important role as diagnostic biomarkers. Three datasets with accession numbers GSE31568, GSE61741 and GSE20994 were downloaded from the Gene Expression Omnibus (GEO) database. MATLAB software was used to analyze differentially expressed miRNAs between cutaneous melanoma plasma samples and normal plasma samples (control). Plasma levels of miR-193b, miR-146b-3p and miR-483-3p were evaluated by the RT-PCR method. Furthermore, linear regression followed by receiver operating characteristic analyses was performed to estimate whether selected plasma miRNAs were able to distinguish between cases and controls. Finally, the data were analyzed by unpaired Mann–Whitney U test using Graph pad prism 8 computer software. Specifically, miR-193b and miR-146b-3p were downregulated in the plasma of melanoma patients compared with control groups which were decreased 5 × \({10}^{6}\)-fold in miR-193b and 58-fold in miR-146b-3p, while miR-483-3p was upregulated 3.5-fold. After receiver operating characteristic (ROC) curve analysis, miR-193b with the most area under the curve (AUC: 1.00, 95% confidence interval 1.00–1.00, p < 0.0001) had the best discriminatory power, and miR-146b-3p had the large area under the curve (AUC: 0.96, 95% confidence interval 0.96–1.00, p < 0.0001) and consequently the high discriminatory power. Between these three miRNAs, miR-193b and miR-146b-3p had a high capacity to distinguish between melanoma patients and control groups that are appropriate to be applied in melanoma diagnosis as an early and noninvasive method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is a malignant tumor that exhibits the most serious clinical symptoms and the highest mortality rate among skin cancers [1,2,3]. The incidence of melanoma cases and deaths increased in 204 countries from 1990 to 2019 [1]. Melanoma represents only 1–2% of all cancer cases in the world and less than 5% of skin cancers but is responsible for approximately 95% of skin cancer deaths [4].
There are three major types of skin cancer: basal cell carcinoma, squamous cell carcinoma and melanoma [5]. Melanocytes in the human skin are able to produce melanin, and altered melanocyte proliferation causes melanoma [6, 7]. The process of melanoma genesis is a linear progression of normal melanocytes through various precursor lesions and ultimately to melanoma [7].
Currently, the diagnosis of melanoma requires removal and analysis of primary melanoma and to determine the presence and stage of metastatic disease when high-risk markers are detected, a sentinel lymph node biopsy is required [8, 9]. This is invasive, costly and time-consuming. Consequently, given the clinical importance of melanoma, some laboratory methods are noninvasive, allowing economical, rapid and repetitive sampling and thus enabling early intervention and dynamic treatment management. Much effort has been put into identifying blood-based biomarkers [8, 9]. Circulating microRNAs (miRNAs) have emerged as potential noninvasive biomarkers for melanoma. To date, several efforts have been made to identify circulating miRNA biomarkers for the diagnostic and prognostic usefulness of melanoma [10].
MiRNAs are stable and easily detectable molecules in biological fluids that appear as potential candidate biomarkers [10]. MiRNAs are a type of small, single-stranded, non-coding RNAs with a length of 18–25 nucleotides that can play the role of oncogenes and tumor suppressors [11, 12]. MiRNAs can be released into the human serum or plasma and can be identified in melanoma cells. Studies evaluating serum or plasma miRNAs as biomarkers in skin melanoma have focused on discriminating melanoma patients from control groups [11, 13].
The aberrant expression of miRNA has been detected in tumors and biofluids [14]. Using molecular biomarkers will suggest the potential to determine melanoma before it is evidently visible or symptomatic, and alleviate easy detection without even minimum surgical operation [15, 16].
In our study, system biology and bioinformatics approaches were used to analyze gene expression data to determine differentially expressed microRNAs (DEMIRs) between control and cutaneous melanoma plasma samples. Three datasets were acquired from the Gene Expression Omnibus (GEO) database of the National Center for Biotechnology Information (NCBI) to identify miRNAs that were differentially expressed that could serve as potential biomarkers for melanoma diagnosis.
We aimed to study the expression of three miRNAs, miR-193b-3p, miR-146b-3p and miR-483-3p in melanoma cancer. We quantified the expression levels of these three miRNAs by stem-loop real-time PCR, which detects miRNA expression levels with higher specificity and sensitivity than conventional detection methods [17, 18]. Twenty-seven normal and paired melanoma cancer specimens were selected for this study. In addition, we analyzed differences in miRNA expression between melanoma patients and control groups.
Materials and Methods
Dataset Analysis
MiRNA expression microarray data related to cutaneous melanoma were downloaded from the Gene Expression Omnibus (GEO) database. The following keywords were used in the GEO database: cutaneous OR skin melanoma AND miRNA expression AND human [organism]. The microarray data platform was Febit Homo sapiens miRbase 13.0 (GPL9040) for three datasets.
We preprocessed and analyzed microarray raw data using MATLAB software (R2014b). First, preprocessing of miRNA expression raw data was performed by transferring all miRNA expression values to log2, and by indicating the normalization step (using the quantile norm function in MATLAB). Then, p-values for two different datasets (control vs. cancer) were estimated using the mat test function in MATLAB [18].
Finally, fold change values were derived based on expression data and p-value. This procedure was repeated for cancer and control sets of data.
Differentially expressed (DE) analysis was performed by comparing miRNA expression between melanoma and control plasma. A volcano plot was drawn to show fold change and p-values of DEMIRNAs between melanoma patients and control groups. Remarkably up- and downregulated miRNAs were described as those with an adjusted p-value < 0.05 and a fold change greater than 2, log2 (fold change) > 1 or < − 1 for up- and downregulated miRNAs, respectively [19].
Study Population
The statistical population was obtained by the Cochran formula of 27 melanoma patients and 27 control groups.
Blood samples of melanoma patients and control groups were collected from Imam Khomeini hospital complex and Razi hospital of Tehran. All procedures performed in studies involving human participants were in accordance with the ethical principles and the national norms and standards for conducting medical research in iran. Approval ID: IR.TUMS.MEDICINE.REC.1400.453, evaluated by Research Ethics Committees of School of Medicine—Tehran University of Medical Sciences. Samples were collected regardless of age and gender, melanoma patients are from stages I to IV. All patients had histological and pathological tests that confirmed melanoma (Table 1).
Samples were collected from the hospital in the form of blood in the EDTA tube. Then blood samples were quickly transferred from the hospital to the laboratory, centrifuged at 7000×g for 10 min, and then plasma isolated and stored at − 70 °C until further use.
RNA Extraction
Total RNA was extracted using the RNX plus™ kit (CinnaGen, Tehran, Iran. Cat No: EX6101) based on the manufacturer’s recommendations. Then, 300 µL of the serum sample was homogenized with 200 µL of the RNX-PLUS solution and incubated at room temperature for 5 min. Chloroform (200 μL) was added to the solution and centrifuged at 4 °C at 10,000×g for 20 min. The supernatant was transferred to another tube and an equal volume of isopropanol was added. The mixture was incubated at − 20 °C for 30 min. Then the mixture was centrifuged at 4 °C at 10,000×g for 20 min and the resulting pellet was washed in ethanol (75%) and centrifuged at 4 °C at 7500×g for 8 min. The supernatant was discarded and the pellet was left to dry at room temperature for a few minutes then dissolved in 40 µL Diethyl pyro carbonate (DEPC)-treated water. The purity and the integrity of the extracted RNA were evaluated by optical density measurements and visual observation of sample electrophoresis on 2% agarose gel and a Nano Drop spectrophotometer [20].
Primers
Reverse primer The universal reverse primer was obtained from an article by Yang et al. [21] and ordered for synthesis by Pishgam Biotech Co., Ltd. (Tehran, IRAN). (Primer sequences are shown in Table 2).
Forward primer Forward primers for each miRNA were designed according to the Shi et al., Busk et al. and Esmaeili-bandboni et al. protocols [22,23,24], and ordered for synthesis by Pishgam Biotech Co., Ltd. (Tehran, IRAN). (Primer sequences are shown in Table 2).
cDNA Synthesis
The universal stem-loop primer of the miRNAs was extracted from an article by Yang et al. [21] and ordered for synthesis by Pishgam Biotech co. Ltd. (Tehran, IRAN). (Primer sequences are shown in Table 2). For using stem-loop in cDNA synthesis, it was heated to 95 °C for 10 m. The heat was reduced slowly to 75 °C then the temperature was held at 75°, 68°, 65° and 62 °C for an hour each, held at 60 °C for several more hours and finally the primer was transferred to a new tube. This step should never need repeating if the primer is never heated above room temperature. The working stocks were kept at − 20 °C.
Following RNA extraction, each sample was reverse transcribed using thermos-resistant H minus M-MULV reverse transcriptase (Pars Tous, Mashhad, Iran. Cat No: C101031). For synthesizing cDNA of miRNA, 7 µL RNA, 5 µL stem-loop, 2 µL thermos-resistant RT enzyme, 4 µL 5× RT buffer and 2 µL dNTP were added together to make up a final volume of 20 µL reaction mix. The reaction mix was incubated in a thermocycler for 20 min at 16 °C, 10 min at 25 °C, 1 h at 47 °C and 10 min at 70 °C at last was chilled on ice. The cDNA was stored at − 70 °C [25].
Real-Time PCR
Each cDNA (2 µL) of each control and melanoma samples was diluted to a volume of 20 µL of PCR mix containing 3 µL of primer, 5 µL of DEPC water and 10 µL of SYBR Green master mix (Ampliqon, Denmark. Cat No: A190303). Primers were purchased from Pishgam Biotech Co (Tehran, Iran). For amplification, an initial denaturation step at 95 °C for 15 min was used. For 40 subsequent cycles, the conditions were denaturation for 30 s at 95 °C, annealing for 30 s at 60 °C and elongation for 30 s at 72 °C. The melting curve was at 60–95 °C [26]. For the amplification of miRNAs, the Corbet Rotor-Gene 3000 system was used. The expression of each miRNA was analyzed in plasma samples of 27 melanoma patients and 27 control groups.
The relative expression of miRNAs in plasma was normalized to the expression of miR-16. Data were analyzed with \(\Delta \Delta CT\) method [27]. Relative expression of each person in melanoma patients and control groups was performed with mean \(\Delta CT\) of control group.
Statistical Analysis
GraphPad Prism version 8.0 (Graph Pad software) and SPSS 26.0 software (IBM) were used for statistical analysis. The data were tested for normality and were found to be different, so the nonparametric Mann–Whitney U test (a nonparametric test that compares the distribution of two unmatched groups) was applied to compare two groups of melanoma patients and control groups. Receiving operating characteristic (ROC) curve analysis was then used to evaluate the discriminatory power of the combinations. Statistical significance was set at p-values < 0.05.
Results
Identification of DEmiRs
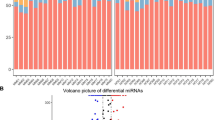
Analysis of the GSE61741 dataset identified 141 DEMIRs between melanoma patients and control groups; these included 85 upregulated and 56 downregulated miRNAs (Fig. 1a), analysis of the GSE20994 dataset identified 273 DEMIRs between melanoma patients and control groups; these included 145 upregulated and 128 downregulated miRNAs (Fig. 1b). Analysis of the GSE31568 dataset identified 11 DEMIRs between melanoma patients and control groups; these included five upregulated and six downregulated miRNAs (Fig. 1c).
Expression of differentially expressed miRNAs (DEMIRs) in the three datasets. a Volcano plot of DEMIRs between melanoma patients and control groups in GSE61741. b Volcano plot of DEMIRs between melanoma patients and control groups in GSE20994. c Volcano plot of DEMIRs between melanoma patients and control groups in GSE31568. (X axis represents fold change and Y axis represents p-value)
Remarkable Downregulation of miR-146b-3p and miR-193b
It was observed that miR-146b-3p and miR-193b expression levels decreased in the melanoma patients contrasted with control groups. After statistical analysis by unpaired Mann–Whitney U test, significant downregulation was observed, p < 0.0001. Furthermore, to contrast mean fold changes of expression levels of miR-146b-3p and miR-193b, among melanoma patients and control groups, it was shown that there was a decreased level of expression in the melanoma patients with the control groups, which was decreased 5 × \({10}^{6}\)-fold in miR-193b (This amount of decreased expression means close to zero expression in melanoma) and 58-fold in miR-146b-3p, respectively (Fig. 2).
Comparative expression of miR-193b, miR-146b-3p and miR-483-3p, comparisons were performed using the Mann–Whiney U test; Y axis represents fold changes using the \(\Delta \Delta CT\) method. The levels of miR-193b and miR-146b-3p decreased in melanoma patients versus control groups. However, all of these findings were significant (p < 0.0001 for miR-193b and miR-146-3p, p < 0.04 for miR-483-3P, p-values less than 0.0001 are summarized with four asterisks and p-values less than 0.05 are summarized with one asterisk)
For miR-483-3p, the statistical analysis by unpaired Mann–Whitney U test demonstrated that the melanoma patients had a higher level of expression compared to the control groups. Furthermore, the mean fold changes demonstrated that the melanoma patients had 3.5-fold higher levels of expression contrasted to the control groups (Fig. 2).
Great Distinguishing Power of microRNA Biomarkers
To measure the distinguishing power of the three microRNAs biomarkers for melanoma diagnosis, receiver operating characteristic (ROC) curve analysis for each of the patients was performed.
As displayed in Fig. 3, miR-193b had the best distinguishing power. For this marker, area under the curve (AUC) in comparison among the melanoma patients and control groups was 1.00 (95% confidence interval [CI] 1.00–1.00; p < 0.0001). In addition, miR-146b-3p had high distinguishing power, for this marker, AUC was 0.96 (95% confidence interval [CI] 0.96–1.00, p < 0.0001). However, miR-483-3p had low distinguishing power, for this marker, AUC was 0.31 (95% confidence interval [CI] 0.31–1.00, p = 0.04). Consequently, miR-193b had the highest and the best distinguishing power for melanoma diagnosis in this study.
Discussion
The prevalence of melanoma is expanding in the world. This illness is treatable when diagnosed in preliminary stages and it might be fatal when detected in developed stages. Though recently new and extremely impressive treatments came into clinical use, the fatality rate of stage IV melanoma patients is still high [28, 29]. Melanoma should be treated in preliminary stages to amplify the possibilities of patient survival. The capability to determine preliminary symptoms of melanoma progression would be very valuable in a clinical setting. Several studies have indicated that miRNA expression is correlated with cell proliferation, metastasis, invasion and response to therapy. Circulating miRNAs, are resistant, extremely specific, sensitive and easily detectable molecules in biological fluids revealed as potential noninvasive biomarkers for diagnosing cancers. These are major characteristics to look for in a potential circulating biomarker [30]. To date, trustworthy biomarker has been distinguished as sensitive or specific enough to be useful for the preliminary discovery of melanoma in each of the stages. Trustworthy circulating biomarker to identify melanoma with distant or regional spread prior to clinical evidence of metastasis might develop therapy and result for melanoma patient.
In this study, we recognized three miRNAs with desirable diagnostic power in the plasma of melanoma patients at distinct stages of the disease. Two of them, miR-146b-3p and miR-193b were significantly downregulated, while one of them, miR-483-3p was significantly upregulated in the plasma of melanoma patients contrasted with control groups. While we observed low considerable levels of miR-193b from plasma samples of melanoma patients compared with control groups, Kardab et al. obtained lower miR-193b levels in melanoma cell lines [29]. Furthermore, other authors observed decreased miR-193b expression in melanoma cell lines and tissue samples [31, 32]. This suggests that miR-193b has a functional role as a tumor suppressor in human melanoma. Concerning miR-193b, there is some evidence that suggests that in some cancer varieties other than melanoma, it may operate as a tumor suppressor. For instance, miR-193b expression was reduced in human ovarian cancer tissues and cell lines compared with controls, miR-193b might be nearly associated with the metastatic potential of ovarian cancer cells via increasing uPA expression [33, 34]. Furthermore, Wu et al. revealed that the expression of miR-193b was notably downregulated in endometrioid adenocarcinoma [35]. Rauhala et al. investigated that miR-193b is a tumor suppressor in prostate cancer [36]. The same authors described the decreased expression of miR-193b in melanoma cell lines and tissues [37]. Chen et al. identified that Mcl-1, an antiapoptotic protein associated with the Bcl-2 family was upregulated in malignant melanoma. They presented that the expression of miR-193b was related conversely to the expression of Mcl-1 in melanoma tissue samples. So overexpression of miR-193b suppresses Mcl-1 levels in some melanoma cell lines [31, 32]. Chen et al. reported that miR-193b overexpression decreases cell proliferation in melanoma cell lines and recommended that miR-193b may regulate cell cycle advancement by downregulating the expression of CCND1 protein [31]. Leivonen et al. identified extinguishing some targets of miR-193b such as AKR1C2 and AKR1C1 suppresses breast cancer cell growth in the MCF-7 cell line [38]. In addition, it was determined that miR-193b instantly targets estrogen receptor a (ERa) and ultimately represses cancer cell growth [38]. It was identified that miR-193b is downregulated in breast cancer tissues and has been illustrated as a tumor suppressor associated with clinical metastasis. Significantly there was a contrary relation among miR-193b and it is a direct target dimethylaminohydrolase (DDAH1) expression in breast cancer cell lines that miR-193b regulates cell proliferation and migration in breast cancer cell line, while DDAH1 knock down represses cell migration [39, 40]. A latter study verified that silencing of miR-193b in colorectal cancer cells (CRC) remarkably reduces cell proliferation and induced apoptosis. In detail, the downregulation of miR-193b evidently activated the expression of SMAD3 and TGF-β in CRC cells [41, 42]. A prior study determined miR-193b was downregulated importantly in hepatocellular carcinoma (HCC) tissues contrasted with normal liver tissues. Furthermore, the expression level of miR-193b was reduced in hepatic cirrhosis [43]. It was determined that miR-193b may have a tumor suppressor role in hepatocellular carcinoma (HCC) progression by direct targeting of Cyclin D1 (CCND1) and ETS1, which causes cell cycle arrest and prevents the invasion and migration of hepatoma cells [44].
Evidently, the tumorigenesis function of miR-193b is associated with the upregulation or downregulation of particular targets, which leads to cancer cell growth, migration, invasion and metastasis in the origination and development of various cancers (Fig. 4).
In this study, miR-146b-3p was downregulated in the plasma of melanoma patients contrasted with control groups. Although, upregulation of miR-146a has been discovered in preliminary and metastatic cutaneous melanoma, and it may be particular for metastatic disease. It was obvious that miR-146a and miR-146b have oncogenic functions in cutaneous melanoma whereas miR-146a promotes both initiation and progression of BRAF/NRAS-mutated cutaneous melanoma by promoting activation of the NOTCH protein and NOTCH1 is implicated in melanoma formation and may improve the metastatic potential of primary melanoma cells through activation of the mitogen-activated protein kinase (MAPK) pathway or the PI3K/Akt pathway. Upregulation of miR-146b expression has been revealed in cutaneous melanoma and emerges to be related to melanoma evolution. MiR-146a-5p was significantly upregulated in fresh frozen tissue samples from conjunctival melanoma and these miRNAs may have the same function in conjunctival melanoma [45]. This suggests that miR-146a and miR-146b may have a similar function in a particular tumor. The mature sequences for miR-146a and miR-146b are extremely preserved across species. Furthermore, the two miRNAs are different in two nucleotides on the 3′-end of the mature strand, not within the seed region [46]. Li et al. found that miR-146b-5p has been revealed as aberrantly downregulated in gliomas, the correlation between its expression level and the grade of tumor malignity remains to be clarified, the consequences showed that the expression of miR-146b-5p reduced with the upgrading of the tumors malignity grade, demonstrating that miR-146b-5p downregulation might play a crucial role in the incidence and malignant advancement of gliomas [47]. Furthermore, Yu et al. established that miR-146b-5p and miR-146b-3p expression were remarkably upregulated in thyroid cancer tissues and cell lines compared with normal thyroid tissue and cells [48]. Mature miRNA-3p and miRNA-5p are created from the identical pre-miRNA progenitor, but more often, one of them is degenerated, though the co-existence of these two species has also been reported [49]. Su et al. showed that miR-146b-3p altered expression is nearly associated with inflammation and thrombosis. Inflammatory factors can cause thrombosis via the miR-146b-3p/P38MAPK/COX-2 pathway [50]. These clear diversities may be due to the complicated functional role of miR-146b-3p in tumor biology.
The third miRNA found upregulated in this study is miR-483-3p, that level was significantly elevated in the plasma of melanoma patients than in control groups. This study investigated the relationship between miR-483-3p and melanoma for the first time. Regarding miR-483-3p, there is some evidence that suggests that it may be upregulated in certain cancers other than melanoma. For instance, miR-483-3p is overexpressed in 30% of the colon, breast and liver cancers tissues and 100% of Wilms’ tumors. Veronese et al. determined an association between miR-483-3p, a miRNA placed within the IGF2 location at chromosome 11p15.5, and human tumorigenesis. This indicated that a critical oncogenic function was correlated with the miR-483-3p within the IGF2 location [51]. Upregulation of miR-483-3p was also found in pancreatic cancers. miR-483-3p expression was conversely coordinated with Dpc4/Smad4 in pancreatic cancer, and upregulated miR-483-3p in pancreatic cancer suppressed the expression levels of Dpc4/Smad4, which in turn increases carcinogenesis [52]. In apparent contradiction with these findings but in according with those of this study, in some cancers, including gastric, pancreatic, and hepatocellular carcinomas, miR-483-3p levels were discovered to be decreased in tissues of patients, and it could have anti-tumor features in those cancers [53]. Accumulated evidence suggested that the roles of miR-483-3p in carcinogenesis were complicated. Furthermore, these data demonstrate that different functions of miR-483-3p in various kinds of cancer depend on cancer types and cellular context.
Conclusions
In conclusion, our study determined three miRNAs, which were first distinguished in the plasma of melanoma patients. miR-193b, miR-146b-3p and miR-483-3p are appropriate diagnostic biomarkers in human melanoma and deserve validation in future prospective clinical trials. The most important limitation of this study was the small and rare number of melanoma cases, which led to spending a lot of time to reach the desired sample size in terms of statistics. According to the research result, miR-193, through particular targets and biological pathways, play remarkable roles in the origination and development of pivotal biological and pathological procedures in a broad range of significant human diseases, especially different cancers. More significantly, miR-193 can be contemplated to be a therapeutic biomarker in cancer treatment by improving or suppressing particular molecular procedures. Therefore, miR-193b is the best one of these three miRNAs with the best discriminatory power in melanoma diagnosis. However, according to the limitations of the study, these three biomarkers should be investigated in other cancers to show the specificity of these biomarkers in melanoma diagnosis with more certainty.
Data Availability
Data are unavailable due to privacy or ethical restrictions.
References
Li, Z., Fang, Y., Chen, H., Zhang, T., Yin, X., Man, J., Yang, X., & Lu, M. (2022). Spatiotemporal trends of the global burden of melanoma in 204 countries and territories from 1990 to 2019: Results from the 2019 global burden of disease study. Neoplasia, 24(1), 12–21. https://doi.org/10.1016/j.neo.2021.11.013
Prasad, R., & Katiyar, S. K. (2014). Down-regulation of miRNA-106b inhibits growth of melanoma cells by promoting G1-phase cell cycle arrest and reactivation of p21/WAF1/Cip1 protein. Oncotarget, 5(21), 10636–10649. https://doi.org/10.18632/oncotarget.2527
Liu, X., Fang, H., Chen, H., Jiang, X., Fang, D., Wang, Y., & Zhu, D. (2012). An artificial miRNA against HPSE suppresses melanoma invasion properties, correlating with a down-regulation of chemokines and MAPK phosphorylation. PLoS ONE. https://doi.org/10.1371/journal.pone.0038659
Bai, R., Huang, H., Li, M., & Chu, M. (2021). Temporal trends in the incidence and mortality of skin malignant melanoma in China from 1990 to 2019. Journal of Oncology, 2021, 9989824. https://doi.org/10.1155/2021/9989824
Banerjee, S., Singh, S. K., Chakraborty, A., Das, A., & Bag, R. (2020). Melanoma diagnosis using deep learning and fuzzy logic. Diagnostics, 10(8), 577. https://doi.org/10.3390/diagnostics10080577
Cichorek, M., Wachulska, M., Stasiewicz, A., & Tymińska, A. (2013). Skin melanocytes: Biology and development. Postepy Dermatologii i Alergologii, 30(1), 30–41. https://doi.org/10.5114/pdia.2013.33376
Varrone, F., & Caputo, E. (2020). The miRNAs role in melanoma and in its resistance to therapy. International Journal of Molecular Sciences, 21(3), 878. https://doi.org/10.3390/ijms21030878
Gogas, H., Eggermont, A. M. M., Hauschild, A., Hersey, P., Mohr, P., Schadendorf, D., Spatz, A., & Dummer, R. (2009). Biomarkers in melanoma. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 20 Suppl 6(Suppl 6), vi8–vi13. https://doi.org/10.1093/annonc/mdp251
Mumford, S. L., Towler, B. P., Pashler, A. L., Gilleard, O., Martin, Y., & Newbury, S. F. (2018). Circulating microRNA biomarkers in melanoma: Tools and challenges in personalised medicine. Biomolecules, 8(2), 21. https://doi.org/10.3390/biom8020021
Carpi, S., Polini, B., Fogli, S., Podestà, A., Ylösmäki, E., Cerullo, V., Romanini, A., & Nieri, P. (2020). Circulating microRNAs as biomarkers for early diagnosis of cutaneous melanoma. Expert Review of Molecular Diagnostics, 20(1), 19–30. https://doi.org/10.1080/14737159.2020.1696194
Lu, T., Chen, S., Qu, L., Wang, Y., Chen, H. D., & He, C. (2019). Identification of a five-miRNA signature predicting survival in cutaneous melanoma cancer patients. PeerJ. https://doi.org/10.7717/peerj.7831
Wang, J., Fang, Y., Liu, Y. F., Wang, X., Wang, X. L., Wang, R. Y., & Meng, Z. D. (2019). MiR-154 inhibits cells proliferation and metastasis in melanoma by targeting AURKA and serves as a novel prognostic indicator. European Review for Medical and Pharmacological Sciences, 23(10), 4275–4284. https://doi.org/10.26355/eurrev_201905_17932
Friedman, E. B., Shang, S., de Miera, E. V., Fog, J. U., Teilum, M. W., Ma, M. W., Berman, R. S., Shapiro, R. L., Pavlick, A. C., Hernando, E., Baker, A., & Osman, I. (2012). Serum microRNAs as biomarkers for recurrence in melanoma. Journal of Translational Medicine, 10(1), 155. https://doi.org/10.1186/1479-5876-10-155
Neagu, M., Constantin, C., Cretoiu, S. M., & Zurac, S. (2020). miRNAs in the diagnosis and prognosis of skin cancer. Frontiers in Cell and Developmental Biology, 8, 71. https://doi.org/10.3389/fcell.2020.00071
Deacon, D. C., Smith, E. A., & Judson-Torres, R. L. (2021). Molecular biomarkers for melanoma screening, diagnosis and prognosis: Current state and future prospects. Frontiers in Medicine. https://doi.org/10.3389/fmed.2021.642380
Dahmke, I. N., Backes, C., Rudzitis-Auth, J., Laschke, M. W., Leidinger, P., Menger, M. D., Meese, E., & Mahlknecht, U. (2013). Curcumin intake affects miRNA signature in murine melanoma with mmu-miR-205-5p most significantly altered. PLoS ONE. https://doi.org/10.1371/journal.pone.0081122
Chen, C., Ridzon, D. A., Broomer, A. J., Zhou, Z., Lee, D. H., Nguyen, J. T., Barbisin, M., Xu, N. L., Mahuvakar, V. R., Andersen, M. R., Lao, K. Q., Livak, K. J., & Guegler, K. J. (2005). Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Research, 33(20), e179–e179. https://doi.org/10.1093/nar/gni178
Ghorbani, M., Pourjafar, F., Saffari, M., & Asgari, Y. (2020). Paclitaxel resistance resulted in a stem-like state in triple-negative breast cancer: A systems biology approach. Meta Gene, 26, 100800. https://doi.org/10.1016/j.mgene.2020.100800
Akiyama, Y., Kiyohara, Y., Yoshikawa, S., Otsuka, M., Kondou, R., Nonomura, C., Miyata, H., Iizuka, A., Ashizawa, T., Ohshima, K., Urakami, K., Nagashima, T., Kusuhara, M., Sugino, T., & Yamaguchi, K. (2018). Immune response-associated gene profiling in Japanese melanoma patients using multi-omics analysis. Oncology Reports, 39(3), 1125–1131. https://doi.org/10.3892/or.2017.6173
Salehi, P., Tafvizi, F., & Kamyab Hesari, K. (2019). Low expression of occludin in the melanoma patient. Iranian Journal of Pathology, 14(4), 272–278. https://doi.org/10.30699/ijp.2019.85213.1801
Yang, L. H., Wang, S. L., Tang, L. L., Liu, B., Ye, W. L., Wang, L. L., Wang, Z. Y., Zhou, M. T., & Chen, B. C. (2015). Universal stem-loop primer method for screening and quantification of microRNA. PLoS ONE, 9(12), e115293. https://doi.org/10.1371/journal.pone.0115293
Shi, R., Sun, Y. -H., Zhang, X. -H., & Chiang, V. L. (2012). Poly(T) adaptor RT-PCR. In: Fan, JB. (eds), Next-generation microRNA expression profiling technology. Methods in Molecular Biology (pp. 53–66). Humana Press, Totowa, NJ
Esmaeili-bandboni, A., Bagheri, J., Bakhshandeh, A. R., Mohammadnejad, J., & Sadroddiny, E. (2018). Serum levels of miR-155, miR-326, and miR-133b as early diagnostic biomarkers for the detection of human acute heart allograft rejection in comparison with serum cardiac troponin T. The Heart Surgery Forum, 21(2), E101–E107. https://doi.org/10.1532/hsf.1887
Busk, P. K. (2014). A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics, 15(1), 1–9. https://doi.org/10.1186/1471-2105-15-29
Barartabar, Z., Moini, N., Abbasalipourkabir, R., Mesbah-Namin, S. A., & Ziamajidi, N. (2022). Investigation of miR-133a, miR-637 and miR-944 genes expression and their relationship with PI3K/AKT signaling in women with breast cancer.
Bahramy, A., Zafari, N., Izadi, P., Soleymani, F., Kavousi, S., & Noruzinia, M. (2021). The role of miRNAs 340-5p, 92a-3p, and 381-3p in patients with endometriosis: A plasma and mesenchymal stem-like cell study. BioMed Research International, 2021, 5298006. https://doi.org/10.1155/2021/5298006
Rao, X., Huang, X., Zhou, Z., & Lin, X. (2013). An improvement of the 2^(− delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostatistics, Bioinformatics and Biomathematics, 3(3), 71–85.
Garbe, C., Peris, K., Hauschild, A., Saiag, P., Middleton, M., Bastholt, L., Grob, J. J., Malvehy, J., Newton-Bishop, J., Stratigos, A. J., & Pehamberger, H. (2016). Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—Update 2016. European Journal of Cancer, 63, 201–217. https://doi.org/10.1016/j.ejca.2016.05.005
Kordaß, T., Weber, C. E. M., Eisel, D., Pane, A. A., Osen, W., & Eichmüller, S. B. (2018). miR-193b and miR-30c-1(*) inhibit, whereas miR-576-5p enhances melanoma cell invasion in vitro. Oncotarget, 9(65), 32507–32522. https://doi.org/10.18632/oncotarget.25986
Wang, Q., Xu, K., Tong, Y., Dai, X., Xu, T., He, D., & Ying, J. (2020). Novel miRNA markers for the diagnosis and prognosis of endometrial cancer. Journal of Cellular and Molecular Medicine, 24(8), 4533–4546. https://doi.org/10.1111/jcmm.15111
Chen, J., Feilotter, H. E., Paré, G. C., Zhang, X., Pemberton, J. G., Garady, C., Lai, D., Yang, X., & Tron, V. A. (2010). MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. American Journal of Pathology, 176(5), 2520–2529. https://doi.org/10.2353/ajpath.2010.091061
Chen, J., Abi-Daoud, M., Wang, A., Yang, X., Zhang, X., Feilotter, H. E., & Tron, V. A. (2013). Stathmin 1 is a potential novel oncogene in melanoma. Oncogene, 32(10), 1330–1337. https://doi.org/10.1038/onc.2012.141
Mitra, A. K., Chiang, C. Y., Tiwari, P., Tomar, S., Watters, K. M., Peter, M. E., & Lengyel, E. (2015). Microenvironment-induced down regulation of miR-193b drives ovarian cancer metastasis. Oncogene, 34(48), 5923–5932. https://doi.org/10.1038/onc.2015.43
Li, H., Xu, Y., Qiu, W., Zhao, D., & Zhang, Y. (2015). Tissue miR-193b as a novel biomarker for patients with ovarian cancer. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 21, 3929–3934. https://doi.org/10.12659/msm.895407
Wu, W., Lin, Z., Zhuang, Z., & Liang, X. (2009). Expression profile of mammalian microRNAs in endometrioid adenocarcinoma. European Journal of Cancer Prevention: Official Journal of the European Cancer Prevention Organization, 18(1), 50–55. https://doi.org/10.1097/CEJ.0b013e328305a07a
Rauhala, H. E., Jalava, S. E., Isotalo, J., Bracken, H., Lehmusvaara, S., Tammela, T. L., Oja, H., & Visakorpi, T. (2010). miR-193b is an epigenetically regulated putative tumor suppressor in prostate cancer. International Journal of Cancer, 127(6), 1363–1372. https://doi.org/10.1002/ijc.25162
Khordadmehr, M., Shahbazi, R., Sadreddini, S., & Baradaran, B. (2019). miR-193: A new weapon against cancer. Journal of Cellular Physiology, 234(10), 16861–16872. https://doi.org/10.1002/jcp.28368
Leivonen, S. K., Mäkelä, R., Östling, P., Kohonen, P., Haapa-Paananen, S., Kleivi, K., Enerly, E., Aakula, A., Hellström, K., Sahlberg, N., & Kristensen, V. N. (2009). Protein lysate microarray analysis to identify microRNAs regulating estrogen receptor signaling in breast cancer cell lines. Oncogene, 28(44), 3926–3936. https://doi.org/10.1038/onc.2009.241
Hulin, J.-A., Tommasi, S., Elliot, D., Hu, D. G., Lewis, B. C., & Mangoni, A. A. (2017). MiR-193b regulates breast cancer cell migration and vasculogenic mimicry by targeting dimethylarginine dimethylaminohydrolase 1. Scientific Reports, 7(1), 13996. https://doi.org/10.1038/s41598-017-14454-1
Li, X.-F., Yan, P.-J., & Shao, Z.-M. (2009). Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene, 28(44), 3937–3948. https://doi.org/10.1038/onc.2009.245
Wu, K., Zhao, Z., Ma, J., Chen, J., Peng, J., Yang, S., & He, Y. (2017). Deregulation of miR-193b affects the growth of colon cancer cells via transforming growth factor-β and regulation of the SMAD3 pathway. Oncology Letters, 13(4), 2557–2562. https://doi.org/10.3892/ol.2017.5763
Zhong, Q., Wang, T., Lu, P., Zhang, R., Zou, J., & Yuan, S. (2014). miR-193b promotes cell proliferation by targeting SMAD3 in human glioma. Journal of Neuroscience Research, 92(5), 619–626. https://doi.org/10.1002/jnr.23339
Salvi, A., Conde, I., Abeni, E., Arici, B., Grossi, I., Specchia, C., Portolani, N., Barlati, S., & De Petro, G. (2013). Effects of miR-193a and sorafenib on hepatocellular carcinoma cells. Molecular Cancer, 12, 162. https://doi.org/10.1186/1476-4598-12-162
Xu, C., Liu, S., Fu, H., Li, S., Tie, Y., Zhu, J., Xing, R., Jin, Y., Sun, Z., & Zheng, X. (2010). MicroRNA-193b regulates proliferation, migration and invasion in human hepatocellular carcinoma cells. European Journal of Cancer, 46(15), 2828–2836. https://doi.org/10.1016/j.ejca.2010.06.127
Larsen, A. C., Mikkelsen, L. H., Borup, R., Kiss, K., Toft, P. B., von Buchwald, C., Coupland, S. E., Prause, J. U., & Heegaard, S. (2016). MicroRNA expression profile in conjunctival melanoma. Investigative Ophthalmology & Visual Science, 57(10), 4205–4212. https://doi.org/10.1167/iovs.16-19862
Paterson, M. R., & Kriegel, A. J. (2017). MiR-146a/b: A family with shared seeds and different roots. Physiological Genomics, 49(4), 243–252. https://doi.org/10.1152/physiolgenomics.00133.2016
Li, Y., Wang, Y., Yu, L., Sun, C., Cheng, D., Yu, S., Wang, Q., Yan, Y., Kang, C., Jin, S., & An, T. (2013). miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Letters, 339(2), 260–269. https://doi.org/10.1016/j.canlet.2013.06.018
Yu, C., Zhang, L., Luo, D., Yan, F., Liu, J., Shao, S., Zhao, L., Jin, T., Zhao, J., & Gao, L. (2018). MicroRNA-146b-3p promotes cell metastasis by directly targeting NF2 in human papillary thyroid cancer. Thyroid, 28(12), 1627–1641. https://doi.org/10.1089/thy.2017.0626
Jagadeeswaran, G., Zheng, Y., Sumathipala, N., Jiang, H., Arrese, E. L., Soulages, J. L., Zhang, W., & Sunkar, R. (2010). Deep sequencing of small RNA libraries reveals dynamic regulation of conserved and novel microRNAs and microRNA-stars during silkworm development. BMC Genomics, 11(1), 52. https://doi.org/10.1186/1471-2164-11-52
Su, Z., & Wu, F. (2020). Inflammatory factors induce thrombosis through the miR-146b-3p/p38MAPK/COX-2 pathway. BioMed Research International, 2020, 8718321. https://doi.org/10.1155/2020/8718321
Veronese, A., Lupini, L., Consiglio, J., Visone, R., Ferracin, M., Fornari, F., Zanesi, N., Alder, H., D’Elia, G., Gramantieri, L., & Bolondi, L. (2010). Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Research, 70(8), 3140–3149. https://doi.org/10.1158/0008-5472.CAN-09-4456
Hao, J., Zhang, S., Zhou, Y., Hu, X., & Shao, C. (2011). MicroRNA 483-3p suppresses the expression of DPC4/SMAD4 in pancreatic cancer. FEBS Letters, 585(1), 207–213. https://doi.org/10.1016/j.febslet.2010.11.039
Yu, X., & Li, Z. (2016). The role of miRNAs in cutaneous squamous cell carcinoma. Journal of Cellular and Molecular Medicine, 20(1), 3–9. https://doi.org/10.1111/jcmm.12649
Funding
This research received no funding.
Author information
Authors and Affiliations
Contributions
ZVM, YA, MAM and AM contributed to the study conception and design. Human samples preparation was performed by SRM, HRM and SH and AM. Material preparation were performed by ZVM and AM. Data collection and analysis were performed by YA, AEB and AM. The first draft of manuscript was written by AM. All authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical principles and the national norms and standards for conducting medical research in Iran. Approval ID: IR.TUMS.MEDICINE.REC.1400.453, evaluated by Research Ethics Committees of School of Medicine—Tehran University of Medical Sciences.
Consent to Participate
Informed consent was obtained from all subjects involved in the study.
Consent to Publish
The authors claim that human research subjects gave their informed permission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadloo, A., Asgari, Y., Esmaeili-Bandboni, A. et al. The Potential of Circulating miR-193b, miR-146b-3p and miR-483-3p as Noninvasive Biomarkers in Cutaneous Melanoma Patients. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00893-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00893-x







