Abstract
Lipidic carriers are composed of natural, synthetic, or physiological lipid/phospholipid materials. The flexibility of lipid-based delivery systems for transferring a variety of molecules such as immunomodulators, antigens, and drugs play a key role in design of effective vaccination and therapeutic strategies against infectious and non-infectious diseases. Genetic and subunit vaccines are two major groups of promising vaccines that have the potential for improving the protective potency against different diseases. These vaccine strategies rely greatly on delivery systems with various functions, including cargo protection, targeted delivery, high bioavailability, controlled release of antigens, selective induction of antigen-specific humoral or cellular immune responses, and low side effects. Lipidic carriers play a key role in local tissue distribution, retention, trafficking, uptake and processing by antigen-presenting cells. Moreover, lipid nanoparticles have successfully achieved to the clinic for the delivery of mRNA. Their broad potential was shown by the recent approval of COVID-19 mRNA vaccines. However, size, charge, architecture, and composition need to be characterized to develop a standard lipidic carrier. Regarding the major roles of lipid-based delivery systems in increasing the efficiency and safety of vaccine strategies against different diseases, this review concentrates on their recent advancements in preclinical and clinical trials.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterial-based delivery systems (e.g., lipid-based, polymer-based, inorganic nanomaterials-based vehicles) show desirable efficiency in the development of prophylactic and therapeutic vaccines such as lipid nanoparticles (LNPs) in the context of coronavirus disease 2019 (COVID-19) [1, 2]. These nanoplatforms have been tested for delivery of vaccines in the form of protein subunits and DNA/mRNA sequences encoding the antigens [3,4,5,6]. The designed nanomaterial-based therapeutic vaccines showed outstanding properties, such as encapsulation efficiency, enhancement of immunogenicity, biocompatibility, stability, and induction of specific CD8+ T-cell responses [1].
One of the potential ways for delivery of plasmid-based vaccines, protein-based vaccines, and conventional vaccines is the use of lipid-based vehicles [7]. Lipidic delivery systems include bilayer lipid vesicles, such as liposomes, nanoliposomes, archaeosomes, vesicular lipid gels, immunovesicles, lipospheres, solid lipid nanoparticles (SLN), tocosomes, and some other micro- and nanocarrier systems [8]. The use of lipidic structures (e.g., liposomes, virosomes, immune-stimulating complexes, gas-filled microbubbles, and emulsions) was efficient for the mucosal delivery of antigens, and the induction of local and systemic immune responses in preclinical and clinical trials [9]. Moreover, lipid-based bioactive delivery systems were employed for the encapsulation and targeted release of vaccine components [1].
The lipid-based nano-delivery of drug/vaccine is a therapeutic option for the treatment of infectious diseases (e.g., COVID-19, MERS, SARS, and Ebola) due to some advantages, such as low cost, easy preparation, increased bioavailability, cellular permeability, and uptake and stability of drug/vaccine [10]. Indeed, lipidation was known as a convenient and useful approach to improve the stability and transport across biological membranes. The lipid core peptide (LCP) system has appeared as a promising lipidation tool due to its flexible features and an effective approach for delivery of DNA/oligonucleotides and peptide drugs by improving their uptake, targeting, and enzymatic stability [11]. On the other hand, lipid-based DNA therapeutics could deliver an encoding gene sequence specifically to the target tissue expressing therapeutic protein of interest in unhealthy cells [12]. For example, lipid carrier systems including liposomes were proven to be the most suitable vehicles for delivery of nucleic acid-based drugs into the target tissues and reduction of their toxicity [13]. Ideal nanocarriers for delivery of DNA and in vitro-transcribed mRNA (IVT-mRNA) to the nasal and pulmonary mucosa in an effective vaccination against infectious diseases (e.g., COVID-19) should be able to protect genetic materials, overcome physical and biological barriers at the airway mucosal site, facilitate transfection in targeted epithelial or antigen-presenting cells, and incorporate adjuvants [14]. Recently, two mRNA-based vaccines encapsulated in lipidic delivery system created by BioNTech/Pfizer and Moderna/National Institute of Allergy and Infectious Diseases (NIAID) have become the first approved mRNA vaccines for human use against COVID-19 [15]. On the other hand, the effects of protein therapeutics are increasing in healthcare. Their safety and efficacy are often limited by instability, short half-life, and immunogenicity. Nanodelivery systems could overcome these limitations, such as covalent attachment of biocompatible polymers (e.g., polyethylene glycol (PEG) and other synthetic or naturally derived macromolecules) and protein nanoencapsulation in colloidal systems (e.g., liposomes and other lipid or polymeric nanocarriers) [16].
Generally, lipid-based delivery systems could act as an effective vector for enhancing the potency of vaccines and inducing effective immune protection against severe infectious and non-infectious diseases. However, the physical and chemical properties of lipidic carriers can influence their efficiency and safety in vaccine development. Characterization of these systems is a complex process. Indeed, size, charge, architecture, and composition need to be characterized to develop a standard lipid nanoparticle. Moreover, the safety and toxicity profiles of each novel nanoparticle should be determined to avoid unpredictable adverse effects. The design of lipidic delivery system is more complex with adding surface modification and with coatings and/or ligands. Thus, it is required to optimize a suitable and potent vaccine/lipid formulation for in vivo studies. This review represents the efficiency of lipid-based delivery systems for development of nucleic acid- and protein-based vaccines in preclinical and clinical trials (Graphical Abstract).
Classification of Lipidic Vaccine Carriers
Generally, lipidic vaccine carriers include bilayer lipid vesicles, solid lipid nanoparticles, tocosomes, and some other micro- and nanocarrier systems as follows. Figure 1 shows some properties of major lipidic delivery systems. Lipidic nanocarriers are composed of natural, synthetic, or physiological lipid/phospholipid materials [8].
Liposomes
Liposomes known as bilayer lipid or phospholipid vesicles are mainly composed of amphiphilic lipid and phospholipid molecules (i.e., natural components in different scales). However, other ingredients (e.g., sterols, polypeptides, antioxidants, and polymers) may be added in their structures for modulation of the bilayer structure, enhancement of their half-life in blood circulation, improvement of their tolerance against reactive oxygen species, and development of a targeting strategy for the lipid vesicles [8]. Liposomes improve the stability and efficacy of bioactive compounds by entrapment, release, and delivery to target cells/tissues [8]. Two-layered liposomes are preferred for the formulations due to ease of cellular endocytosis. Cholesterol and polyethylene glycol are used to stabilize liposomes and to avoid immune cell attack, respectively [17]. The first use of liposomes in mRNA vaccines was demonstrated in 1978 by delivery of rabbit globin mRNA sequences to mouse lymphocyte cells [17]. Liposomes and lipid nanoparticles have been developed to improve subunit vaccines against infectious diseases for several decades, such as tuberculosis (TB) subunit vaccines [18, 19]. However, several factors should be optimized to increase the efficacy of liposomes, such as the liposome size, surface charge, and composition of the lipid bilayers [19]. Depending on the preferred effect, ligands such as drug, peptide, cytokine, RNA or nucleotide, and antibody were conjugated onto or loaded within liposomes in different ways [20].
Liposomal vaccines were developed to target specific immune cell types for the induction of certain immune responses [21]. Cationic liposomes as an adjuvant or a delivery system could increase the potential of different subunit vaccines (e.g., TB) due to their enhanced interaction with the negatively charged immune cells [21, 22]. Cationic liposomes combined with other immunostimulatory factors such as TDB (trehalose 6, 60-dibehenate), MPL (monophosphoryl lipid A), TDM (trehalose dimycolate), and Poly I:C showed strong electrostatic interactions with antigen-presenting cells (APCs) and thus induced both humoral and cellular immune responses as well as a strong memory response [22]. Two approved liposomal vaccine formulations for antigen delivery are available, including Inflexal® V (influenza vaccine) and Epaxal® (hepatitis A vaccine). Both of these formulations used virosome-based technology in which viral proteins were bonded to the surface of a liposome carrier [14]. Up to now, various methods have been used to improve the stability of liposome formulations during storage including freeze-drying, spray-drying, supercritical fluid technology, and lyophilization [14].
Nanoliposomes
Nanoliposomes or lipidic nanovesicles known as colloidal nanostructures are composed of lipid or phospholipid molecules and possess the physicochemical properties similar to liposomes. However, nanoliposomes and liposomes are different in their size and surface area. Nanoliposomes have higher potency than liposomes in enhancing solubility and bioavailability, improving the controlled release of cargo, and accurate targeting of the encapsulated vaccine compounds [8].
Solid Lipid Nanoparticles (SLNs)
Solid lipid nanoparticles known as nano-sized colloidal carriers are composed of accurate ratios of lipid, surfactants, and bioactive compounds. They are efficient drug carriers due to their small size and lipid core (i.e., the solid lipids including triglycerides, acetyl alcohol, emulsifying wax, beeswax, carnauba wax, cholesterol, and cholesterol butyrate) [8].
Immunostimulatory Complexes (ISCOMs)
The immunostimulatory complexes known as ISCOMs (size: 40–60 nm) are vaccine carriers (e.g., hydrophobic antigens) with potent adjuvant properties as used in clinical trials. These cage-like particles with a hollow center are composed of saponin adjuvant Quil A, a protein antigen, cholesterol, and phospholipid in certain ratios that are self-assembled in solution. ISCOM-based vaccines significantly increase both humoral and cellular immune responses [8]. ISCOMATRIX is a vaccine carrier and adjuvant which is more applicable than ISCOMs due to its potency to remove the limitation of hydrophobic antigens. Several research groups published the use of different antigens, such as antigens derived from human immunodeficiency virus (HIV), human papilloma virus (HPV), Newcastle disease, and influenza for forming ISCOMs and ISCOMATRIX vaccines [8].
Tocosome
Tocosome is a vesicular and colloidal bioactive carrier that is mainly composed of the phosphorylated form of alpha-tocopherol (Alpha-tocopherol phosphate: TP). This component is available naturally in human tissues, some animal tissues, and certain food compounds. Also, tocosomes can possess proteins, polymers, and sterols in their structures. TP molecule has an inhibitory effect against tumors. Formulations of tocosomes containing various phospholipid molecules and different combinations of cholesterol were successfully used for the entrapment and controlled release of the anticancer drugs [8].
Vaccine Formulation Based on Lipidic Carrier
Lipids and their derivatives (especially the cationic/ionizable lipid materials with one or more amino groups) have been widely applied for in vivo delivery of vaccines due to encapsulation of vaccine compounds (e.g., mRNA vaccines) for their protection from enzymatic degradation and effective delivery of vaccine molecules into the cell cytosol through endocytosis processes [8]. However, the efficacy of vaccine delivery could be affected by modification of fatty acids in the hydrophobic tails. Moreover, the helper lipids could stabilize the structures of lipidic nanocarriers and facilitate endosomal escape. On the other hand, the PEG-lipid conjugates with a hydrophilic outer layer could stabilize the nanocarriers and extend the circulation time after in vivo administration [8]. As reported, lipid nanoparticles (LNPs) could protect the mRNA against degradation, help in endocytosis and endosomal escape, and incorporate adjuvants to activate immune system. Moreover, LNPs were targeted to specific cell types by decorating their surfaces with specific ligands. However, it should be noted that some cationic lipids (containing three domains: the polar headgroup, hydrophobic moiety, and linker) showed toxicity, and the repeated use of PEG-lipid induced an immune response against PEG [23]. Adjuvant activity was shown for the engineered ionizable lipids containing cyclic amino head groups, isocyanide linker, and two unsaturated alkyl tails. Covering the surface of lipidic nanocarriers with immune cell receptors could facilitate their uptake by the desired type of immune cells. For instance, the nanocarriers were designed to target the lymph node (with high concentration of APCs) based on their size and surface composition. Indeed, the nanoparticles with diameters less than about 150 nm could enter the lymphatic capillaries, and were subsequently drained to the peripheral lymphatics [8]. Moreover, inclusion of certain polymers to the lipid vesicles prevented opsonization by the cells of the immune system mainly in the liver and spleen leading to the stable vesicles in intravenous injection. On the other hand, the lipidic nanoparticles were not actively targeted toward dendritic cells (DCs). Thus, DC uptake was enhanced by modification of the surfaces of vesicles with suitable molecules, such as antibodies or peptides targeting integrins or the c-type lectin receptor [8]. It was reported that while adjuvant-free antigens tend to induce humoral response, delivery complexes (delivery with immune stimulatory complexes) form depot effect for efficient antigen uptake, and presentation by professional APCs. The physicochemical properties of the delivery systems determine both the efficiency and the mechanism of antigen uptake which in turn play critical roles in antigen-specific immune activation. On the other hand, immune stimulatory complexes lead to activation and maturation of innate immune cells for generation of targeted acquired immunity [24].
Generally, surface engineering of nanocarriers with different lipids increases the target specificity, reduces cytotoxicity, extends circulation half-life in vivo, and improves transfection efficiency of the nanocarriers. However, the concentration and composition of different lipids should be exactly controlled especially in gene delivery, because these factors directly influence the efficiency of nanocarriers [25].
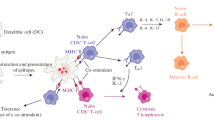
Different genetic and subunit vaccines such as mRNA-based vaccines, DNA-based vaccines, and protein-based vaccines could be formulated with lipidic carriers as follows. Subunit vaccines designed as nanoparticle formulations could improve antigen uptake by APCs and enhance immunogenicity against nanoparticles or adjuvant molecules co-delivered by the nanoparticles. These vaccine platforms are biodegradable and biocompatible with minimal toxicity as compared to traditional microorganism-based vaccines. Moreover, nanoparticle vaccines showed higher ability to increase cross-presentation of subunit antigens and induce strong cellular immunity than traditional vaccines. The nanoparticle vaccines can be considered as a promising approach in clinical trials against cancer and intracellular infections [e.g., HIV, TB and Hepatitis C (HCV)] [26]. Figure 2 shows vaccine delivery using lipidic carriers.
Vaccine delivery using lipidic carriers: Different vaccine constructs (e.g., DNA, RNA, peptide, and protein) can be loaded in lipidic carriers and enter the target cells. The exogenous (i.e., protein or peptide) or endogenous (i.e., the expressed DNA or RNA) antigens undergo the endosomal or proteasomal pathways in antigen-presenting cells, present on the MHC I or MHC II molecules, activate the CD8+ or CD4+ T cells, and stimulate humoral or cellular immune responses
Lipid-Based Delivery Systems (Lipid Nanoparticles) and mRNA-Based Vaccine
The mRNA vaccines possess many advantages as compared to other subunit vaccines. For instance, they are relatively safe and also induce different types of immune responses leading to the activation of CD4+ and CD8+ T cells [17]. However, the mRNA vaccines have some disadvantages such as degradation by nucleases in vivo. For this purpose, different mRNA delivery systems were designed to protect the mRNA molecules from degradation and to promote their cellular uptake into the targeted cells [27]. Nanoparticles (NPs) based delivery systems such as polymers and liposomes are effective tools to target the mRNA molecules safely to the APCs. Hybrid NP delivery platforms with the adjuvant potency were also used to enhance various immune responses and subsequently the efficiency of vaccines [17]. Monslow et al. reported that mRNA encoding gE antigen of Varicella-zoster virus (VZV) formulated with lipid-based nanoparticles induced higher immune responses than live attenuated VZV in preclinical trials [28]. In addition, lipidic nanoparticles-formulated mRNA vaccine encoding multiple conserved antigens of Influenza virus showed protection against challenge with a panel of Group 1 Influenza A viruses in mice [29]. Lo et al. also reported that a lipid-based mRNA vaccine encoding the soluble glycoprotein of Hendra virus provided protection against Nipah virus challenge in Syrian hamsters [30]. Moreover, lipidic carriers were effective in cancer vaccine development. For example, Arya et al. indicated that the ovalbumin (OVA) mRNA-liposome nanocomplex significantly inhibited B16-OVA tumor progression and increased mouse survival without clear toxicity [31]. Also, Zhang et al. showed that a minimalist nanovaccine with C1 lipid nanoparticle (LNP) effectively increased mRNA delivery and antigen presentation with a self-adjuvant feature through activating TLR4 signaling. The C1 LNP mRNA nanovaccines showed significant in vivo efficacy in both tumor prevention and therapeutic vaccine trials [32].
For COVID-19 mRNA vaccines, the mRNA coding for spike protein was encapsulated in a solid lipid structure composed of ionizable lipids (i.e., Ionizable lipid-based nanoparticles (iLNPs) [17]. This lipid structure is neutral (or slightly charged) at physiological pH and positive at acidic pH (e.g., in endosomes) and contains cholesterol (for complexation), helper lipids (for stabilization of nanoparticles), and PEGylated lipids (for reduction of non-specific interactions) [27]. In addition, Moyo et al. developed a tetravalent iLNP-mRNA vaccine “HIVconsvM” against HIV, which induced strong T-cell responses in mice [33]. On the other hand, the use of cationic lipid-based nanoparticles for mRNA vaccine delivery induced strong immune responses in mice similar to iLNPs [34]. For instance, the mRNA encoding cytokeratin 19 delivered by cationic liposome/protamine complex increased cellular immune responses and anti-tumor activity in a Lewis lung cancer model [35]. The researchers showed that lipid-based nanoparticles containing cholesterol analogs have higher potency of gene transfection [36]. For example, the mRNA vaccine formulation containing DOTAP/cholesterol nanoparticles reduced Influenza A viral titers and morbidity in mice [37]. However, different approaches were used to enhance the immunologic activity of lipid-based nanoparticles such as the use of lipid and polylactic acid (PLA) nanoparticles in mRNA vaccine for increasing Th1 response [38; and/or the use of hybrid PLGA-core/lipid-shell nanoparticles in mRNA vaccine along with a TLR7 agonist for stimulating immune responses in mice [39].
Lipid-Based Delivery Systems (Lipid Nanoparticles) and DNA-Based Vaccine
Several lipidic vectors were utilized for gene delivery including cationic lipids, ionizable lipids, lipidoids (lipid-like compounds containing tertiary amines), gene-lipid conjugates, and functional LNPs. Cationic lipids were used to deliver nucleic acids in gene therapy more than two decades ago. The size of the lipoplex (cationic lipid: nucleic acid complex) is a major factor for lipofection efficiency in vitro. It was reported that larger liposomes are eliminated from the blood circulation more rapidly than smaller liposomes. Moreover, positively charged liposomes have a shorter half-life than neutral or negative liposomes [40]. Ionizable lipids can self-assemble into nanoparticles after mixing with polyanionic nucleic acids. Indeed, ionizable cationic lipids (e.g., DLin-KC2-DMA or DLin-MC3-DMA) enhance nucleic acid delivery and subsequently the therapeutic efficacy of gene therapy. Moreover, nucleic acid conjugation with lipids (i.e., gene-lipid conjugates) could improve gene therapy in vivo [40]. Several functional LNPs have been developed to enhance targeted gene delivery using different strategies such as modification of the nanoparticles with tumor-specific ligands (e.g., iron-saturated transferrin, folic acid, RGD and anisamide) to enhance intracellular uptake and escape from endosomal/lysosomal vesicles using pH-sensitive functional groups applied to the LNPs (e.g., pH-sensitive linkers including diorthoester, orthoester, vinyl ether, phosphoramidate, hydrazine, and beta-thiopropionate) [40].
Liposomes and some other vesicular systems were used as delivery systems for DNA vaccines [14]. The preventive and therapeutic potential of DNA vaccines delivered by lipids (lipofection) was investigated in treatment of infectious diseases, cancers, or autoimmune disorders. Cationic and neutral lipids may play the role of adjuvants, as well. Cationic polyprenyl derivatives along with helper lipids were effective for cell transfection and for immunization of animals [41]. Mucker et al. showed that Andes virus or Zika virus DNA vaccines formulated with lipid nanoparticle (LNP) could significantly increase neutralizing antibodies in rabbits and non-human primates as compared to unformulated DNA vaccine [42]. The plasmid DNA could be either electrostatically complexed on the surface of cationic liposomes or encapsulated in the aqueous core by a dehydration–rehydration procedure. Cationic liposomes could increase transfection efficiency in vitro, while non-ionic or anionic liposomes could enhance antibody responses in animal models [14].
On the other hand, surface modifications with antigenic components or targeting ligands could significantly augment immune responses of liposome-based vaccines (e.g., liposomes coated with glycol chitosan) [14]. For instance, surface-modified cationic liposomes such as phosphatidylcholine, dioleoyl phosphatidylethanolamine, and cholesterol induced stronger humoral, cellular, and mucosal immune responses after intranasal administration in mice against hepatitis than unmodified liposomes [43]. Several studies indicated the use of liposomes as DNA vaccine carriers to induce efficient immune responses against respiratory pathogens. For instance, Rosada et al. demonstrated a single intranasal immunization with liposome-based formulations of plasmid DNA encoding heat shock protein 65 (HSP65) against M. tuberculosis led to a significant reduction of bacterial load in lungs of mice [44]. Furthermore, intranasal immunization with liposome-based DNA vaccine induced complete protection against challenge with influenza virus [45]. On the other hand, liposomes conjugated to cell penetrating peptides (CPPs) and transferrin (Tf) ligand were developed to deliver plasmid DNA in brain cells based on targeting molecular recognition of transferrin receptor overexpressed on the blood–brain barrier (BBB) with enhanced internalization ability of CPPs. CPP-Tf-conjugated liposomes demonstrated high potency to overcome the BBB and penetrate the brain of mice [46]. Vaxfectin®-adjuvanted plasmids were used to enhance humoral responses of DNA vaccines, as well. Vaxfectin is a combination of cationic lipid with neutral lipid. A tetravalent DNA vaccine with and without Vaxfectin adjuvant compound was studied in a Phase I clinical trial in Dengue virus-seronegative healthy volunteers. The tetravalent dengue DNA vaccine (TVDV) was safe and well tolerated and induced significantly anti-dengue IFN-gamma responses in a dose-dependent approach [47].
Niosomes known as non-ionic surfactant-based vesicles possess some advantages, such as cost-effective manufacturing, large-scale production, and stability. Niosomes were applied as carriers for delivery of plasmid DNA, small interference RNAs (siRNAs), and aptamers into target cells due to their structural similarities to liposomes [14]. Cationic niosomes showed ~ 95% DNA transfection efficiency in vitro [48]. Furthermore, successful transfection of human tyrosinase gene was reported by cationic niosomes in vivo [49]. Mannolysated niosomes encapsulated with plasmid DNA encoding HBsAg (Hepatitis B surface antigen) were reported to induce protective immunity against hepatitis B as both DNA vaccine carrier and adjuvant for oral immunization [50].
Lipid-Based Delivery Systems (Lipid Nanoparticles) and Protein/Peptide-Based Vaccine
Proteins and peptides are more desirable therapeutic molecules than small molecular drugs because of their high selectivity and efficacy and low side effects. Due to their poor stability and limited permeability through gastrointestinal tract and epithelia, they are usually injected through parenteral route. However, the development of oral formulations for therapeutic peptides and proteins is necessary. Recently, researches focused on developing novel strategies to overcome these barriers, including enteric coating, enzyme inhibitors, permeation enhancers, nanoparticles, and intestinal microdevices. Some of them were achieved to clinical trials and even marketing [51]. In general, lipid-based nanocarriers (e.g., oil-in-water nanoemulsions, self-emulsifying drug delivery systems (SEDDS), solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), liposomes and micelles) were known as the most promising formulation approaches [52]. Lipid-based delivery systems have been used to deliver active compounds through overcoming issues of pure active compounds, such as rapid release and metabolism, poor solubility, low stability, poor bioavailability, poor bioaccessibility, and toxicity [53]. For example, to increase the immunogenicity of the model subunit vaccine, ovalbumin (OVA) was combined with platycodin (PD), a saponin adjuvant and both of them were loaded into liposomes. Mice treated with the non-toxic OVA- and PD-loaded liposomes (OVA-PD-Lipos) showed a significantly enhanced immune response [54]. Lipid nanoparticles especially nanostructured lipid carriers (NLC) are a promising approach for the formulation of peptides and proteins with poor aqueous solubility [55]. The importance of lipid-based colloidal carriers and their pharmaceutical implications in the delivery of peptides and proteins was evaluated for oral and parenteral administration. However, the nanoencapsulation of biomacromolecules in colloidal particles protects them against the gastrointestinal environment, and increases their transmucosal transport. This ability is related to various mechanisms depending on the nanocarrier composition, such as mucoadhesion, particle internalization phenomenon, and permeation enhancing effect [55]. For example, the size of particles smaller than 1 μm may pass across the intestinal mucosa and thus facilitate the absorption of the bioactive drugs from the gut lumen. Moreover, hydrophobic nanoparticles are usually transported through the gut-associated lymphoid tissue, whereas particles with a more hydrophilic nature are transported across the regular enterocytes [55]. Nanocarriers with a size < 200 nm and a mucoinert surface (e.g., PEG or zwitterionic surfaces) show high mucus permeating properties [52]. Lipid-based nanocarriers facilitate paracellular and lymphatic drug uptake, induce endocytosis and transcytosis, or simply fuse with the cell membrane releasing their cargo into the systemic circulation. Different studies indicated the potential of these delivery systems in vivo [52]. On the other hand, lipid-protein conjugation is a novel strategy due to having both lipids and proteins in one delivery system and subsequently causing better synergistic effects in the body. Its major results are higher stability, better mechanical strength, controlled release, higher circulation time, targeted delivery, less cytotoxicity, higher loading capacity, co-encapsulation, and better bioavailability [53].
Lipid-Based Delivery of Genetic and Subunit Vaccines in Preclinical and Clinical Trials
Lipid-based nanoparticles are efficient candidates for vaccine delivery which were achieved to clinical trials especially for design of therapeutic cancer vaccines [1]. LNPs consist of four components, such as ionizable cationic lipids, PEGylated lipids, cholesterol, and helper lipids [56]. Ionizable lipids function as binding reagents for nucleic acids and help mRNA escape from the endosome/lysosome into the cytoplasm (e.g., Dlin-MC3-DMA from Alnylam Pharmaceuticals, SM-102 from Moderna, and ALC-0159 from Pfizer/BioNTech are all under patent protection). PEGylated lipids and cholesterol maintain LNP stability and influence the biodistribution of LNPs [39, 57]. Helper lipids [e.g., 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC) used in the mRNA-1273 and BNT162b2 COVID-19 vaccines and dioleoyl phosphatidylethanolamine (DOPE)] were reported to assist endosome escape [39]. Liposomal RNA vaccines also mediated durable objective responses in immune checkpoint blockade-experienced patients with advanced melanoma when optimized to target immature DCs in lymphoid tissues and to drive tumor-associated antigen presentation on both major histocompatibility complex I or II (MHC I or II) [39]. Clinical trials with direct administration of synthetic mRNAs encoding tumor antigens demonstrated safety and induction of tumor-specific immune responses. Indeed, as compared to the naked mRNAs, their formulations with chemical carriers led to more internalization of mRNA in dendritic cells for better immune responses and dose reduction [58].
Liposomes and liposome-derived nanovesicles (e.g., archaeosomes and virosomes) are known as major carrier systems in vaccine development [59]. For example, liposomes can encapsulate antigens, protect the antigen from degradation, fuse with cell membranes, present antigens to APCs, and induce adaptive immunity. For the first time, in 1974, liposomes were applied as a safe and effective adjuvant in human vaccination protocols. After that, various liposome-based vaccine systems were achieved to clinical trials [1]. The use of cationic lipids, neutral lipids, anionic lipids, and PEG-lipids in liposomes showed specific structure–functional properties. For example, the ionizable cationic lipid components and neutral lipids (as helper lipids) were used to improve the transfection efficiency of DNA or mRNA vaccines [60,61,62]. Moreover, anionic lipids are less toxic compared to cationic lipids in vivo inducing a continuous and potent immune response [63].
On the other hand, different modified cationic lipids could induce high CD8+ and CD4+ T-cell responses [64]. For instance, PEG-modified nanoparticles provided a biocompatible platform for gene transfer enhancing the circulation time and the stability of vaccines in vivo [65]. Recently, the use of liposome-targeted delivery with specific ligands or by targeting molecules to the relevant receptors on APCs is of interest. For example, lymph node-targeted delivery of vaccines (e.g., melittin-lipid nanoparticles) led to higher humoral and cellular immune responses especially for cancer immunotherapy [66]. Different LNP-complexed mRNA vaccines were designed to deliver mRNA, and activate a systemic immune response including a) heterocyclic LNPs to activate immunity through the mRNA-mediated STING pathway, b) mRNA loaded into cationic lipid-based nanoparticles to enhance transfection efficiency, and c) a nucleoside-modified mRNA-LNP vaccine to induce potent immune responses [67,68,69].
Preclinical and clinical studies demonstrated that mRNA delivered intramuscularly with LNPs produces the increased immune responses [57]. Most of mRNA-based vaccine candidates are currently being tested in clinical trials [1]. For instance, lipid NP-formulated mRNA vaccines against influenza achieved to clinical trials (NCT03076385, NCT03345043) after preclinical studies in primates and mice. These vaccines were shown to induce humoral immune response against H10N8 and H7N9 influenza viruses in humans [17]. As known, the mRNA vaccines against COVID-19 infection were approved by the U.S. Food and Drug Administration [1].
In general, depending on the chemical properties, water-soluble antigens (proteins, peptides, nucleic acids, carbohydrates, haptens) are entrapped within the aqueous inner space of liposomes, whereas lipophilic compounds (lipopeptides, antigens, adjuvants, linker molecules) are intercalated into the lipid bilayer, and antigens or adjuvants can be attached to the liposome surface either by adsorption or stable chemical linking [59]. Effective mRNA vaccines require both mRNA delivery and antigen expression to enable antigen-specific immunity [70]. Efforts to generate lipids with adjuvant effect have also provided promising results. It was known that cyclic dinucleotides may activate the stimulator of the interferon genes (STING) pathway, which activates IFN secretion and may enhance immune responses [67, 71].
Various functions can be achieved using strategies, such as molecule modification, cargo selection, and nanoparticle design [56]. The adjuvant and delivery property are influenced by formulation parameters, including size, surface charge, stability, lamellarity, composition and method of preparation [24]. The size of the particle plays a crucial role in determining the distribution and antigen uptake of the vaccine formulation following immunization. Some studies explained the role of particle size on immune stimulation [24]. LNP particle size without altering lipid composition showed that while small diameter LNPs were significantly less immunogenic in mice, all the tested particle sizes induced an increased immune response in non-human primates [70]. For mRNA vaccines, LNP uptake by APCs is necessary for generating antigen-specific immunity. The mRNA particles with the size of 500–5000 nm are significantly taken up by macrophages, whereas particles with the size of 20–200 nm are importantly taken up by DCs. The mRNA particles with the size of 80–100 nm elicit APC uptake and antigen expression. Subcutaneous administration (of mRNA vaccine) shows that smaller lipid particles leave the injection site more readily than larger particles, allowing them to drain more efficiently to lymph nodes [70]. However, an optimum size is important to facilitate both efficient lymph node drainage and cellular interactions in subcutaneous administration (e.g., too small particles are not efficient). It is required modulating particle size of lipid-based systems in intramuscular injection. Particle size was shown to affect cell recruitment of nanoparticles. For example, emulsion droplets with diameter 160 nm can recruit a greater number of immune cells to the injection site (or the highest number of antigen-positive immune cells within the draining lymph node) compared to smaller particles of identical composition (20 and 90 nm) for mRNA vaccines [70]. However, the kinetics of LNP entry into the cell may change within the size range. Moreover, it should be considered that the smaller-scale murine lymphatics are more sensitive to particle size within the range than the larger primate lymphatics. Thus, optimal mRNA vaccine particle size determined in rodents may not translate to primates [70]. However, the route of immunization, nature of antigens, and the composition of particles can influence the strength and type of immune responses, as well. Lipid composition determines the stability and immunomodulatory properties of lipid vesicles. Unsaturated lipids with a low transient point are used to release vesicles easily, while saturated lipids are generally used for stable formulations. Use of helper lipids further adds to the stability of lipid particle [24]. On the other hand, LNP therapeutic agents for gene therapy are currently used in clinical trials, such as ALN-TTRsc (targeting TTR for treatment of transthyretin-mediated amyloidosis) and ALN-PCS02 (targeting proprotein convertase subtilisin/kexin type 9 (PCSK9) to lower cholesterol for treatment of hypercholesterolemia). SNALP technology is one of the most widely used lipid-based nucleic acid delivery approaches (i.e., lipid vesicles encapsulating nucleic acids) for systemic administration in clinical trials [40]. Table 1 shows preclinical and clinical studies of genetic and subunit vaccines delivered by lipid-based vehicles.
Conclusion
This study shows the importance of Lipid-based delivery systems especially LNPs for protection of vaccine constructs against nucleases and proteases, continuous release of antigens, and enhancement of immunogenicity. These systems need to be optimized for increasing the potency of cargo delivery, cell targeting, safety, and immune stimulation. At present, the success of LNPs in development of COVID-19 mRNA vaccines has attracted a special interest for researchers working on other infectious or non-infectious diseases. Also, lipidic carriers are effectively used to deliver drugs as well as vaccines with high bioavailability and low side effects in clinical studies. Thus, lipidic delivery systems possess suitable and vital properties for the development of novel drug and vaccine formulations.
Future Perspective
Recently, a large range of compounds and technologies are available to design and develop formulations that can induce potent immune responses against different pathogens. Lipid-based nanoparticles (LNPs) play an important role due to natural adjuvant properties. LNPs can be easily decorated with small molecules or glycans targeting the vaccine to specific type of immune cells and finally enhancing the potency of the immune response. In addition, adjuvants or antigens can be encapsulated into lipid nanoparticles. Some processes were developed to control the size of LNPs independent of lipid composition. It is interesting that mice require an average particles size (~ 100 nm) for generating high antibody levels. In contrast, all particle sizes (~ 60–150 nm) generate strong immune responses in non-human primates. The impact of LNP biophysical parameters on immunogenicity is an important step for enabling rapid development of potent vaccines against novel growing diseases. Recent advances in lipid gene delivery systems have significantly improved the efficacy and the level of expression of targeted genes. The improved structure–activity design has increased the potential of LNPs in gene therapy in tumors, and other disorders.
Although, effective vaccine encapsulation and delivery systems with simple formulations play an important role in vaccine development; but however, optimization of the safety profile and enhancement of the vaccination efficacy are still required for various preclinical and clinical trials. Some studies demonstrate that hybrid delivery platforms increase the efficiency of vaccines for boosting a variety of immune responses. Among the all-available delivery systems, lipidic carriers show one of the most advanced platforms for vaccine delivery and targeting in vivo. Lipidic delivery systems include bilayer lipid vesicles such as liposomes, nanoliposomes, archaeosomes, vesicular lipid gels, immunovesicles, lipospheres, solid lipid nanoparticles (SLN), tocosomes, and some other micro- and nanocarrier systems. The lipid-based nano-delivery of drug/vaccine is a therapeutic option for the treatment of infectious diseases (e.g., COVID-19, MERS, SARS, and Ebola) due to some advantages, such as low cost, easy preparation, increased bioavailability, cellular permeability, and uptake and stability of drug/vaccine. Moreover, lipid-based nanoparticles are a major platform for the design of multicomponent adjuvant systems. Immunotherapy with lipid adjuvants can add to the effective disease management strategies alone and in combination. Although, the loaded antigen or adjuvant with the nanoparticle and other adjuvants could be more useful than a simple mixture, but its manufacturing is commonly complex and difficult. The design of lipidic delivery system is more complex with adding surface modification and with coatings and/or ligands. The use of synthetic coatings and ligands can influence the biocompatibility, biodistribution, and toxicity of lipid-based formulations and thus it needs an exact assay of the interaction of nanoparticles with tissues or cells.
A balance between efficiency, production cost, and simple manufacturing is required to use lipidic delivery systems for development of effective multicomponent subunit vaccines. Furthermore, for using LNPs in a clinical trial, a better understanding of certain mechanisms and techniques is required including the interaction of lipid vectors and gene expression and the safety and immunogenicity profile of vectors. By overcoming these barriers, efficient delivery of genes will be performed by LNPs for clinical use. For instance, lipid-based delivery systems are potent vehicles to target the mRNA molecules safely to the antigen-presenting cells (APCs). Based on the studies, incorporation of the plasmids into the lipid carriers leads to their delivery through the mucosal surfaces or the transdermal route. The efficiency of the transdermal route as an effective administration is dependent on the composition of the lipid vehicles. However, characterization of these systems is a complex process. Size, charge, architecture, and composition need to be characterized to develop a standard lipid nanoparticle. In addition, the safety and toxicity profiles of each novel nanoparticle should be determined to avoid unpredictable adverse effects. Generally, the lipid carriers possess the ability to improve antigen stability and presentation to immunocompetent cells (depending on their specific properties, such as composition, size, and surface properties), to overcome biological barriers (e.g., skin or mucosa), to provide controlled and slow release of antigens, and finally to induce strong immune responses provided by coformulated adjuvants.
References
Liang, J., & Zhao, X. (2021). Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development. Cancer Biology & Medicine, 18(2), 352–371.
Ftouh, M., Kalboussi, N., Abid, N., Sfar, S., Mignet, N., & Bahloul, B. (2021). Contribution of nanotechnologies to vaccine development and drug delivery against respiratory viruses. Hindawi PPAR Research, 2021, 1–28.
Bolhassani, A. (2019). Improvements in chemical carriers of proteins and peptides. Cell Biology International, 43(4), 437–452.
Hosseinzadeh, S., & Bolhassani, A. (2015). Immunostimulant properties of chemical delivery systems in vaccine development. Current Drug Delivery, 12(4), 360–368.
Bolhassani, A., Safaiyan, S., & Rafati, S. (2011). Improvement of different vaccine delivery systems for cancer therapy. Molecular Cancer, 10, 3.
Kim, D., Wu, Y., Kim, Y. B., & Oh, Y. K. (2021). Advances in vaccine delivery systems against viral infectious diseases. Drug Delivery and Translational Research, 11, 1401–1419.
Baca-Estrada, M. E., Foldvari, M., Babiuk, S. L., & Babiuk, L. A. (2000). Vaccine delivery: Lipid-based delivery systems. Journal of Biotechnology, 83, 91–104.
Raoufi, E., Bahramimeimandi, B., Salehi-Shadkami, M., Chaosri, P., & Mozafari, M. R. (2021). Methodical design of viral vaccines based on avant-garde nanocarriers: A multi-domain narrative review. Biomedicines, 9, 520.
Corthésy, B., & Bioley, G. (2018). Lipid-based particles: Versatile delivery systems for mucosal vaccination against infection. Frontiers in Immunology, 9, 431.
Ansari, M. A., Almatroudi, A., Alzohairy, M. A., AlYahya, S., Alomary, M. N., Al-Dossary, H. A., & Alghamdi, S. (2020). Lipid-based nano delivery of Tat-peptide conjugated drug or vaccine-promising therapeutic strategy for SARS-CoV-2 treatment. Expert Opinion on Drug Delivery, 17(12), 1671–1674.
Zhong, W., Skwarczynski, M., & Toth, I. (2009). Lipid core peptide system for gene, drug, and vaccine delivery. Australian Journal of Chemistry, 62, 956–967.
Buck, J., Grossen, P., Cullis, P. R., Huwyler, J., & Witzigmann, D. (2019). Lipid-based DNA therapeutics: Hallmarks of non-viral gene delivery. ACS Nano, 13, 3754–3782.
Barba, A. A., Bochicchio, S., Dalmoro, A., & Lamberti, G. (2019). Lipid delivery systems for nucleic-acid-based-drugs: From production to clinical applications. Pharmaceutics, 11, 360.
Tang, J., Cai, L., Xu, C., Sun, S., Liu, Y., Rosenecker, J., & Guan, S. (2022). Nanotechnologies in delivery of DNA and mRNA vaccines to the nasal and pulmonary mucosa. Nanomaterials, 12, 226.
Chatzikleanthous, D., O’Hagan, D. T., & Adamo, R. (2021). Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: Toward multicomponent vaccines. Molecular Pharmaceutics, 18, 2867–2888.
Moncalvo, F., Martinez Espinoza, M. I., & Cellesi, F. (2020). Nanosized delivery systems for therapeutic proteins: Clinically validated technologies and advanced development strategies. Frontiers in Bioengineering and Biotechnology, 8, 89.
Okay, S., Özcan, O. O., & Karahan, M. (2020). Nanoparticle-based delivery platforms for mRNA vaccine development. AIMS Biophysics, 7(4), 323–338.
Tretiakova, D. S., & Vodovozova, E. L. (2022). Liposomes as adjuvants and vaccine delivery systems. Biochemistry, 16(1), 1–20.
Luwi, N. E. M., Ahmad, S., Azlyna, A. S. N., Nordin, A., Sarmiento, M. E., Acosta, A., Nor Norazmi, M., Uskoković, V., Mohamud, R., & Kadir, R. (2022). Liposomes as immunological adjuvants and delivery systems in the development of tuberculosis vaccine: A review. Asian Pacific Journal of Tropical Medicine, 15(1), 7–16.
Fobian, S. F., Cheng, Z., & ten Hagen, T. L. M. (2022). Smart lipid-based nanosystems for therapeutic immune induction against cancers: Perspectives and outlooks. Pharmaceutics, 14, 26.
De Serrano, L. O., & Burkhart, D. J. (2017). Liposomal vaccine formulations as prophylactic agents: Design considerations for modern vaccines. Journal of Nanobiotechnology, 15, 83.
Khademi, F., Taheri, R. A., Momtazi-Borojeni, A. A., Farnoosh, G., Johnston, T. P., & Sahebkar, A. (2018). Potential of cationic liposomes as adjuvants/delivery systems for tuberculosis subunit vaccines. Reviews of Physiology, Biochemistry and Pharmacology, 175, 47–69.
Reichmuth, A. M., Oberli, M. A., Jaklenec, A., Langer, R., & Blankschtein, D. (2016). mRNA vaccine delivery using lipid nanoparticles. Therapeutic Delivery, 7(5), 319–334.
Sabur, A., Asad, M., & Ali, N. (2016). Lipid based delivery and immuno-stimulatory systems: Master tools to combat leishmaniasis. Cellular Immunology, 309, 55–60.
Bose, R. J. C., Lee, S. H., & Park, H. (2016). Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomaterials Research, 20, 34.
Sahdev, P., Ochyl, L. J., & Moon, J. J. (2014). Biomaterials for nanoparticle vaccine delivery systems. Pharmaceutical Research, 31, 2563–2582.
Pichon, C., & Perche, F. (2021). Design and delivery of messenger RNA-based vaccines. Biochemistry, 43(4), 4–7.
Monslow, M. A., Elbashir, S., Sullivan, N. L., Thiriot, D. S., Ahl, P., Smith, J., Miller, E., Cook, J., Cosmi, S., Thoryk, E., Citron, M., Thambi, N., Shaw, C., Hazuda, D., & Vora, K. A. (2020). Immunogenicity generated by mRNA vaccine encoding VZV gE antigen is comparable to adjuvanted subunit vaccine and better than live attenuated vaccine in nonhuman primates. Vaccine, 38, 5793–5802.
Freyn, A. W., da Silva, J. R., Rosado, V. C., Bliss, C. M., Pine, M., Mui, B. L., Tam, Y. K., Madden, T. D., de Souza Ferreira, L. C., Weissman, D., Krammer, F., Coughlan, L., Palese, P., Pardi, N., & Nachbagauer, R. (2020). A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Molecular Therapy, 28, 1569–1584.
Lo, M. K., Spengler, J. R., Welch, S. R., Harmon, J. R., Coleman-McCray, J. D., Scholte, F. E. M., Shrivastava-Ranjan, P., Montgomery, J. M., Nichol, S. T., Weissman, D., & Spiropoulou, C. F. (2020). Evaluation of a single-dose nucleoside-modified messenger RNA vaccine encoding Hendra virus-soluble glycoprotein against lethal Nipah virus challenge in Syrian hamsters. Journal of Infectious Diseases, 221(4), S493–S498.
Arya, S., Lin, Q., Zhou, N., Gao, X., & Huang, J. D. (2020). Strong immune responses induced by direct local injections of modified mRNA-lipid nanocomplexes. Molecular Therapy: Nucleic Acids, 19, 1098–1109.
Zhang, H., You, X., Wang, X., Cui, L., Wang, Z., Xu, F., Li, M., Yang, Z., Liu, J., Huang, P., Kang, Y., Wu, J., & Xia, X. (2021). Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through toll-like receptor 4 signaling. Proceedings of the National Academy of Sciences, 118(6), e2005191118.
Moyo, N., Wee, E. G., Korber, B., Bahl, K., Falcone, S., Himansu, S., Wong, A. L., Dey, A. K., Feinberg, M., & Hanke, T. (2020). Tetravalent immunogen assembled from conserved regions of HIV-1 and delivered as mRNA demonstrates potent preclinical T-cell immunogenicity and breadth. Vaccines, 8, 360.
Lou, G., Anderluzzi, G., Schmidt, S. T., Woods, S., Gallorini, S., Brazzoli, M., Giusti, F., Ferlenghi, I., Johnson, R., Roberts, C. W., O’Hagan, D. T., Baudner, B. C., & Perrie, Y. (2020). Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: The impact of cationic lipid selection. Journal of Controlled Release, 325, 370–379.
Mai, Y., Guo, J., Zhao, Y., Ma, S., Hou, Y., & Yang, J. (2020). Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cellular Immunology, 354, 104143.
Eygeris, Y., Patel, S., Jozic, A., & Sahay, G. (2020). Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Letters, 20, 4543–4549.
Van Hoecke, L., Verbeke, R., De Vlieger, D., Dewitte, H., Roose, K., Van Nevel, S., Krysko, O., Bachert, C., Schepens, B., Lentacker, I., & Saelens, X. (2020). mRNA encoding a bispecific single domain antibody construct protects against influenza A virus infection in mice. Molecular Therapy Nucleic Acids, 20, 777–787.
Coolen, A. L., Lacroix, C., Mercier-Gouy, P., Delaune, E., Monge, C., Exposito, J. Y., & Verrier, B. (2019). Poly (lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials, 195, 23–37.
Yang, J., Arya, S., Lung, P., Lin, Q., Huang, J., & Li, Q. (2019). Hybrid nanovaccine for the co-delivery of the mRNA antigen and adjuvant. Nanoscale, 11, 21782–21789.
Zhao, Y., & Huang, L. (2014). Lipid nanoparticles for gene delivery. Advances in Genetics, 88, 13–36.
Rak, M., Góra-Sochacka, A., & Madeja, Z. (2021). Lipofection-based delivery of DNA vaccines. Methods in Molecular Biology, 2183, 391–404.
Mucker, E. M., Karmali, P. P., Vega, J., Kwilas, S. A., Wu, H., Joselyn, M., Ballantyne, J., Sampey, D., Mukthavaram, R., Sullivan, E., Chivukula, P., & Hooper, J. W. (2020). Lipid nanoparticle formulation increases efficiency of DNA-vectored vaccines/immunoprophylaxis in animals including transchromosomic bovines. Scientific Reports, 10, 8764.
Khatri, K., Goyal, A. K., Gupta, P. N., Mishra, N., Mehta, A., & Vyas, S. P. (2008). Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine, 26, 2225–2233.
Rosada, R. S., de la Torre, L. G., Frantz, F. G., Trombone, A. P. F., Zárate-Bladés, C. R., Fonseca, D. M., Souza, P. R. M., Brandão, I. T., Masson, A. P., Soares, E. G., Ramos, S. G., Faccioli, L. H., Silva, C. L., Santana, M. H. A., & Coelho-Castelo, A. A. M. (2008). Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunology, 9, 38.
Wong, J. P., Zabielski, M. A., Schmaltz, F. L., Brownlee, G. G., Bussey, L. A., Marshall, K., Borralho, T., & Nagata, L. P. (2001). DNA vaccination against respiratory influenza virus infection. Vaccine, 19, 2461–2467.
dos Santos Rodrigues, B., Lakkadwala, S., Kanekiyo, T., & Singh, J. (2019). Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. International Journal of Nanomedicine, 14, 6497–6517.
Danko, J. R., Kochel, T., Teneza-Mora, N., Luke, T. C., Raviprakash, K., Sun, P., Simmons, M., Moon, J. E., De La Barrera, R., Martinez, L. J., Thomas, S. J., Kenney, R. T., Smith, L., & Porter, K. R. (2018). Safety and immunogenicity of a tetravalent Dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase I clinical trial. American Journal of Tropical Medicine and Hygiene, 98(3), 849–856.
Rose, J. K., Buonocore, L., & Whitt, M. A. (1991). A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. BioTechniques, 10, 520–525.
Manosroi, J., Khositsuntiwong, N., Manosroi, W., Götz, F., Werner, R. G., & Manosroi, A. (2010). Enhancement of transdermal absorption, gene expression and stability of tyrosinase plasmid (pMEL34)-loaded elastic cationic niosomes: Potential application in vitiligo treatment. Journal of Pharmaceutical Sciences, 99, 3533–3541.
Jain, S., Singh, P., Mishra, V., & Vyas, S. P. (2005). Mannosylated niosomes as adjuvant-carrier system for oral genetic immunization against hepatitis B. Immunology Letters, 101, 41–49.
Zhu, Q., Chen, Z., Paul, P. K., Lu, Y., Wu, W., & Qi, J. (2021). Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharmaceutica Sinica B, 11(8), 2416–2448.
Haddadzadegan, S., Dorkoosh, F., & Bernkop-Schnürch, A. (2022). Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Advanced Drug Delivery Reviews, 182, 114097.
Dissanayake, T., Sun, X., Abbey, L., & Bandara, N. (2022). Recent advances in lipid-protein conjugate-based delivery systems in nutraceutical, drug, and gene delivery. Food Hydrocolloids for Health, 2, 100054.
Zhao, J. H., Zhang, Q. B., Liu, B., Piao, X. H., Yan, Y. L., Hu, X. G., Zhou, K., Zhang, Y. T., & Feng, N. P. (2017). Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. International Journal of Nanomedicine, 12, 4763–4772.
Martins, S., Sarmento, B., Ferreira, D. C., & Souto, E. B. (2007). Lipid-based colloidal carriers for peptide and protein delivery-liposomes versus lipid nanoparticles. International Journal of Nanomedicine, 2(4), 595–607.
Huang, S., Zhu, Y., Zhang, L., & Zhang, Z. (2022). Recent advances in delivery systems for genetic and other novel vaccines. Advanced Materials, 2107946
Hassett, K. J., Benenato, K. E., Jacquinet, E., Lee, A., Woods, A., Yuzhakov, O., Himansu, S., Deterling, J., Geilich, B. M., Ketova, T., Mihai, C., Lynn, A., McFadyen, I., Moore, M. J., Senn, J. J., Stanton, M. G., Almarsson, Ö., Ciaramella, G., & Brito, L. A. (2019). Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Molecular Therapy: Nucleic Acids, 15, 1–18.
Midoux, P., & Pichon, C. (2015). Lipid-based mRNA vaccine delivery systems. Expert Review of Vaccines, 14(2), 221–234.
Schwendener, R. A. (2014). Liposomes as vaccine delivery systems: A review of the recent advances. Therapeutic Advance in Vaccines, 2(6), 159–182.
Jayaraman, M., Ansell, S. M., Mui, B. L., Tam, Y. K., Chen, J., Du, X., Butler, D., Eltepu, L., Matsuda, S., Narayanannair, J. K., Rajeev, K. G., Hafez, I. M., Akinc, A., Maier, M. A., Tracy, M. A., Cullis, P. R., Madden, T. D., Manoharan, M., & Hope, M. J. (2012). Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angewandte Chemie, 51, 8529–8533.
Cullis, P. R., & Hope, M. J. (2017). Lipid nanoparticle systems for enabling gene therapies. Molecular Therapy, 25, 1467–1475.
Marty, R., N’Soukpoe-Kossi, C. N., Charbonneau, D., Weinert, C. M., Kreplak, L., & Tajmir-Riahi, H. A. (2009). Structural analysis of DNA complexation with cationic lipids. Nucleic Acids Research, 37, 849–857.
Courant, T., Bayon, E., Reynaud-Dougier, H. L., Villiers, C., Menneteau, M., Marche, P. N., & Navarro, F. P. (2017). Tailoring nanostructured lipid carriers for the delivery of protein antigens: Physicochemical properties versus immunogenicity studies. Biomaterials, 136, 29–42.
Zhu, D., Hu, C., Fan, F., Qin, Y., Huang, C., Zhang, Z., Lu, L., Wang, H., Sun, H., Leng, X., Wang, C., Kong, D., & Zhang, L. (2019). Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials, 206, 25–40.
Sekiya, T., Yamagishi, J., Gray, J. H. V., Whitney, P. G., Martinelli, A., Zeng, W., Wong, C. Y., Sugimoto, C., Jackson, D. C., & Chua, B. Y. (2017). PEGylation of a TLR2-agonist-based vaccine delivery system improves antigen trafficking and the magnitude of ensuing antibody and CD8(+) T cell responses. Biomaterials, 137, 61–72.
Yu, X., Dai, Y., Zhao, Y., Qi, S., Liu, L., Lu, L., Luo, Q., & Zhang, Z. (2020). Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic antitumor immune response. Nature Communications, 11, 1110.
Miao, L., Li, L., Huang, Y., Delcassian, D., Chahal, J., Han, J., Shi, Y., Sadtler, K., Gao, W., Lin, J., Doloff, J. C., Langer, R., & Anderson, D. G. (2019). Delivery of mRNA vaccines with heterocyclic lipids increases antitumor efficacy by STING-mediated immune cell activation. Nature Biotechnology, 37, 1174–1185.
Sayour, E. J., Grippin, A., De Leon, G., Stover, B., Rahman, M., Karachi, A., Wummer, B., Moore, G., Castillo-Caro, P., Fredenburg, K., Sarkisian, M. R., Huang, J., Deleyrolle, L. P., Sahay, B., Carrera-Justiz, S., Mendez-Gomez, H. R., & Mitchell, D. A. (2018). Personalized tumor RNA loaded lipid-nanoparticles prime the systemic and intratumoral milieu for response to cancer immunotherapy. Nano Letters, 18, 6195–6206.
Pardi, N., Hogan, M. J., Naradikian, M. S., Parkhouse, K., Cain, D. W., Jones, L., Moody, M. A., Verkerke, H. P., Myles, A., Willis, E., LaBranche, C. C., Montefiori, D. C., Lobby, J. L., Saunders, K. O., Liao, H. X., Korber, B. T., Sutherland, L. L., Scearce, R. M., Hraber, P. T., … Weissman, D. (2018). Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. Journal of Experimental Medicine, 215, 1571–1588.
Hassett, K. J., Higgins, J., Woods, A., Levy, B., Xia, Y., Hsiao, C. J., Acosta, E., Almarsson, O., Moore, M. J., & Brito, L. A. (2021). Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. Journal of Controlled Release, 335, 237–246.
Li, K., Qu, S., Chen, X., Wu, Q., & Shi, M. (2017). Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. International Journal of Molecular Sciences, 18(2), 404.
D’Souza, S., Rosseels, V., Denis, O., Tanghe, A., De Smet, N., Jurion, F., Palfliet, K., Castiglioni, N., Vanonckelen, A., Wheeler, C., & Huygen, K. (2002). Improved tuberculosis DNA vaccines by formulation in cationic lipids. Infection and Immunity, 70, 3681–3688.
Wang, D., Christopher, M. E., Nagata, L. P., Zabielski, M. A., Li, H., Wong, J. P., & Samuel, J. (2004). Intranasal immunization with liposome-encapsulated plasmid DNA encoding influenza virus hemagglutinin elicits mucosal, cellular and humoral immune responses. Journal of Clinical Virology: Official Publication of the Pan America Society of the Clinical Virology, 31(1), 99–106.
Shirai, S., Shibuya, M., Kawai, A., Tamiya, S., Munakata, L., Omata, D., Suzuki, R., Aoshi, T., & Yoshioka, Y. (2020). Lipid nanoparticles potentiate CpG-oligodeoxynucleotide-based vaccine for influenza virus. Frontiers in Immunology, 10, 3018.
Qiao, C., Liu, J., Yang, J., Li, Y., Weng, J., Shao, Y., & Zhang, X. (2016). Enhanced non-inflammasome mediated immune responses by mannosylated zwitterionic-based cationic liposomes for HIV DNA vaccines. Biomaterials, 85, 1–17.
Brignole, C., Marimpietri, D., Di Paolo, D., Perri, P., Morandi, F., Pastorino, F., Zorzoli, A., Pagnan, G., Loi, M., Caffa, I., Erminio, G., Haupt, R., Gambini, C., Pistoia, V., & Ponzoni, M. (2010). Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer Research, 70, 9816–9826.
Li, L., Yang, S., Song, L., Zeng, Y., He, T., Wang, N., Yu, C., Yin, T., Liu, L., Wei, X., Wu, Q., Wei, Y., Yang, L., & Gong, C. (2018). An endogenous vaccine based on fluorophores and multivalent immunoadjuvants regulates tumor micro-environment for synergistic photothermal and immunotherapy. Theranostics, 8, 860.
Casares, S., Brumeanu, T. D., & Richie, T. L. (2010). The RTS, S malaria vaccine. Vaccine, 28(31), 4880–4894.
Lal, H., Cunningham, A. L., & Heineman, T. C. (2015). Adjuvanted herpes zoster subunit vaccine in older adults. New England Journal of Medicine, 373(16), 1576–1577.
Yu, Y., Wang, D., Abula, S., Hu, Y., Zhao, X., Huang, Y., Liu, J., Wu, Y., Wang, D., Tao, Y., & Pan, H. (2013). The immunological adjuvant activity of gypenosides liposome against Newcastle disease vaccine. International Journal of Biological Macromolecules, 60, 116–121.
Zhao, X., Fan, Y., Wang, D., Hu, Y., Guo, L., Ruan, S., Zhang, J., & Yuan, J. (2011). Immunological adjuvant efficacy of glycyrrhetinic acid liposome against Newcastle disease vaccine. Vaccine, 29, 9611–9617.
Lin, Y., Deng, M., Tseng, L., Jiang, P. R., Jan, T. R., Hsieh, F. I., & Liu, D. Z. (2011). Adjuvant effect of liposome in chicken result from induction of nitric oxide. Biomedical Materials, 6, 015011.
Onuigbo, E., Okore, V., Ofokansi, K., Okoye, J. O. A., Nworu, C. S., Esimone, C. O., & Attama, A. A. (2012). Preliminary evaluation of the immunoenhancement potential of Newcastle disease vaccine formulated as a cationic liposome. Avian Pathology, 41, 355–360.
Tseng, L., Liang, H., Deng, M., Lee, K. M., Pan, R. N., Yang, J. C., Huang, Y. Y., & Liu, D. Z. (2010). The influence of liposomal adjuvant on intranasal vaccination of chickens against Newcastle disease. The Veterinary Journal, 185, 204–210.
Fan, Y., Wang, D., Hu, Y., Liu, J., Han, G., Zhao, X., Yuan, J., Liu, C., Liu, X., & Ni, X. (2012). Liposome and epimedium polysaccharide-propolis flavone can synergistically enhance immune effect of vaccine. International Journal of Biological Macromolecules, 50, 125–130.
Pang, Y., Zhang, Y., Wang, H., Jin, J., Piao, J., Piao, J., Liu, Q., & Li, W. (2013). Reduction of Salmonella enteritidis number after infections by immunization of liposome-associated recombinant SEFA. Avian Diseases, 57, 627–633.
Cheng, G., Zhao, X., Yan, W., Wang, W., Zuo, X., Huang, K., Liu, Y., Chen, J., Wang, J., Cong, W., Liu, M., Gao, H., Chen, J., Lu, Y., & Zheng, Z. (2007). Alpha interferon is a powerful adjuvant for a recombinant protein vaccine against foot-and-mouth disease virus in swine, and an effective stimulus of in vivo immune response. Vaccine, 25, 5199–5208.
Nishimura, M., Kohara, J., Kuroda, Y., Hiasa, J., Tanaka, S., Muroi, Y., Kojima, N., Furuoka, H., & Nishikawa, Y. (2013). Oligomannose-coated liposome-entrapped dense granule protein 7 induces protective immune response to Neospora caninum in cattle. Vaccine, 31, 3528–3535.
Thakur, A., Aagaard, C., Stockmarr, A., Andersen, P., & Jungersen, G. (2013). Cell-mediated and humoral immune responses after immunization of calves with a recombinant multiantigenic Mycobacterium avium subsp. paratuberculosis subunit vaccine at different ages. Clinical and Vaccine Immunology, 20, 551–558.
Hansen, J., Lindenstrom, T., Lindberg-Levin, J., Aagaard, C., Andersen, P., & Agger, E. (2012). CAF05: Cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunology, Immunotherapy, 61, 893–903.
Fan, Y., Stronsky, S. M., Xu, Y., Steffens, J. T., van Tongeren, S. A., Erwin, A., Cooper, C. L., & Moon, J. J. (2019). Multilamellar vaccine particle elicits potent immune activation with protein antigens and protects mice against Ebola virus infection. ACS Nano, 13, 11087–11096.
Bazzill, J. D., Stronsky, S. M., Kalinyak, L. C., Ochyl, L. J., Steffens, J. T., van Tongeren, S. A., Cooper, C. L., & Moon, J. J. (2019). Vaccine nanoparticles displaying recombinant Ebola virus glycoprotein for induction of potent antibody and polyfunctional T cell responses. Nanomedicine, 18, 414–425.
Herzog, C., Hartmann, K., Künzi, V., Kürsteiner, O., Mischler, R., Lazar, H., & Glück, R. (2009). Eleven years Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine, 27(33), 4381–4387.
Moser, C., Muller, M., Kaeser, M. D., Weydemann, U., & Amacker, M. (2013). Influenza virosomes as vaccine adjuvant and carrier system. Expert Review of Vaccines, 12(7), 779–791.
Drane, D., Maraskovsky, E., Gibson, R., Mitchell, S., Barnden, M., Moskwa, A., Shaw, D., Gervase, B., Coates, S., Houghton, M., & Basser, R. (2009). Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: A phase I study in healthy volunteers. Human Vaccines, 5(3), 151–157.
Stanberry, L. R., Simon, J. K., Johnson, C., Robinson, P. L., Morry, J., Flack, M. R., Gracon, S., Myc, A., Hamouda, T., & Baker, J. R. (2012). Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W805EC combined with approved seasonal influenza antigens. Vaccine, 30(2), 307–316.
Brunel, F., Darbouret, A., & Ronco, J. (1999). Cationic lipid DC-Chol induces an improved and balanced immunity able to overcome the unresponsiveness to the hepatitis B vaccine. Vaccine, 17, 2192–2203.
Joseph, A., Itskovitz-Cooper, N., Samira, S., Flasterstein, O., Eliyahu, H., Simberg, D., Goldwaser, I., Barenholz, Y., & Kedar, E. (2006). A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoylspermine (CCS)-I. Immunogenicity and efficacy studies in mice. Vaccine, 24, 3990–4006.
Klinguer, C., Beck, A., De-Lys, P., Bussat, M. C., Blaecke, A., Derouet, F., Bonnefoy, J. Y., Nguyen, T. N., Corvaïa, N., & Velin, D. (2001). Lipophilic quaternary ammonium salt acts as a mucosal adjuvant when co-administered by the nasal route with vaccine antigens. Vaccine, 19, 4236–4244.
Perche, F., Benvegnu, T., Berchel, M., Lebegue, L., Pichon, C., Jaffrès, P. A., & Midoux, P. (2011). Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine, 7, 445–453.
Bordon, Y. (2020). An RNA vaccine for advanced melanoma. Nature Reviews Immunology, 20, 517.
Sahin, U., Oehm, P., Derhovanessian, E., Jabulowsky, R. A., Vormehr, M., Gold, M., Maurus, D., Schwarck-Kokarakis, D., Kuhn, A. N., Omokoko, T., Kranz, L. M., Diken, M., Kreiter, S., Haas, H., Attig, S., Rae, R., Cuk, K., Kemmer-Brück, A., Breitkreuz, A., … Türeci, O. (2020). An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature, 585, 107.
Martinon, F., Krishnan, S., Lenzen, G., Magné, R., Gomard, E., Guillet, J. G., Lévy, J. P., & Meulien, P. (1993). Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. European Journal of Immunology, 23, 1719–1722.
Mai, Y., Guo, J., Zhao, Y., & Yang, J. (2020). Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cellular Immunology, 354, 104143.
Hou, X., Zaks, T., Langer, R., & Dong, Y. (2021). Lipid nanoparticles for mRNA delivery. Nature Reviews: Materials, 6, 1078–1094.
Li, K., Qu, S., Chen, X., Wu, Q., & Shi, M. (2017). Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. International Journal of Molecular Sciences, 18, 404.
Zhuang, X., Qi, Y., Wang, M., Yu, N., Nan, F., Zhang, H., Tian, M., Li, C., Lu, H., & Jin, N. (2020). mRNA vaccines encoding the HA protein of influenza a H1N1 virus delivered by cationic lipid nanoparticles induce protective immune responses in mice. Vaccines, 8, 123.
Erasmus, J. H., Khandhar, A. P., O’Connor, M. A., Walls, A. C., Hemann, E. A., Murapa, P., Archer, J., Leventhal, S., Fuller, J. T., Lewis, T. B., Draves, K. E., Randall, S., Guerriero, K. A., Duthie, M. S., Carter, D., Reed, S. G., Hawman, D. W., Feldmann, H., Gale, M., … Fuller, D. H. (2020). An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Science Translational Medicine, 12, eabc9396.
Laczkó, D., Hogan, M. J., Toulmin, S. A., Hicks, P., Lederer, K., Gaudette, B. T., Castaño, D., Amanat, F., Muramatsu, H., Oguin, T. H., Ojha, A., Zhang, L., Mu, Z., Parks, R., Manzoni, T. B., Roper, B., Strohmeier, S., Tombácz, I., Arwood, L., … Pardi, N. (2020). A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in Mice. Immunity, 53, 724.
Goswami, R., Chatzikleanthous, D., Lou, G., Giusti, F., Bonci, A., Taccone, M., Brazzoli, M., Gallorini, S., Ferlenghi, I., Berti, F., O’Hagan, D. T., Pergola, C., Baudner, B. C., & Adamo, R. (2019). Mannosylation of LNP results in improved potency for self-amplifying RNA (SAM) vaccines. ACS Infectious Disease, 5, 1546–1558.
Pardi, N., Parkhouse, K., Kirkpatrick, E., McMahon, M., Zost, S. J., Mui, B. L., Tam, Y. K., Karikó, K., Barbosa, C. J., Madden, T. D., Hope, M. J., Krammer, F., Hensley, S. E., & Weissman, D. (2018). Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nature Communications, 9, 3361.
Pardi, N., Secreto, A. J., Shan, X., Debonera, F., Glover, J., Yi, Y., Muramatsu, H., Ni, H., Mui, B. L., Tam, Y. K., Shaheen, F., Collman, R. G., Karikó, K., Danet-Desnoyers, G. A., Madden, T. D., Hope, M. J., & Weissman, D. (2017). Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nature Communications, 8, 14630.
Zhang, N. N., Li, X. F., Deng, Y. Q., Zhao, H., Huang, Y. J., Yang, G., Huang, W. J., Gao, P., Zhou, C., Zhang, R. R., Guo, Y., Sun, S. H., Fan, H., Zu, S. L., Chen, Q., He, Q., Cao, T. S., Huang, X. Y., Qiu, H. Y., … Qin, C. F. (2020). A thermostable mRNA vaccine against COVID-19. Cell, 182, 1271–1283.
McKay, P. F., Hu, K., Blakney, A. K., Samnuan, K., Brown, J. C., Penn, R., Zhou, J., Bouton, C. R., Rogers, P., Polra, K., Lin, P. J. C., Barbosa, C., Tam, Y. K., Barclay, W. S., & Shattock, R. J. (2020). Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nature Communications, 11, 3523.
Pardi, N., Hogan, M. J., Pelc, R. S., Muramatsu, H., Andersen, H., DeMaso, C. R., Dowd, K. A., Sutherland, L. L., Scearce, R. M., Parks, R., Wagner, W., Granados, A., Greenhouse, J., Walker, M., Willis, E., Yu, J. S., McGee, C. E., Sempowski, G. D., Mui, B. L., … Weissman, D. (2017). Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature, 543, 248–251.
Richner, J. M., Himansu, S., Dowd, K. A., Butler, S. L., Salazar, V., Fox, J. M., Julander, J. G., Tang, W. W., Shresta, S., Pierson, T. C., Ciaramella, G., & Diamond, M. S. (2017). Modified mRNA vaccines protect against Zika virus infection. Cell, 169, 176.
Aldosari, B. N., Alfagih, I. M., & Almurshedi, A. S. (2021). Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics, 13, 206.
Lutz, J., Lazzaro, S., Habbeddine, M., Schmidt, K. E., Baumhof, P., Mui, B. L., Tam, Y. K., Madden, T. D., Hope, M. J., Heidenreich, R., & Fotin-Mleczek, M. (2017). Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines, 2, 29.
Liang, F., Lindgren, G., Lin, A., Thompson, E. A., Ols, S., Röhss, J., John, S., Hassett, K., Yuzhakov, O., Bahl, K., Brito, L. A., Salter, H., Ciaramella, G., & Loré, K. (2017). Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Molecular Therapy, 25, 2635–2647.
Pardi, N., Hogan, M. J., Pelc, R. S., Muramatsu, H., Andersen, H., DeMaso, C. R., Dowd, K. A., & Sutherland, L. L. (2017). Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature, 543, 248–251.
Richner, J. M., Jagger, B. W., Shan, C., Fontes, C. R., Dowd, K. A., Cao, B., Himansu, S., Caine, E. A., Nunes, B. T. D., Medeiros, D. B. A., Muruato, A. E., Foreman, B. M., Luo, H., Wang, T., Barrett, A. D., Weaver, S. C., Vasconcelos, P. F. C., Rossi, S. L., Ciaramella, G., … Diamond, M. S. (2017). Vaccine mediated protection against Zika virus-induced congenital disease. Cell, 170, 273–283.
Meyer, M., Huang, E., Yuzhakov, O., Ramanathan, P., Ciaramella, G., & Bukreyev, A. (2018). Modified mRNA-based vaccines elicit robust immune responses and protect Guinea pigs from Ebola virus disease. Journal of Infectious Diseases, 217, 451–455.
John, S., Yuzhakov, O., Woods, A., Deterling, J., Hassett, K., Shaw, C. A., & Ciaramella, G. (2018). Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine, 36, 1689–1699.
Magini, D., Giovani, C., Mangiavacchi, S., Maccari, S., Cecchi, R., Ulmer, J. B., De Gregorio, E., Geall, A. J., Brazzoli, M., & Bertholet, S. (2016). Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge. PLoS ONE, 11, e0161193.
Lee, K., Kim, S. Y., Seo, Y., Kim, M. H., Chang, J., & Lee, H. (2020). Adjuvant incorporated lipid nanoparticles for enhanced mRNA-mediated cancer immunotherapy. Biomaterials Science, 8, 1101–1105.
Wahane, A., Waghmode, A., Kapphahn, A., Dhuri, K., Gupta, A., & Bahal, R. (2020). Role of lipid-based and polymer-based non-viral vectors in nucleic acid delivery for next-generation gene therapy. Molecules, 25, 2866.
Aldrich, C., Leroux-Roels, I., Huang, K. B., Bica, M. A., Loeliger, E., Schoenborn-Kellenberger, O., Walz, L., Leroux-Roels, G., von Sonnenburg, F., & Oostvogels, L. (2021). Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase I trial. Vaccine, 39(8), 1310–1318.
Brazzoli, M., Magini, D., Bonci, A., Buccato, S., Giovani, C., Kratzer, R., Zurli, V., Mangiavacchi, S., Casini, D., Brito, L. M., De Gregorio, E., Mason, P. W., Ulmer, J. B., Geall, A. J., & Bertholet, S. (2016). Induction of broad-based immunity and protective efficacy by self-amplifying mRNA vaccines encoding influenza virus hemagglutinin. Journal of Virology, 90, 332–344.
Bogers, W. M., Oostermeijer, H., Mooij, P., Koopman, G., Verschoor, E. J., Davis, D., Ulmer, J. B., Brito, L. A., Cu, Y., Banerjee, K., Otten, G. R., Burke, B., Dey, A., Heeney, J. L., Shen, X., Tomaras, G. D., Labranche, C., Montefiori, D. C., Liao, H. X., … Barnett, S. W. (2015). Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. Journal of Infectious Diseases, 211, 947–955.
Brito, L. A., Chan, M., Shaw, C. A., Hekele, A., Carsillo, T., Schaefer, M., Archer, J., Seubert, A., Otten, G. R., Beard, C. W., Dey, A. K., Lilja, A., Valiante, N. M., Mason, P. W., Mandl, C. W., Barnett, S. W., Dormitzer, P. R., Ulmer, J. B., Singh, M., … Geall, A. J. (2014). A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Molecular Therapy, 22, 2118–2129.
Maruggi, G., Chiarot, E., Giovani, C., Buccato, S., Bonacci, S., Frigimelica, E., Margarit, I., Geall, A., Bensi, G., & Maione, D. (2017). Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine, 35, 361–368.
Garcia, A. B., Siu, E., Sun, T., Exler, V., Brito, L., Hekele, A., Otten, G., Augustijn, K., Janse, C. J., Ulmer, J. B., Bernhagen, J., Fikrig, E., Geall, A., & Bucala, R. (2018). Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nature Communication, 9(1), 2714.
Geall, A. J., Verma, A., Otten, G. R., Shaw, C. A., Hekele, A., Banerjee, K., Cu, Y., Beard, C. W., Brito, L. A., Krucker, T., O’Hagan, D. T., Singh, M., Mason, P. W., Valiante, N. M., Dormitzer, P. R., Barnett, S. W., Rappuoli, R., Ulmer, J. B., & Mandl, C. W. (2012). Non-viral delivery of self-amplifying RNA vaccines. Proceedings of the National Academy of Science, 109, 14604–14609.
Chahal, J. S., Fang, T., Woodham, A. W., Khan, O. F., Ling, J., Anderson, D. G., & Ploegh, H. L. (2017). An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Science and Reports, 7(1), 252.
Erasmus, J. H., Khandhar, A. P., Guderian, J., Granger, B., Archer, J., Archer, M., Gage, E., Fuerte-Stone, J., Larson, E., Lin, S., Kramer, R., Coler, R. N., Fox, C. B., Stinchcomb, D. T., Reed, S. G., & Van Hoeven, N. (2018). A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Molecular Therapy, 26, 2507–2522.
Miyazaki, J., Nishiyama, H., Yano, I., Nakaya, A., Kohama, H., Kawai, K., Joraku, A., Nakamura, T., Harashima, H., & Akaza, H. (2011). The therapeutic effects of R8-liposome-BCG-CWS on BBN-induced rat urinary bladder carcinoma. Anticancer Research, 31, 2065–2071.
Yaguchi, K., Ohgitani, T., Noro, T., Kaneshige, T., & Shimizu, Y. (2009). Vaccination of chickens with liposomal inactivated avian pathogenic Escherichia coli (APEC) vaccine by eye drop or coarse spray administration. Avian Diseases, 53, 245–249.
Wang, C., Zhuang, Y., Zhang, Y., Luo, Z., Gao, N., Li, P., Pan, H., Cai, L., & Ma, Y. (2012). Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine elicited antitumor immunity by enhancing TLR3-IRF3 signaling and type I interferons in dendritic cells. Vaccine, 30, 4790–4799.
Thomann, J., Heurtault, B., Weidner, S., Brayé, M., Beyrath, J., Fournel, S., Schuber, F., & Frisch, B. (2011). Antitumor activity of liposomal ERBB2/HER2 epitope peptide-based vaccine constructs incorporating TLR agonists and mannose receptor targeting. Biomaterials, 32, 4574–4583.
Tai, W., Roberts, L., Seryshev, A., Gubatan, J. M., Bland, C. S., Zabriskie, R., Kulkarni, S., Soong, L., Mbawuike, I., Gilbert, B., Kheradmand, F., & Corry, D. B. (2011). Multistrain influenza protection induced by a nanoparticulate mucosal immunotherapeutic. Mucosal Immunology, 4, 197–207.
O’Hagan, D. T., Ott, G. S., De Gregorio, E., & Seubert, A. (2012). The mechanism of action of MF59: An innately attractive adjuvant formulation. Vaccine, 30(29), 4341–4348.
Funding
None.
Author information
Authors and Affiliations
Contributions
AB performed literature search, draft writing, and manuscript revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Research Involving Human and Animal Rights
This article does not contain any studies with human participants or animals performed by author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bolhassani, A. Lipid-Based Delivery Systems in Development of Genetic and Subunit Vaccines. Mol Biotechnol 65, 669–698 (2023). https://doi.org/10.1007/s12033-022-00624-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00624-8