Abstract
The role of the transcription factor creA-mediating carbon catabolite repression in Trichoderma orientalis EU7-22 was investigated for cellulase and hemicellulase production. The binary vector pUR5750G/creA::hph was constructed to knock out creA by homologous integration, generating the ΔcreA mutant Trichoderma orientalis CF1D. For strain CF1D, the filter paper activities (FPA), endoglucanase activities (CMC), cellobiohydrolase activity(CBH), β-glucosidase activity (BG), xylanase activity (XYN), and extracellular protein concentration were 1.45-, 1.15-, 1.71-, 2.51-, 2.72, and 1.95-fold higher in inducing medium and were 6.41-, 7.50-, 10.27-, 11.79-, 9.25-, and 3.77-fold higher in glucose repressing medium, respectively, than those in the parent strain after 4 days. SDS–PAGE demonstrated that the extracellular proteins were largely secreted in the mutant CF1D. Quantitative reverse-transcription polymerase chain reaction indicated that the expressions of cbh1, cbh2, eg1, eg2, bgl1, xyn1, and xyn2 were significantly increasing for the mutant CF1D not only in the inducing medium but also in the repressing medium. Those results indicated that creA was a valid target gene in strain engineering for improved enzyme production in T. orientalis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is a renewable resource, which can be used to produce environmental-friendly biofuels, chemicals, polymers, and materials [1]. Conversion of lignocellulosic biomass into fermentable sugars mainly depends on the degradation of cellulolytic enzymes such as cellulase and hemicellulase, which are produced by many filamentous fungi including Trichoderma, Aspergillus, Penicillium, and Acremonium [2,3,4]. The cellulases include three major groups of enzymes: endoglucanases, which randomly attack internal glycosidic linkages; cellobiohydrolases, which produce cellobiose from the reducing and non-reducing ends of the cellulose chain; and β-glucosidases, which convert cellobiose into glucose [3, 4]. In addition to cellulases, a number of hemicellulases like xylanases, β-xylosidases, α-arabinosidases, α-glucuronidases, α-galactosidases, ferulic acid esterases, and acetyl xylan esterases are needed for a complete hydrolysis of lignocellulosic substrates. Xylanases is the most important enzyme among the hemicellulases, which randomly cleave the β-1,4 backbone of the complex plant cell wall polysaccharide xylan [4].
However, carbon catabolite repression (CCR) is an important mechanism for controlling metabolic processes in prokaryotic and eukaryotic microorganisms. Filamentous fungi yield large amounts of biomass-degradation enzymes that are regulated by CCR to mainly control carbon assimilation [5]. The Cys2His2-type transcription factor creI/creA has been shown to act as a repressor for mediating CCR, which binds to the promoter region of target genes via the consensus motif 5′-SYGGRG-3′, whose function in vivo has been shown in Trichoderma reesei [5, 6], Aspergillus nidulans [7], Aspergillus niger [8], and Neurospora crassa [9]. Production of cellulolytic enzymes could be induced by cellulose, but are strongly repressed by hydrolysate-glucose, the major end-product.
In this study, the transcription factor creA of the Trichoderma orientalis EU7-22 was investigated to evaluate the production of cellulase and hemicellulase not only in cellulose-inducing medium but also in glucose repressing medium.
Materials and Methods
Strains
Trichoderma orientalis EU7-22, which was isolated and preserved in this laboratory, was cultivated on potato dextrose agar (PDA) for 5 days. Spores were collected in sterile water and then filtered. The number of spores were counted using a hemocytometer, inoculated in the potato dextrose liquid medium (1 × 105 total spores per mL), incubated at 30 °C for 34 h with 180 rpm agitation on a rotary shaker, and then transferred to submerged fermentation medium with 10% inoculum for enzyme production. Escherichia coli DH5a was used for vector construction. Agrobacterium tumefaciens AGL1 was used to mediate the transformation.
Plasmid Construction
The gene creA (GenBank no. JQ238603) from the parent strain T. orientalis EU7-22 was knocked out for enzyme and protein analyses. The T-DNA binary vector pUR5750G/creA::hph was constructed on the backbone of pUR5750G (Fig. 1a). The first homologous arm sequence (fragment I = 1942 bp) of creA upstream was amplified by PCR with the primers CreA-QC1F (containing Kpn I) and CreA-QC1R (containing Sac I), and then, it was inserted into the Kpn I/Sac I sites of pUR5750G. Then, the second homologous arm sequence (fragment II = 1487 bp) of creA downstream was amplified by PCR with the primers CreA-QC2F (containing XbaI) and CreA-QC2R (containing HindIII), and then, it was also inserted to the XbaI/HindIII sites of pUR5750G. The primer sequences used in this study are listed in Table 1, and the schematic maps of the primer locus for the knockout of creA are shown in Fig. 1b.
Obtaining the ΔcreA Transformants
The binary vector pUR5750G/creA::hph was transferred into the parental strain T. orientalis EU7-22 via Agrobacterium tumefaciens-mediated transformation (ATMT) [10]. The putative visible transformants were first picked and transferred to a PDA agar plate containing hygromycin B. The transformants were then cultured on PDA agar plates without hygromycin B for three times. Then, the monoconidial cultures were transferred to PDA plates containing 100 μg/mL hygromycin B for determining the stability of the transformants. Genomic DNA of the transformants was extracted from all available mycelia according to the method of Penttilä et al. [11]. The gene fragments were analyzed by PCR amplification using the primer CreA-F&-R(1209 bp), hph-F&-R (811 bp), and HtrpC-YZF and CreA-YZR (2691 bp). The positive mutant strain was analyzed by PCR (Fig. 2).
Identifying T. orientalis CF1D transformants. (a primers CreA-F and CreA-R test; b primers Hph-F and Hph-R test; c primers HtrpC-F and CreA-YZRtest; lanes 1 and 2 represent the genomic DNA as the template from the parental strain T. orientalis EU7-22 and the T. orientalis CF1D transformant, respectively. Ma: 200 bp DNA Ladder Marker; Mb: GeneRuler™ Mix Marker)
Cellulase and Xylanase Preparation
Experiments were conducted in 250-mL Erlenmeyer flasks. The inducing medium (IM, pH 5.2) was 50 mL, containing Avicel (PH101, 50 μm particle size, FMC Corporation) inducer substrate (2%, w/v), wheat bran (1%, w/v), 0.5% peptone, 0.05% CaCl2, 0.05% MgSO4, 0.4% Tween-80, and 0.25% KH2PO4.
The repressing medium (RM) was based on the IM supplemented with 3.0% glucose. The submerged fermentation conditions were 30 °C for 4 days with 180 rpm agitation on a rotary shaker.
Enzyme Activities Assay and SDS–PAGE Analysis
Crude enzyme was first centrifuged (6000 rpm for 10 min) to remove the cells and solid material. The enzyme activity of the supernatant was then determined. The filter paper activity (FPA) and endoglucanase activities (CMC) were measured as described by Ghose [12], and a standard curve of d-glucose was used as a reference. One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 µmol of reducing sugar per minute, and was expressed as U/mL. The cellobiohydrolase activity (CBH) and β-glucosidase activity (BG) were assayed as described by Saha [13], and a standard curve of p-nitrophenol (pNP) was used as a reference. One unit of enzyme activity was defined as the amount of enzyme that released 1 μmol pNP per minute in the reaction and expressed as U/mL. Xylanase activity (XYN) was assayed according to the method of Bailey et al. [14] by measuring the total reducing sugars released from 1% (w/v) beechwood xylan (Sigma, St. Louis, USA, X4252) (1.0 mL) in citrate buffer (50 mM, pH 4.8). One unit of xylanase activity was defined as the amount of enzyme that produced 1 μmol xylose per minute and was expressed as U/mL.
The protein concentration of crude enzyme was determined with a Bradford protein assay kit (Sangon Biotech Co. Ltd., Shanghai, China). The supernatant was denaturized with boiling water for 10 min and directly analyzed with SDS–PAGE on a 13% polyacrylamide gel stained with Coomassie brilliant blue. The protein marker (SM0431) was purchased from the company Fermentas. Every lane contained an equal volume of the protein samples.
Transcription Analysis
Approximately 200 mg of the fermentation sample was grounded to a fine powder under liquid nitrogen and then transferred to a 50-mL Corning tube on ice. RNA was extracted from the parental strain sample and ΔcreA strain with Trizol reagent (Takara, Japan). The quality of the extracted total RNA was identified by agarose gel electrophoresis, and the concentration of mRNA was measured by spectroscopy (Nanodrop 2000, Thermo Fisher Scientific). Reverse transcription was carried out using the PrimeScript® RT reagent kit (Takara, Japan). Relative expression levels of cellobiohydrolase-encoding gene cbh1 (JQ238604) and cbh2 (JQ238605), endoglucanase-encoding gene eg1 (JQ238606) and eg2 (JQ238607), β-glucosidase-encoding gene bgl1 (JQ904600), xylanase-encoding gene xyn1 (JQ238610) and xyn2 (JQ238611) were calculated in comparison with the expression of the 18S rRNA gene by real-time PCR (ABI Step One Plus). The primers are listed in Table 1.
Results
Obtaining the ΔcreA Mutant Strain
After A. tumefaciens AGL1-mediated transformation of pUR5750G/creA::hph into T. orientalis EU7-22, three transformants were selected and verified to retain their mitotic stability. Genomic DNA from the three transformants were tested by PCR analysis. However, only one of the transformants was positive. Firstly, the primers CreA-F&-R were used to detect creA, which was present in the parental strain but was absent in the ΔcreA mutant (Figs. 1b, 3a). At the same time, the primers Hph-F&-R were used to detect hph, which was absent in the parental strain but was present in the ΔcreA mutant (Figs. 1b, 2b). Then, the primers HtrpC-F and CreA-YZR were used to verify that the hph cassettes were successfully inserted into the creA locus in the ΔcreA mutant (Figs. 1b, 3c). Therefore, the mutant was named T. orientalis CF1D. The phenotype of the parental strain and T. orientalis CF1D were grown on PDA for 3 days and are shown in Fig. 3. The ΔcreA mutant grew slower and denser than the parental strain.
Deletion of creA Increased Cellulolytic Enzyme Production
The parental strain T. orientalis EU7-22 and mutant T. orientalis CF1D were cultivated for cellulase and xylanase analysis. In T. orientalis CF1D, the FPA, CMC, CBH, BG, and XYN activities were 1.45-, 1.15-, 1.71-, 2.51-, and 2.72-fold higher, respectively, than that of the parental stain when cultured in IM for 4 days (Fig. 4a). However, when both strains were cultured in RM for 4 days, the cellulase and hemicellulase activities were higher in T. orientalis CF1D (Fig. 4b). The FPA, CMC, CBH, BG, and XYN activities were 6.41-, 7.50-, 10.27-, 11.79-, and 9.25-fold higher, respectively, than that in the parental strain. Furthermore, 98.58% of the glucose was utilized by the parental strain, while only 71.58% glucose was utilized by T. orientalis CF1D in the RM.
Relative expression analysis of enzyme activities and genes (a enzyme activities for T. orientalis EU7-22 and T. orientalis CF1D after 4 days of cultivation in inducing medium. b Enzyme activities for T. orientalis EU7-22 and T. orientalis CF1D after 4 days of cultivation in repressing medium. c Gene relative expression fold analysis for T. orientalis EU7-22 and T. orientalis CF1D cultivated in inducing medium. d Gene relative expression fold analysis for T. orientalis EU7-22 and T. orientalis CF1D cultivated in repressing medium. Inducing medium: 2% Avicel with 1% wheat bran. Repressing medium: 2% Avicel with 1% wheat bran and 3% glucose)
Compared to the IM, the FPA, CMC, CBH, BG, and XYN activities of the parental strain decreased by 85.67, 88.38, 84.39, 81.04, and 89.07% in the RM, respectively. The FPA, CMC, CBH, BG, and XYN activities of T. orientalis CF1D decreased by 36.71, 24.08, 0.06, 0.11, and 62.87% in the RM, respectively. However, the FPA, CMC, CBH, BG, and XYN activities of T. orientalis CF1D in RM were higher than that of the parental strain in the IM. With the Avicel inducer in the RM, production of cellulase and hemicellulase was repressed in the parental strain but was derepressed in T. orientalis CF1D.
Increased Expression Levels of Cellulolytic Enzymes Correlated with Increased Enzymatic Activity in the ΔcreA Strain
To determine whether increased cellulolytic enzyme activities in the T. orientalis CF1D were due to a higher level of cellulolytic genes, the major genes were analyzed by qRT-PCR. As predicted, the expression levels of cbh1, cbh2, eg1, eg2, bgl1, xyn1, and xyn2 were significantly higher in the ΔcreA mutant than in the parental strain not only when cultured in the IM but also in the RM. The cbh1, cbh2, eg1, eg2, bgl1, xyn1, and xyn2 genes were 6.34-, 2.33-, 3.56-, 1.33-, 5.27-, 1.57-, and 27.57-fold higher in IM culture, respectively (Fig. 4c),whereas they were 62.98-, 47.94-, 33.64-, 46.71-, 67.04-, 15.30-, and 129.63-fold higher in the RM culture, respectively, than that in the parental stain (Fig. 4d).
Effect of ΔcreA on Extracellular Protein Secretion
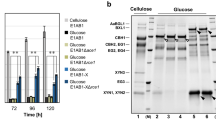
Extracellular proteins secreted into the culture medium were analyzed by SDS–PAGE. When grown in IM, T. orientalis CF1D secreted 1.95-fold more extracellular protein than that of the parent strain (Fig. 5a, c), which also contributed to the increase in cellulase and hemicellulase. When grown on RM, T. orientalis CF1D secreted 3.77-fold more extracellular protein than that of the parent strain (Fig. 5b, c), which also contributed to the increase in cellulase and hemicellulase. Compared to IM, the protein concentration for the parent strain and T. orientalis CF1D showed a 62.67 and 27.94% decrease, respectively. However, T. orientalis CF1D secreted 1.41-fold more extracellular protein in RM than that for the parental strain in IM.
Secreted protein of T. orientalis EU7-22 and T. orientalis CF1D after 4 days of cultivation. (a T. orientalis EU7-22 and CF1D in inducing medium. b T. orientalis EU7-22 and CF1D in repressing medium. c Secreted protein concentration for T. orientalis EU7-22 and CF1D in inducing or repressing medium)
Discussion
The effects of creA on cellulase and hemicellulase activity as well as the amount of extracellular protein produced in the mutant strain were investigated. Portnoy et al. [5] elucidated the cre1 regulatory range in the fungus T. reesei (anamorph of Hypocrea jecorina) by profiling transcription in wild-type and Δcre1 mutant strains. Analysis by genome-wide microarrays revealed 2.8% of the transcripts whose expression was regulated in at least by one of the four experimental conditions: 47.3% of which were repressed by cre1, whereas 29.0% were actually induced by cre1, and 17.2% were only affected by the growth rate but were also cre1 independent.
It was observed in this study that creA deletion has clear effects on the colony morphology of T. orientalis. The colonies were smaller and produced fewer aerial hyphae and spores than colonies of the parental strain. The same effect could be detected similar to the phenotypes of A. niger [8], T. reesei [9], N. crassa [15] creA/cre1 mutants because cre1 could affect the growth rate of fungi [5]. Higher cellulase and xylanase activities (Fig. 4a, b) and amounts of corresponding mRNAs (Fig. 4c, d), which were observed both for the IM and RM, were detected in the strain CF1D-based the ΔcreA transformant than those in the parental strain. In the RM, this was an expected result, since loss of creA released the strain from carbon catabolite repression. The cre1 directly suppressed cbh1 transcription by binding to two closely spaced 5′-CCCCAC-3′ motifs in the cbh1 promoter region in H. jecorina [6, 16]. Deletion of cre1 caused an increase in the cbh1 transcript levels under repressing conditions [17]. The cre1 involved indirectly in the control of cbh2 expression by regulating the main inducer XYR1 [16]. We also observed differences in the transcript levels of genes encoding cellulase and xylanase such as cbh1, cbh2, egl1, egl2, bgl1, xyn1, and xyn2. The relative transcript ratio of the cbh1, egl1, bgl1, and xyn2 genes was significantly increased in the transformant relative to the wild-type strain. Mach-Aigner et al. [16] reported that the xylanase and cellulase activator gene xyr1 is under the control of the cre1 repressor. This could provide an explanation for our results since the creA gene deletion also had a strong effect in a medium inducing the cellulase and hemicellulase genes.
However, it was also noteworthy that even though the deletion of creA led to increased production of proteins both in the IM and RM (Fig. 5). The production levels of cellulase and xylanase in glucose-based RM were still lower than the levels obtained from the CF1D strain in the IM (Figs. 4, 5). The large effect on protein production detected in this work suggested that creA also has a role in modulating the levels of gene expression and then regulating protein secretion [5]. In any case, this result shows that deletion creA is a relevant strategy for improving even highly developed industrial strains.
Conclusions
This study focused on the C2H2-type transcription factor creA from T. orientalis EU7-22 to study cellulase and hemicellulase production. The homologous integration vector pUR5750G/creA::hph was constructed to knockout creA gene. And we obtained the mutant strain T. orientalis CF1D, which exhibited higher cellulase and hemicellulase activity with largely extracellular secretion protein not only in cellulose-inducing medium but also in glucose repressing medium. The qRT-PCR showed that the expression levels of cbh1, cbh2, eg1, eg2, bgl1, xyn1, and xyn2 were significantly higher in the ΔcreA mutant than in the parent strain. Therefore, deletion of creA is a relevant strategy for improving industrial strains.
References
Kamm, B., & Kamm, M. (2007). Biorefineries—Multi product processes. Advances in Biochemical Engineering/Biotechnology, 105, 175–204.
Kumar, R., Singh, S., & Singh, O. V. (2008). Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. Journal of Industrial Microbiology and Biotechnology, 35, 377–391.
Kovács, K., Szakacs, G., & Zacchi, G. (2009). Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broths and washed mycelia of Trichoderma reesei and Trichoderma atroviride. Bioresource Technology, 100, 1350–1357.
Sipos, B., Benko, Z., Dienes, D., Réczey, K., Viikari, L., & Siika-aho, M. (2010). Characterisation of specific activities and hydrolytic properties of cell-wall degrading enzymes produced by Trichoderma reesei Rut C30 on different carbon sources. Applied Biochemistry and Biotechnology, 161, 347–364.
Portnoy, T., Margeot, A., Linke, R., Atanasova, L., Fekete, E., Sándor, E., et al. (2011). The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: A master regulator of carbon assimilation. BMC Genomics, 12(269), 1–12.
Takashima, S., Iikura, H., Nakamura, A., Masaki, H., & Uozumi, T. (1996). Analysis of Cre1 binding sites in the Trichoderma reesei cbh1 upstream region. FEMS Microbiology Letters, 145, 361–366.
Cubero, B., & Scazzocchio, C. (1994). Two different, adjacent and divergent zinc finger binding sites are necessary for CREA-mediated carbon catabolite repression in the proline gene cluster of Aspergillus nidulans. EMBO Journal, 13, 407–415.
Drysdale, M. R., Kolze, S. E., & Kelly, J. M. (1993). The Aspergillus niger carbon catabolite repressor encoding gene, creA. Gene, 130, 241–245.
Sun, J., & Glass, N. L. (2011). Identification of the CRE-1 cellulolytic regulon in Neurospora crassa. PLoS ONE, 6, e25654.
Long, C. N., Cheng, Y. J., Gan, L. H., Liu, J., & Long, M. N. (2013). Identification of a genomic region containing a novel promoter resistant to glucose repression and over-expression of β-glucosidase gene in Hypocrea orientalis EU7-22. International Journal of Molecular Sciences, 14(4), 8479–8490.
Penttilä, M., Nevalainen, H., Rättö, M., Salminen, E., & Knowles, J. (1987). A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene, 61, 155–164.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Saha, B. C. (2014). Production, purification and properties of endoglucanase from a newly isolated strain of Mucor circinelloides. Process Biochemistry, 39, 1871–1876.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology, 23, 257–270.
Nakari-Setälä, T., Paloheimo, M., Kallio, J., Vehmaanperä, J., Penttilä, M., & Saloheimo, M. (2009). Genetic modification of carbon catabolite repression in Trichoderma reesei for improved protein production. Applied and Environment Microbiology, 75, 4853–4860.
Mach-Aigner, A. R., Pucher, M. E., Steiger, M. G., Bauer, G. E., Preis, S. J., & Mach, R. L. (2008). Transcriptional regulation of xyr1, encoding the main regulator of the xylanolytic and cellulolytic enzyme system in Hypocrea jecorina. Applied and Environment Microbiology, 74, 6554–6562.
Ries, L., Belshaw, N. J., Ilmén, M., Penttilä, M. E., Alapuranen, M., & Archer, D. B. (2014). The role of CRE1 in nucleosome positioning within the cbh1 promoter and coding regions of Trichoderma reesei. Applied Microbiology and Biotechnology, 98, 749–762.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31170067, 21303142), Jiangxi Province Science Foundation for Youths (Grant No. 20161BAB214177).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Long, C., Cheng, Y., Cui, J. et al. Enhancing Cellulase and Hemicellulase Production in Trichoderma orientalis EU7-22 via Knockout of the creA . Mol Biotechnol 60, 55–61 (2018). https://doi.org/10.1007/s12033-017-0046-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-0046-3