Abstract
Cancer is one of the major malignant diseases in the world. Current anti tumor agents are restricted during the chemotherapy due to their poor solubility in aqueous media, multidrug resistance problems, cytotoxicity, and serious side effects to healthy tissues. Development of targeted drug nanocarriers would enhance the undesirable effects of anticancer drugs and also selectively deliver them to cancerous tissues. Variety of nanocarriers such as micelles, polymeric nanoparticles, liposomes nanogels, dendrimers, and carbon nanotubes have been used for targeted delivery of anticancer agents. These nanocarriers transfer loaded drugs to desired sites through passive or active efficacy mechanisms. Chitosan and its derivatives, due to their unique properties such as hydrophilicity, biocompatibility, and biodegradability, have attracted attention to be used in nanocarriers. Grafting cancer-specific ligands onto the Chitosan nanoparticles, which leads to ligand–receptor interactions, has been successfully developed as active targeting. Chitosan-conjugated components also respond to external or internal physical and chemical stimulus in targeted tumors that is called environment triggers. In this study, mechanisms of targeted tumor deliveries via nanocarriers were explained; specifically, chitosan-based nanocarriers in tumor-targeting drug delivery were also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is essentially a genetic disease characterized by increased cellular proliferation, reduced cell death, or usually a combination of both. Rapid proliferation of cancer cells, reduces cell death, tissue infiltration, establishment of a blood supply to the tumor, metastasis to secondary sites in the body and dysfunction of affected organs [1]. The ultimate goal of cancer therapeutics is to increase the survival time and the quality of life of the patient by reducing the unintended harmful side effects [2].
The common cancer treatments are chemotherapy, radiation, and surgery with chemotherapy being the major treatment modality [3]. Use of chemotherapy has proven beneficial in improving survival rate for cancer to some extent [4]. However, conventional chemotherapeutic agents are limited by their undesirable properties, such as poor solubility, narrow therapeutic window, and non-specific site of action following oral/intravenous administration and cytotoxicity to normal tissues, which may be the cause of treatment failure in cancer [5, 6].
In the last three decades, therefore, nanopreparations have been evaluated to improve the delivery of potential therapeutic molecules to cancer sites and to improve their stability in systemic circulations while minimizing the exposure to normal tissues to reduce unwanted effects [4]. More recently, researchers have shown interest in water-soluble stimuli-responsive polymeric systems that have a phase transition in response to external physical and chemical stimulus such as temperature, pH, irradiation, specific ion, and magnetic field [7].
Biodegradable nanoparticles are frequently used to improve the therapeutic value of various water-soluble and insoluble medicinal drugs and bioactive molecules by improving bioavailability, solubility, and retention time. These nanoparticle–drug formulations reduce the patient expenses and risks of toxicity [8]. An exciting potential solution in cancer treatments is to encapsulate the drug in a biocompatible material that can be injected into the blood stream with the intention of delivering drug to a tumor site [7]. Polysaccharides, lipids, surfactants, and dendrimers have received increasing attention due to their outstanding physical and biological properties [9]. Natural and synthetic polymers have been well explored in drug delivery. Among them, chitosan is the well-known polymer which has many applications in biomedical field [7].
Nanocarriers and Their Application in Chemotherapy
The critical bottleneck of conventional cancer chemotherapeutics includes high toxicity of most anticancer drugs. This behavior is due to indiscriminate distribution of drugs toward disease and healthy cells following by systemic administration. In addition, anticancer drugs often suffer from poor solubility in water; thus need to use organic solvents or detergents for clinical applications, resulting in undesirable side effects such as venous irritation and respiratory distress [10]. Designing a distinct carrier system that encapsulates a large quantity of drugs and specifically targets tumor cells is, therefore, indispensable for successful cancer therapy [11]. Recently, carrier-based approaches have been implemented to bypass the majority of the challenges in drug delivery. However, the selection, design, and development of the specific carrier system is a state-of-art and requires thorough understanding of the physicochemical properties of drug substances and its behavior in physiological conditions [12]. Among the various approaches, nanocarriers (particularly in the size range from 10 to 100 nm) offer some unique properties such as high surface area-to-volume ratio. Nanocarriers can also be designed to carry therapeutic molecules that distinguish them from other cancer therapeutics [13]. Several types of nanocarriers including micelles, liposomes, dendrimers, carbon nanotubes, nanocrystals, polymeric nanoparticles, and nanogels have been evaluated for cancer chemotherapy [14, 15].
The principal advantages of nanocarriers include their increased solubilization potential, superior encapsulation, altered absorption pathways, prevention of metabolic degradation within gastro-intestinal tract, chemical versatility of materials eligible for nanomedicines, flexibility in surface functionalization, drug- and disease-specific tailor-made design capability, targeting potential, and ability to incorporate a wide variety of drug substances [12]. Among these systems, nanoparticle-mediated delivery provides a number of advantages including small particle size, increased drug efficacy, lowered toxicity, enhanced drug solubility and stability, and ability to achieve steady-state therapeutic levels over an extended time frame [16]. More recently, nanoparticles have attracted attention as a way of resolving the toxicity problems of conventional cancer therapeutics. Nanotechnology confers targeting favorable properties such as relatively high accumulation in tumor sites and long blood circulation time on nanoparticles (NPs). Specifically, (NPs) can be easily accumulated at tumor site with high concentration due to the pathophysiological differences between normal tissues and tumor tissues known as enhanced permeability and retention (EPR) effect [17]. Nanoparticles have made remarkable improvements in the solubility, stability, biocompatibility, release profile, and non-specific toxicity of the drugs, owing to their physicochemical and biological properties [17].
Enhanced Permeability and Retention (EPR) Effect
When a solid tumor reaches a given size, the normal vasculature present in its vicinity is not sufficient to provide all the required oxygen supply for its further proliferation. As cells start to die, they secrete growth factors that trigger the budding of new blood vessels from the surrounding capillaries [18]. This process, known as angiogenesis, promotes the rapid development of new, irregular blood vessels that present a discontinuous epithelium and lack the basal membrane of normal vascular structures [19]. The defective vascular architecture, created due to the rapid vascularization necessary to serve fast-growing cancers, coupled with poor lymphatic drainage allows an EPR effect [20]. The nanometer size of nanoparticles is suitable for preventing renal clearance in kidney and long circulation in blood flow. Particularly, anticancer drug-encapsulated nanoparticles could easily penetrate the fenestrate blood vessels in angiogenic tumor site. Consequently, they have presented higher tumor tissue accumulation compared to natural anticancer drugs, which is denoted as the EPR effect [21, 22].
Elimination of Drug Resistance
Major impediment to successful chemotherapy is the emergence of multidrug resistance (MDR), which is a state of resilience against structurally and mechanistically unrelated drugs that may be intrinsic (i.e., prevailing prior to chemotherapeutic treatment) or acquired, during chemotherapeutic treatment, as a response to chemotherapy [23, 24]. Multidrug resistance is usually associated with the overexpression of ATP-binding cassette (ABC) transporters, including P-glycoprotein (Pgp) and multidrug-resistant proteins (MRP). [25]. The MRP family of proteins found predominantly in intracellular organelles and is thought to play an important role in exocytosis [25]. It is estimated that approximately 500,000 new cases of cancer will eventually exhibit MDR phenotype each year, which can either be acquired from drug treatment or be intrinsic, pre-existing in the cancer cells. This defense mechanism contributes to therapeutic failure and tumor relapses in over 90 % of patients. Two major strategies have been developed regarding how MDR may be modulated: (1) Increasing intracellular concentrations of anticancer drugs in MDR cells and (2) Down-regulation of Pgp expression in MDR cells [26]. To overcome MDR, higher doses or frequency of dosing of the chemotherapeutic agents are required, thus resulting in serious side effects or toxicity [27, 28].
Recently, the prevalence of drug–drug interactions in cancer patients treated with oral anticancer drugs is reported which is alarming in the sense that conventional drug delivery system (both oral and intravenous) is dangerous to patients [12, 29]. As an alternative strategy to circumvent MDR, by incorporating drugs to nanocarriers can be taken up by cells through an endocytic pathway and escape the effect of Pgp efflux pumps due to their passive accumulation in tumor tissues with leaky vasculature through the EPR effect [25]. Nanoparticles can bypass drug efflux by ABC transporters, as they are internalized via either non-specific or specific endocytosis which results in a higher intracellular accumulation of the drug [30]. Nanoparticles are excellent platforms to enhance the therapeutic efficacy of anticancer agents at the target site of action due to their passive and active tumor-targeting abilities, which can reduce systemic toxicity and potentially circumvent the problem of drug resistance [31].
Increase of Hydrophobic Drugs Solubility
Large numbers of chemical drugs for cancer therapy are hydrophobic, and two methods are used to introduce hydrophobic drugs into polymeric nanoparticles: (1) physically loading on to polymeric nanoparticles and (2) chemical conjugation to polymeric nanoparticles [32]. The physical loading technique has been widely used with amphiphilic nanoparticles; however, problems, such as the instability of nanoparticles during blood circulation causing a burst of release and loss of the loaded drugs, have been encountered. The chemical conjugation technique allows the conjugation of hydrophobic drugs to hydrophilic polymers, which can then self-assemble to form spherical nanoparticles in aqueous conditions [33]. To deliver poorly water-soluble drugs is one of the most challenging goals for formulation scientists [34]. Most of the cancer therapeutic drugs that exhibit strong cytotoxic activity against the variety of cancer types have hydrophobic structure such as paclitaxel (PTX), camptothecin (CPT), doxorubicin (DOX), and Pyrrolidine dithiocarbamate (PDTC) [22, 35, 36].
Importantly, nano-sized polymeric carriers like nanoparticles, micelles, or nanogels have been extensively applied. This is due to the fact that nano-sized polymeric carriers enhance the drug solubility and stability in vivo by encapsulating the hydrophobic drug into the nano-size drug carriers. In Fact, drug is encapsulated into the hydrophobic cores and the drug carriers are covered with hydrophilic and biocompatible polymer shells [37]. Furthermore, nano-sized polymeric carrier-encapsulated drugs have exhibited a prolonged circulation time in vivo by avoiding the reticuloendothelial system (RES), and the prolonged circulation time of the polymeric carriers allows the encapsulated hydrophobic drug such as CPT to extravasate and accumulate into the tumor tissue. Therefore, a disorganized vasculature and defective vascular architecture develop in tumor tissue [37]. Polymeric micelles are self-assemblies of amphiphilic block copolymers in aqueous media. The high potential of polymeric micelles as drug carriers lies in their unique characteristics such as nanoscale size, thermodynamic stability, and unique core–shell architecture [38, 39]. PEGylation (Polyethylene glycol) of nanocarriers can improve the solubility and stability of them in an aqueous solution while minimizing opsonization during circulation in the bloodstream. This will significantly increase the circulation time of the nanocarriers, thereby enhancing the in vivo tumor accumulation of the drug nanocarriers. Several other biocompatible and hydrophilic polymers with flexible main chains have been explored as hydrophilic shells for nanocarriers including poly(acrylamide), poly(vinylpyrrolidone), and poly(vinyl alcohol) [30].
Targeted Drug Delivery in Tumor Therapeutics
Localization of the chemotherapy drugs to a specific area will have a twofold effect. Firstly, optimal site delivery will decrease the overall dosage needed and in turn produce a more effective treatment. Secondly, as a consequence of decreasing the overall drug amount, drug-induced side effects will be prevented or extremely limited [5]. Nanomedicine technology offers NPs unique active/passive targeting properties that can convey drugs to specific target site [40]. This ability distinguishes NPs as favorable tools for conventional cancer therapeutics to overcome their limitations like nonselective toxicity and drug resistance [41].
Tumor-targeted drug delivery takes advantage of the differences between malignant and healthy tissues. The environment of the tumor changes as the tumor grows. Due to increased metabolism and growth rates, the oxygen supply becomes insufficient and glucose is converted to lactate which in turn, decreases the interstitial pH of the tumor tissue. Together, hypoxia and glucose depletion trigger the formation of new blood vessels (angiogenesis) which is crucial for the proliferation, migration, and maintenance of the tumor [42]. The ability to target treatment to very specific cancer cells also uses a cancer’s own structure in that many cancers overexpress particular antigens, even on their surface. This makes them ideal targets for drug delivery as long as the targets for a particular cancer cell type can be identified with confidence and are not expressed in significant quantities anywhere else in the body [20].
Further development of the ‘‘multifunctional approach” involved the addition of certain stimuli-sensitive functions to long-circulating and targeted pharmaceutical nanocarriers. Certain stimuli, intrinsically characteristic of the pathological zone or applied to this zone from the outside of the body, could beneficially modify the properties of the drug in a nanocarrier system. Stimuli typical for pathological tissues themselves include pH and redox conditions; temperature can serve as a local stimulus both within the tissue and from the outside; ultrasound, irradiation, heat, and magnetic field could be applied only ‘‘artificially” and mainly from the outside [43]. Figure 1 shows several stimuli-responsive nanocarriers that have been used in tumor treatment. For the case (a), there are several biodegradable nanoparticles such as PLGA, PLA, chitosan, gelatin, and polycaprolactone are used in drug delivery. For the case (b), different compositions of phospholipids [1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol,1,2-distearoyl-sn-glycero-3 phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PE G 2000), and 1, 2-distearoyl-sn glycero-3-phosphoethanolamine-N-[folate(polyethylene glycol)-2000] (DSPE-PEG2000-folate) were used to prepare liposomes for targeted drug delivery. Poly (amidoamine) spherical dendrimers (PAMAM) with ethylene-diamine (EDA) as a tetravalent initiator core, which are conjugated with anticancer drugs such as 5-fluorouracil (5-FU) is an example for case (c). Chitosan-based nanogels applied in many cases in controlled drug delivery and cell imaging. Chitosan-derived micelles are commonly used for the preparation of amphiphilic nanocarriers to tumor therapy. To achieve the efficient drug delivery, the nanocarrier must simultaneously meet two pairs of challenges: (a) the nanocarrier must retain the drug very tightly without any release during the transport in the blood compartments, but must be able to efficiently release the drug once reaching the intracellular target to exert its pharmaceutical action; (b) the nanocarrier must be “slippery” or “stealthy” while in the blood compartments to effectively evade the RES screening, particularly the capture by liver and spleen for a long blood circulation time. As the blood circulation time of the nanocarrier increases, so does its opportunity passing the hyperpermeable tumor blood vessel and extravasation into the tumor [44]. Nanocarriers (NCs) have no propulsive force, and their transit in blood circulation is totally counted upon the blood flowing [45]. The elevated vascular permeability of tumors, typically referred to as the enhanced permeation and retention (EPR) effect, is providing NCs with accessibility to the target during circulation [46].
Passive Targeting
Tumor targeting consists of “passive targeting” and “active targeting.” Passive targeting refers to the extravagation of nanocarriers through leaky tumor capillary fenestrations that results from abnormalities of angiogenesis at the tumor site, resulting in accumulation and retention. This phenomenon was recognized as the EPR effect [3].
The tumor vasculature has leaky architecture between endothelial cells and poor lymphatic drainage systems due to abnormally rapid propagating ratio of tumor tissue. The poor lymphatic system induces different differential interstitial pressure between center and periphery of tumor. Therefore, NPs ranging from 10 to 200 nm can easily penetrate the leaky vessel wall around tumor and then retain in tumor region. Furthermore, the NPs can accumulate with high concentration in the tumor for long time due to the insufficient venous and lymphatic clearance. A large number of studies have already shown that the EPR effect is mainly attributable to cancer-targeting ability of NPs [17]. Passive targeting is a random distribution and happens to almost all carriers, rather than a selective delivery. Therefore, EPR-based passive targeting of NCs, as compared to conventional small drug molecules, improves the efficacy of drug delivery on systemic level [47]. Nanocarriers by passive targeting internalize to the cells through the endocytic routes that are shown in Fig. 2. In the case of non-targeted nanoparticles, the uptake route depends on their physical attributes including particle size, shape, and surface charge, and also on the type of cell line. Cationic nanoparticles ~100 nm in size derived from polylactide-co-polyethylene glycol (PLA-PEG) have been found to internalize exclusively via clathrin-mediated endocytosis (CME). Poly (l-lysine), which is a cationic polymer functionalized at the surface of poly (lactide-co-glycolide) (PLGA) nanoparticles, has also been found to significantly enhance cellular uptake via CME. It seems that the high rate of cellular internalization via CME under normal conditions concluding the main route of internalization for nanoparticles of sizes ~100 nm. Clathrin-independent endocytosis (CIE) does not require the presence of coat proteins for vesicle formation and internalization; however, the actin and actin-associated proteins are important players for vesicle formation during CIE. Clathrin-independent endocytosis is the internalization route described preferentially for polyplexes of self-branched and trisaccharide-substituted chitosan oligomer nanoparticles (SBTCO) for the delivery of DNA. Recent studies suggest that CIE is involved in a novel mechanism for the uptake of nanoparticles that was described as a type of macropinocytosis. Caveolae are flask-shaped invaginations (60–80 nm) of plasma membrane that participate in different cellular processes including cholesterol homeostasis, endocytosis of proteins, and signal transduction. Negative surface charges have been found to trigger cellular internalization predominantly via caveolae. The intracellular destinations of caveolae have been the subject of controversy for many years. Nevertheless, it has emerged that in endothelial cells, caveolae are able to perform transendothelial transport, which may be exploited for the release of nanoparticles in subendothelial tissues [48].
Nanocarriers by passive targeting internalize to the cells through the endocytic routes [48]
Active Targeting Nanocarriers
The accumulation of drugs in tumor tissue does not always guarantee the successful therapy. Therefore, a more effective mechanism should be employed to enable the therapeutic agents to reach their molecular targets. Cancer cells often overexpress some specific antigens or receptors on their surfaces, which can be utilized as targets in modern nanomedicine. The ligands, with specific affinity toward a particular receptor or molecule differentially expressed at the target site, are displayed on the surface of nanocarriers, resulting in the preferential accumulation and uptake at the site of action [48].
Active targeting can be achieved by chemical modification of nano-sized drug carriers with targeting components which, in turn, precisely recognize and specifically interact with receptors on the targeted tissue [11]. The term “active targeting” was intended to distinguish delivery systems with ligands from those without [46]. The active targeting process occurs only after passive accumulation of drug carriers in tumors [49]. Active targeting based on antibodies or receptor-mediated targeting with cancer-specific ligands has been developed to make target tumor cells more selective [50]. The targeting ligands are conjugated at the surface of the nanocarrier for binding to appropriate receptors expressed at the target site [3]. Ligand–receptor interactions are possible only when the two components are in close proximity (<0.5 nm) [49].
For active targeting approaches, NPs have been fused to various targeting moieties, including antibodies, engineered antibody fragment, peptides, small molecules, and DNA/RNA aptamers [17]. Among all the studied intelligent polymers, temperature- and pH-responsive polymeric systems have drawn more attention due to their importance in the physiology of the body [7]. Figure 3 compares the different modes of drug delivery to tumor sites. As clearly observed, due to the removal of pure drug from the cancerous tissue and returning to the vessel, its efficacy is lower than that of the conjugated drug with nanocarriers. This figure also indicates differences between passive and active targeting via nanocarrier.
Chitosan-Based Nanocarriers in Tumor-Targeted Drug Delivery
For drug applications, nanoparticles offer the advantage of controlled release, therapeutic impact and targeted delivery. The targeting capability of nanoparticles depends on the particle size, surface charge, surface modification, and hydrophobicity. Active ingredients are usually hydrophobic and therefore display poor water solubility, which prevents contact with cells and tissues [51, 52].
Considerable research has been directed toward developing efficient chitosan-based NP drug delivery systems. In comparison with the other biological polymers, positive charges target the chitosan carriers to the negatively charged cell membrane and have mucoadhesive properties to increase the uptake of nanoparticles and prolong the retention time of chitosan in the targeted locations [53, 54]. The adhesive properties of chitosan in a swollen state might involve some mechanisms like adhesion by hydration, hydrogen bonding, and ionic interactions. Effective adhesion has been shown for epithelial tissues and in the mucus coat present on the surface of the tissues [55]. The reactive functional groups of chitosan can be chemically modified with various functional moieties to improve the properties of origin chitosan and offer the wide developmental opportunities for pharmaceutical and biomedical applications of chitosan [17]. Various hydrophobic cancer drugs, such as doxorubicin, paclitaxel, docetaxel, camptotecin, and cisplatin, can be physically encapsulated or chemically conjugated to the CNPs, and they can specifically deliver the anticancer drugs into tumor sites. The results of the CNPs as carriers for cancer drugs and imaging probe show prolonged blood circulation and highly tumor-targeted delivery in cell and animal models as compared to the other NPs [17].
A research showed that the prolonged blood circulation of the chitosan-based NPs induced higher EPR efficiency of the NPs. That also indicated that particle size, particle shape, and surface charge of NPs also affected their blood circulation time and EPR effect [56]. Ultimately, in order to achieve efficient tumor targeting of NPs via the EPR effect, NPs should be designed to improve their serum stability leading to enhanced blood circulation, thus giving a better chance to penetrate the tumor blood vessels and accumulate in tumor [17]. Table 1 presents chitosan-based nanocarriers which are used as passive targeting agents for several anticancer hydrophobic drugs.
An important aspect for the application of chitosan in drug delivery systems is the fate of the chitosan in the body after absorption or injection [57]. Grafting the hydrophobic and hydrophilic segments to the chitosan backbone would give rise to amphiphilic graft copolymers, which can form self-assembled micelles in water [58]. This procedure is based on reversible cross-linking by electrostatic interaction (between protonized-NH3 + and an anion such as tripolyphosphate) instead of chemical cross-linking. It avoids the potential toxicity of reagents and the possibility of damaging the drugs, especially with biological agents [59].
Components Conjugated Chitosan Nanocarrier to Cancer-Targeting Drug Delivery
Effective tumor-targeted drug delivery systems require four key requirements: retain, evade, target, and release [66]. Chitosan is a linear polyamine containing a number of free amine and two hydroxyl groups (primary or secondary functional groups) in its structure, which are readily available for cross-linking and its cationic nature allows for ionic cross-linking with multivalent anions. It also has the ability to control the release of active agents and avoid the use of hazardous organic solvents while fabricating particles, since it is soluble in aqueous acidic solution [67]. The functionalization is carried out on the primary amine group or on the hydroxyl group. As mentioned previously, chitosan is only soluble in acidic solutions of pH below 6.5, required to insure the protonation of the primary amine. In such cases, the presence of positive charges on the chitosan skeleton increases the repulsion between the different polymer chains and facilitating their solubilization [57]. Several targeting components were directly conjugated to chitosan nanoparticles as targeting moieties, and some of them are mentioned below.
Glycol Chitosan Nanoparticles
Glycol chitosan (GC) is a chitosan derivative with ethylene glycol groups on its backbone, and its water solubility is highly enhanced by these glycol groups [33]. When hydrophobic molecules, such as 5β-cholanic acid or protophorphyrin IX, were conjugated to a GC polymer, the resulting amphiphilic conjugates formed self-assembled hydrophobic GC nanoparticles (HGCs) with hydrophilic GC shells and hydrophobic cores under aqueous conditions. These HGCs harbored various anticancer drugs in their hydrophobic inner cores and showed prolonged circulation in the blood and specific delivery of drugs to tumors for cancer therapy [33].
Chitosan-g-poly(N-isopropylacrylamide) Nanocarriers
Derivatives of chitosan which have undergone hydrophobic modification can be used to form nanoparticles via self-assembly. Derivatives of chitosan, for example, can be formed by processes involving polymerizing N-isopropylacrylamide monomers initiated by ammonium nitrate in the presence of chitosan or 6-O-cholesterol-modified chitosan [51].
Several research groups reported the preparation of pH- and temperature-sensitive polymers based on poly (N-isopropylacrylamide) for biomedical applications [68]. Poly(N-isopropylacrylamide) (PNIPAAm) homopolymer and its copolymer are examples of thermosensitive polymers [69].
Rejinold et al. described the formulation of a highly stable thermo-responsive nano constructs of curcumin using chitosan-g-PNIPAAm with extensive physic-chemical characterization, cytotoxicity, and cell uptake studies. In addition, the effect of this formulation on cell viability and apoptosis of both cancer cells and normal cells were investigated [7].
Chitosan-Bound Fe3O4 Nanoparticles
Magnetic nanoparticles, which can be targeted to tumor site in a magnetic field, gained importance in cancer therapy in recent years. Magnetic nanoparticles include metallic, bimetallic, and superparamagnetic iron oxide nanoparticles, which have reactive surface that can be coated with biocompatible polymers and loaded with therapeutic agents [70].
Magnetite Fe3O4 was chosen as a magnetic nucleus because of its high initial magnetic susceptibility and magnetic saturation. Chitosan was selected as the biocompatible and non-toxic polymeric shell on the magnetic nanoparticles, responsible for targeted delivery of ftorafur, doxorubicin, bortezomib, epirubicin, and other anticancer agents [71, 72].
O-Carboxymethyl Chitosan Nanoparticles
Carboxymethyl chitosan derivatives are widely used for biomedical applications because of their non-toxic and biodegradable properties [63]. O-Carboxymethyl chitosan (CMCS) is a water-soluble chitosan derivative, and the carboxyl group on CMCS makes it to obtain high capacity to bind Ca2+. This property could deprive the divalent ions from extracellular matrix and increase the paracellular permeability of the epithelium. On the other hand, the pKa of CMCS is 2.0–4.0. Negatively charged CMCS is able to form polyelectrolyte complex with positively charged CS via electrostatic interaction and maintain the stability of nanostructures in gastro-intestinal (GI) tract, which may provide a great potential to overcome the limitation of CS and expand the drugs absorption site beyond duodenum [69]. O-CMC can be prepared by reacting chitosan with monochloroacetic acid in isopropyl alcohol as a solvent at room temperature [63].
Efficacy Mechanisms of Chitosan Nanocarriers in Targeted Tumor Therapeutics
The concept of stimuli-responsive drug delivery was first suggested in the late 1970s with the use of thermosensitive liposomes for the local release of drugs through hyperthermia [74]. Stimuli-responsive polymers are polymers which respond to external or internal changes in chemical and physical conditions in targeted tissues or cells [69].
The use of stimuli-responsive nanocarriers offers an interesting opportunity for drug delivery as programmable delivery systems in the optimization of cancer therapy. In the stimuli-responsive delivery system, the anticancer agent can be released by an appropriate stimulus (for example, pH, glucose, light, and temperature). Stimuli-responsive nanocarriers are constructed of the right material composition to engineer nanocarriers that can respond specifically to the pathological “triggers” that occur in the selected targeted site, as the disease establishes and progresses [3].
Ligand–Receptor Chitosan Nanocarriers
Nanoparticles can enter tumor sites via either passive or active targeting approaches. One of the effective targeting delivery approaches is to utilize some ligand–receptor interactions [75]. Receptor-targeted nanocarrier delivery mechanism has been shown to improve therapeutic responses both in vitro and in vivo [76]. A variety of ligands have been investigated including folate, transferrin, antibodies, peptides, and aptamers [76]. For example, Folic acid is a ligand that is useful for targeting cell membrane and enhancing nanoparticle endocytosis via the folate receptor. It is a stable, inexpensive, and generally poorly immunogenic chemical with a high affinity for the folate receptor [77, 78]. Because the folate receptor overexpresses on many human epithelial cancer cell surfaces, including cancers of the ovary, kidney, uterus, colon, and lung, conjugation of drugs and macromolecules with folic acid can enhance their uptake and targeting ability. Folic acid conjugates, which are covalently derivative via folate’s Ɣ-carboxyl moiety, can maintain a high affinity to the folate receptor; the mechanism of cellular uptake of folic acid conjugates by folate receptors is as effective as that of folic acid chemical. They can enter cells by folate receptor-mediated endocytosis and move through many organelles by vesicular trafficking, which can supply materials to release into cell cytoplasm. Later, the unligated folate receptor may recycle to the cell surface to transport more folic acid conjugates [79].
In the case of hepatocyte-targeted delivery systems, some polymers attached with galactosylated or lactosylated conjugates have been used to prepare nanoparticles for active targeting delivery of anticancer drugs. This is due to the fact that asialoglyco-protein receptors on hepatoma cells can specifically bind with certain types of ligands containing specific terminals such as β-d-galactose and N-acetylgalactosamine residues [75].
Somatostatin (SST) is a neuropeptide that demonstrates a powerful inhibitory effect against several endocrine systems. SST was originally isolated as an endocrine inhibitor of pituitary growth hormone secretion and is now recognized as a hormone capable of regulating fundamental processes, such as secretion, cell division, proliferation, and apoptosis [80, 81].
Somatostatin receptors (SSTRs) are members of the superfamily of G-protein-coupled receptors (GPCR), which are widely distributed in a variety of tumors and cancer cell lines, including small cell lung cancer, neuroendocrine tumors, prostate cancer, breast cancer, colorectal carcinoma, gastric cancer, and hepatocellular carcinoma [76]. Table 2 shows several investigated chitosan derivatives nanoparticles-conjugated ligands to achieve their specific receptors.
pH-Sensitive Tumor Chitosan Nanocarriers
The existing pH of tumor tissue has been considered as an ideal trigger for the selective release of anticancer drugs in tumor tissues and/or within tumor cells [3]. pH-Sensitive drug delivery system, which will stabilize anticancer drugs at physiological pH and unload the drugs through sensing pH descend in the interstitial space of solid tumors (pH 6.8–7.2) and intracellular endosomal compartments such as endosomes (pH 5–6) and lysosomes (pH 4.5–5.5), is believed to be advantageous for tumor targeting and endosomal escape [23]. This pH difference is caused by hypoxia that up-regulates glycolysis, under aerobic and anaerobic conditions followed by the production of lactate and protons (H+) in extracellular microenvironments [84, 85].
These variations of pH within cells and in a tumor can be strategies for pH-sensitive drug delivery at local microenvironments. This behavior is not only due to decrease the side cytotoxicity but also to promote the efficacy of chemotherapy. Chitosan has been widely used to prepare pH-sensitive nanogels due to its many desirable properties [3]. The amino groups in chitosan are protonated at a certain pH range, and so chitosan can be responsive to external pH stimulation. As the pKa of chitosan is 6.3, its pH response is near or slightly acidic. It has been reported that the microenvironment inside solid tumors has an acidic pH of 5–6.8 at which tumors have a lower extracellular pH than normal tissues [86]. Figure 4 shows the pH-sensitive chitosan-conjugated nanocarrier interactions.
A copolymer of chitosan and N-isopropylacrylamide carrier exhibit pH-sensitive responses to tumor pH in which, the release rates were enhanced below pH 6.8, with paclitaxel-loaded nanoparticles showing anticancer activity in tumor-bearing mice. Chitosan that was chemically modified to glycol chitosan (GCS) by a facile synthetic method is used to formulate a nanogel system composed of GCS grafted with multiple 3-diethylaminopropyl groups. The DOX-loaded nanogels showed an enhanced DOX release at tumor extracellular (pH 6.8), due to the protonation of 3-diethylaminopropyl group. Wu et al. [87] developed a class of chitosan-based hybrid nanogels by in situ immobilization of CdSe quantum dots in the chitosan-poly (methacrylic acid) (chitosan PMAA) semi-IPN networks. Both chitosan and PMAA chains are pH-sensitive, while the CdSe QDs are designed as an optical identification code for biosensing and cellular imaging. In the typical abnormal pH range of 5–7.4, this system exhibits a significant change in the physicochemical environment of the embedded QDs for converting chemical/biochemical signals to optical signals and regulates the release of anticancer drug temozolomide (TMZ) trapped in the nanogel. Furthermore, pH-responsive nanogels composed of glycol chitosan (GCS) grafted with functional 3-diethylaminopropyl (DEAP) groups were fabricated. This system was destabilized due to the protonation of DEAP. At physiological pH, the nanogel exhibited self-assembly, and when the pH decreased to tumor extracellular pH (pH 6.8), the nanogel was destabilized due to the protonation of DEAP and accelerated DOX release from nanogels [29]. Chitosan-grafted PNIPAAm can offer the improvement of biodegradability and potential of pH-responsive hydrogel [88].
Thermo-Responsive Tumor Chitosan Nanocarriers
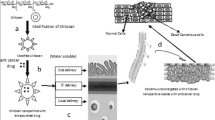
Temperature-sensitive nanocarriers can be applied in many pathological areas which in turn demonstrate distinct hyperthermia. In addition, there exist various means to heat the required area in the body [43]. In a thermo-responsive polymer, the change in conformation can be observed as a change in solubility caused by alterations in the way the polymer chain interacts with solvent. In aqueous systems, this refers to a change in the wettability of the polymer chains, brought about by alteration of the hydrogen bonding. Uncharged polymer chains are soluble in water due to the stable formation of hydrogen bonds with water molecules. The efficiency of intramolecular H-bonding decreases with increasing temperature, resulting in phase separation above the lower critical solution temperature (LCST) or cloud-point [5] (Fig. 5). Because of the precipitation of the polymer, when the temperature is above the LCST (in the tumor) the nanocarrier structure is damaged and the drug is released [89]. Ideally, thermosensitive nanocarriers should retain their load at body temperature (~37 °C) and rapidly deliver the drug within a locally heated tumor (~40–42 °C) to counteract rapid blood-passage time and washout from the tumor [74].
Poly (N-isopropylacrylamide) (PNIPAAm) homopolymer and its copolymers are the best examples of thermosensitive polymers. These polymers exhibit a LCST in aqueous solution and experience a reversible change in volume and shape in response to the changes in temperature in the vicinity of the LCST [69]. The LCST of the PNIPAAm copolymer can be manipulated to be above the normal human body temperature (37 °C) by incorporating co-monomer units, such as N,N-dimethylacrylamide [90, 91]. Chitosan-g-poly (N-isopropyl acrylamide) (PNIPAM) with extensive physicochemical characterization, cytotoxicity, and cell uptake is one of highly stable thermo-responsive nano constructs of cancer drug studies. In addition, the effect of this formulation on cell viability and apoptosis of both cancer cells and normal cells were investigated [7]. Poly (N-vinylcaprolactam) (PNVCL) is another well-studied polymer which shows a well-defined response toward temperature as compared to PNIPAAm [92].
Magneto-Responsive Chitosan Nanocarriers
Superparamagnetic nanoparticles exhibiting higher magnetization and good biocompatibility are of particular interest, as magnetic drug-targeting carriers for hyperthermia can be expected to facilitate intravenous delivery of drug to the desired site (e.g., tumor site) using an external magnetic field [69]. Treatment would involve controlled guidance of drug-laden, magneto-responsive material using a large external magnetic field to enrich these particles at a cancerous area (Fig. 6a).Changes in external magnetic flux can then act to deform the carriers to release the drug (Fig. 6b) [5]. A number of superparamagnetic iron oxide nanoparticle (SPION) systems have been coated with targeting ligands (active targeting). Under the influence of external magnets, it is possible to guide nanoparticles to a particular targeted site. The increasing local temperature obtained by using SPION in an alternating magnetic field allowed the elimination of the tumor. This principle is called the “magnetic thermal ablation” [93]. Gradient magnetic field (AC) targeting can accelerate ligand uptake into tumor cells and facilitate temperature-sensitive nanocarriers to drug release. Possibly, AC magnetic field would also enhance receptor-mediated uptake into tumor cells and thus enhance the final step, tumor cell killing [94]. Drug-loaded magnetic particulates also represent a promising alternative strategy in overcoming the blood brain barrier (BBB) potential for the treatment of several neurological disorders such as Alzheimer’s disease and brain tumor [95]. Developed magnetic chitosan microspheres contain oxantrazole (MCM-OX), an anticancer drug, for the treatment of brain tumors.
In most cases, Chitosan has been selected as a polymeric shell on the magnetic nanoparticles, responsible for the temperature, pH, and magnetic changes during targeted drug delivery [11, 71]. Klostergaard and Seeney applied SPIONs which were modified with o-carboxymethyl chitosan (OCMCS) and folic acid (FA) to attempt to improve their biocompatibility and ability to target specific tumor cells, simultaneously evading the RES [96].
Photo-Sensitive Chitosan Nanocarriers
Photodynamic therapy (PDT) is becoming widely known for its application in cancer therapy using chemical photo-sensitizer and light irradiation at certain wavelength onto target tumor tissues [97]. The light irradiation causes the photo-sensitizer to generate species of cytotoxic singlet oxygen (1O2) through the photochemical reactions between photo-sensitizer and the surrounding molecular oxygen that destroys tumor cells through apoptosis or necrosis [97, 98]. Because light of UV and visible wavelengths is readily absorbed by the skin, these systems may present some limitations. For this reason, other polymers sensitive to infrared or near-infrared lights are studied [99, 100]. The drug delivery and the release from nanocarriers may also be triggered by external ultrasound. Ultrasonic waves can be used to induce either thermal or mechanical effects. Local heating can be induced using high-intensity focused ultrasound (HIFU), inducing phase transition of the polymers, which involves the drug release from nanocarriers [89].
Chlorin e6 (Ce6) is known as a photo-sensitizer because of its hydrophobicity, activity near-infrared wavelengths range, tendency which in turn, acts in deep tissue layers, and high singlet oxygen generation efficiency [101]. Lee et al. applied Ce6-loaded GC nanoparticles (HGC-Ce6) and Ce6-conjugated chitosan nanoparticles (GC-Ce6), and compared the in vitro and in vivo characteristics of these two nanoparticles for PDT in cancer therapy [33].
Protoporphyrin IX (PpIX) is another photo-sensitizer that its accumulation in tumor tissues provides an intense fluorescence signal that also can be employed in photodynamic imaging (PDI) [102]. Lee et al. designed the PpIX-conjugated GC nanoparticles (PpIXeGCe NPs) based on cellular on/off system for synchronous photodynamic imaging (PDI) and PDT in cancer treatment [97].
Pheophorbide a (PheoA), a second generation PDT agent with a high singlet oxygen quantum yield and a high extinction coefficient in the near-infrared region, was conjugated with GC via bioreducible disulfide linkages to prepare new self-quenchable PheoA-ss-GC nanoparticles (PheoA-ss-CNPs), which demonstrated both switchable photo activity and triggered release of photo-sensitizer for more effective PDT [98]. Table 3 lists the available chitosan-based nanocarriers that are sensitive to the physical stimulus including pH, temperature, magneto, and photo.
Future Challenges and Opportunities
Understanding the physicochemical, molecular, and physiological processes of nanoparticles is imperative for nanomedicine to become a reliable and sustainable treatment modality. Further studies are needed to determine the biodistribution of nanoparticles after skin and GI tract exposure. Many pre-clinical studies have demonstrated reduced toxicity profile while incorporated as immune suppressants (i.e., rapamycin and cyclosporine) as well as a variety of anticancer drugs (i.e., paclitaxel and geldanamycin) into nanocarrier systems in rodent studies. In spite of the scientific knowledge gained in recent years in nanotoxicology, scientists are still not able to precisely anticipate the behavior and biokinetics of nanoparticles [107].
A drawback of nanoparticles for drug delivery applications is that they are most likely to be rapidly cleared by macrophages or the RES before they arrive at the desired site [108]. In addition to this, surface-modified superparamagnetic nanoparticles should have water-soluble and specific functionalized groups on their surface to facilitate their conjugation with the drug. Thus, in drug delivery applications, the nanoparticle used in drug carrier should be characterized by a functionalized magnetic core and a biodegradable polymer shell. By using this approach, the possible side effects of an anticancer drug can be minimized. It can be explained by the fact that the drug will be covered by the polymer shell during the delivery process [69].
Nanoparticles under 150 nm can avoid RES in liver and spleen and thus maintain a prolonged circulation time in the body. However, larger size (150–300 nm) NPs, especially composed with rigid components such as quantum dots and iron oxide NPs, are rapidly captured and filtered by RES and kidney. On the contrary, micro-sized red blood cells can freely pass through biological filtration and circulate due to their deformability and flexibility [109, 110].
Deformable and long-circulating NPs may be found useful in the fields of molecular imaging and drug delivery. Therefore, deformability and flexibility of NPs can be considered as another key factor that affects biodistribution and tumor-targeting efficiency [17].
Nanoscale stimuli-responsive devices may be sensitive to specific endogenous stimuli, such as a lowered interstitial pH, a higher glutathione concentration, or an increased level of certain enzymes such as matrix metalloproteinases. At the cellular level, pH sensitivity can either trigger the release of the transported drug into late endosomes or lysosomes, or promote the escape of the nanocarriers from the lysosomes to the cell cytoplasm [74]. The potential application of chitosan is hindered by its limited solubility in aqueous media. Thus, chitosan is chemically modified to improve the polymer processability, solubility, antimicrobial activity, and the ability to interact with other substances. Introducing a carboxymethyl group is the most advantageous method of increasing the solubility of chitosan at neutral and alkaline pH without affecting other important characteristics [63]. The choice of an appropriate nanocarrier is not obvious, because several factors may simultaneously affect biodistribution and targeting. Therefore, successful targeting strategies must be determined experimentally on a case-by-case basis, which is laborious. Systemic therapies using nanocarriers require methods that can overcome non-specific uptake by mononuclear phagocytic cells and by non-targeted cells. Improved production and therapeutic efficacy of targeted nanocarriers has been established in multiple animal models of cancer [111, 112].
Conclusion
Current chemotherapy agents are often associated with some challenges such as nonselective distribution, cytotoxicity, short circulation half-life, and unwanted side effects to normal tissues. To overcome these drawbacks, nano-sized carriers have been investigated to improve their permeability, retention effect, and delivery properties via passive and active mechanisms. Chitosan is a natural polysaccharide with a primary amine and carboxyl groups that make it very efficient material in antitumor therapeutics applications. The functionalization of chitosan-based nanocarriers is carried out on the primary amine group or on the hydroxyl group. Chitosan nanocarriers bearing biorecognition molecules, known as ligands, are recognized by cancerous cell surface receptors aiming to increase specific cell uptake. Finally, after reviewing the literature, it can be concluded that the use of stimuli-responsive chitosan nanocarriers offers an opportunity for targeted drug delivery in the optimization of cancer therapy.
References
Sarkar, F. H., Banerjee, S., & Li, Y. (2007). Pancreatic cancer: Pathogenesis, prevention and treatment. Toxicology and Applied Pharmacology, 224, 326–336.
Byrne, J. D., Betancourt, T., & Brannon-Peppas, L. (2008). Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced Drug Delivery Reviews, 60, 1615–1626.
Manchun, S., Dass, C. R., & Sriamornsak, P. (2012). Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sciences, 90, 381–387.
Patel, N. R., Pattni, B. S., Abouzeid, A. H., & Torchilin, V. P. (2013). Nanopreparations to overcome multidrug resistance in cancer. Advanced Drug Delivery Reviews, 65, 1748–1762.
Chan, A., Orme, R. P., Fricker, R. A., & Roach, P. (2013). Remote and local control of stimuli responsive materials for therapeutic applications. Advanced Drug Delivery Reviews, 65, 497–514.
Pulkkinen, M., Pikkarainen, J., Wirth, T., Tarvainen, T., Haapa-aho, V., Korhonen, H., et al. (2008). Three-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin–biotin technology: Formulation development and in vitro anticancer activity. European Journal of Pharmaceutics and Biopharmaceutics, 70, 66–74.
Sanoj Rejinold, N., Sreerekha, P. R., Chennazhi, K. P., Nair, S. V., & Jayakumar, R. (2011). Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. International Journal of Biological Macromolecules, 49, 161–172.
Lakshmanan, V.-K., Snima, K. S., Bumgardner, J., Nair, S., & Jayakumar, R. (2011). Chitosan-based nanoparticles in cancer therapy. In R. Jayakumar, M. Prabaharan, & R. A. A. Muzzarelli (Eds.), Chitosan for biomaterials, vol. 243: Advances in polymer science (pp. 55–91). Berlin: Springer.
Liu, Z., Jiao, Y., Wang, Y., Zhou, C., & Zhang, Z. (2008). Polysaccharides-based nanoparticles as drug delivery systems. Advanced Drug Delivery Reviews, 60, 1650–1662.
Torchilin, V. P. (2004). Targeted polymeric micelles for delivery of poorly soluble drugs. CMLS. Cellular and Molecular Life Sciences, 61, 2549–2559.
Park, J. H., Saravanakumar, G., Kim, K., & Kwon, I. C. (2010). Targeted delivery of low molecular drugs using chitosan and its derivatives. Advanced Drug Delivery Reviews, 62, 28–41.
Thanki, K., Gangwal, R. P., Sangamwar, A. T., & Jain, S. (2013). Oral delivery of anticancer drugs: Challenges and opportunities. Journal of Controlled Release, 170, 15–40.
Wang, M. D., Shin, D. M., Simons, J. W., & Nie, S. (2007). Nanotechnology for targeted cancer therapy. Expert Review of Anticancer Therapy 7, 833–837.
Jabr-Milane, L. S., van Vlerken, L. E., Yadav, S., & Amiji, M. M. (2008). Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treatment Reviews, 34, 592–602.
Venkatesan, P., Puvvada, N., Dash, R., Prashanth Kumar, B. N., Sarkar, D., Azab, B., et al. (2011). The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials, 32, 3794–3806.
He, M., Zhao, Z., Yin, L., Tang, C., & Yin, C. (2009). Hyaluronic acid coated poly(butyl cyanoacrylate) nanoparticles as anticancer drug carriers. International Journal of Pharmaceutics, 373, 165–173.
Jee, J.-P., Na, J. H., Lee, S., Kim, S. H., Choi, K., Yeo, Y., et al. (2012). Cancer targeting strategies in nanomedicine: Design and application of chitosan nanoparticles. Current Opinion in Solid State and Materials Science, 16, 333–342.
Bates, D. O., Hillman, N. J., Williams, B., Neal, C. R., & Pocock, T. M. (2002). Regulation of microvascular permeability by vascular endothelial growth factors*. Journal of Anatomy, 200, 581–597.
Bertrand, N., Wu, J., Xu, X., Kamaly, N., & Farokhzad, O. C. (2014). Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Advanced Drug Delivery Reviews, 66, 2–25.
Brannon-Peppas, L., & Blanchette, J. O. (2004). Nanoparticle and targeted systems for cancer therapy. Advanced Drug Delivery Reviews, 56, 1649–1659.
Torchilin, V. (2011). Tumor delivery of macromolecular drugs based on the EPR effect. Advanced Drug Delivery Reviews, 63, 131–135.
Koo, H., Min, K. H., Lee, S. C., Park, J. H., Park, K., Jeong, S. Y., et al. (2013). Enhanced drug-loading and therapeutic efficacy of hydrotropic oligomer-conjugated glycol chitosan nanoparticles for tumor-targeted paclitaxel delivery. Journal of Controlled Release, 172, 823–831.
Yin, Q., Shen, J., Zhang, Z., Yu, H., & Li, Y. (2013). Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Advanced Drug Delivery Reviews, 65, 1699–1715.
Livney, Y. D., & Assaraf, Y. G. (2013). Rationally designed nanovehicles to overcome cancer chemoresistance. Advanced Drug Delivery Reviews, 65, 1716–1730.
Jin, Y.-H., Hu, H.-Y., Qiao, M.-X., Zhu, J., Qi, J.-W., Hu, C.-J., et al. (2012). pH-Sensitive chitosan-derived nanoparticles as doxorubicin carriers for effective anti-tumor activity: preparation and in vitro evaluation. Colloids and Surfaces B, 94, 184–191.
Mohamed, S., Zeino, M., Kadioglu, O., Volm, M., & Efferth, T. (2014). Overcoming of P-glycoprotein-mediated multidrug resistance of tumors in vivo by drug combinations. Synergy, 1, 44–58.
Shapira, A., Livney, Y. D., Broxterman, H. J., & Assaraf, Y. G. (2011). Nanomedicine for targeted cancer therapy: Towards the overcoming of drug resistance. Drug Resistance Updates, 14, 150–163.
Garbuzenko, O. B., Saad, M., Pozharov, V. P., Reuhl, K. R., Mainelis, G., & Minko, T. (2010). Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. In Proceedings of the National Academy of Sciences.
Oh, N. M., Oh, K. T., Baik, H. J., Lee, B. R., Lee, A. H., Youn, Y. S., et al. (2010). A self-organized 3-diethylaminopropyl-bearing glycol chitosan nanogel for tumor acidic pH targeting: In vitro evaluation. Colloids and Surfaces B, 78, 120–126.
Saraswathy, M., & Gong, S. (2013). Different strategies to overcome multidrug resistance in cancer. Biotechnology Advances, 13, 1397–1407.
Palakurthi, S., Yellepeddi, V. K., & Vangara, K. K. (2012). Recent trends in cancer drug resistance rever-sal strategies using nanoparticles. Expert opinion on drug delivery, 9, 287–301.
Choi, K. Y., Chung, H., Min, K. H., Yoon, H. Y., Kim, K., Park, J. H., et al. (2010). Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials, 31, 106–114.
Lee, S. J., Koo, H., Jeong, H., Huh, M. S., Choi, Y., Jeong, S. Y., et al. (2011). Comparative study of photosensitizer loaded and conjugated glycol chitosan nanoparticles for cancer therapy. Journal of Controlled Release, 152, 21–29.
Fahr, A., & Liu, X. (2007). Drug delivery strategies for poorly water-soluble drugs. Expert opinion on drug delivery, 4, 403–416.
Huo, M., Zhang, Y., Zhou, J., Zou, A., Yu, D., Wu, Y., et al. (2010). Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. International Journal of Pharmaceutics, 394, 162–173.
Fan, L., Li, F., Zhang, H., Wang, Y., Cheng, C., Li, X., et al. (2010). Co-delivery of PDTC and doxorubicin by multifunctional micellar nanoparticles to achieve active targeted drug delivery and overcome multidrug resistance. Biomaterials, 31, 5634–5642.
Min, K. H., Park, K., Kim, Y.-S., Bae, S. M., Lee, S., Jo, H. G., et al. (2008). Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. Journal of Controlled Release, 127, 208–218.
Gao, J., Ming, J., He, B., Fan, Y., Gu, Z., & Zhang, X. (2008). Preparation and characterization of novel polymeric micelles for 9-nitro-20(S)-camptothecin delivery. European Journal of Pharmaceutical Sciences, 34, 85–93.
Ye, Y.-Q., Chen, F.-Y., Wu, Q.-A., Hu, F.-Q., Du, Y.-Z., Yuan, H., et al. (2009). Enhanced cytotoxicity of core modified chitosan based polymeric micelles for doxorubicin delivery. Journal of Pharmaceutical Sciences, 98, 704–712.
Torchilin, V. P. (2000). Drug targeting. European Journal of Pharmaceutical Sciences, 11(Supplement 2), S81–S91.
Ranganathan, R., Madanmohan, S., Kesavan, A., Baskar, G., Krishnamoorthy, Y. R., Santosham, R., et al. (2012). Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. International Journal of Nanomedicine, 7, e1060.
Altintas, I., Kok, R. J., & Schiffelers, R. M. (2012). Targeting epidermal growth factor receptor in tumors: From conventional monoclonal antibodies via heavy chain-only antibodies to nanobodies. European Journal of Pharmaceutical Sciences, 45, 399–407.
Torchilin, V. (2009). Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. European Journal of Pharmaceutics and Biopharmaceutics, 71, 431–444.
Sun, Q., Radosz, M., & Shen, Y. (2012). Challenges in design of translational nanocarriers. Journal of Controlled Release, 164, 156–169.
Florence, A. T. (2007). Pharmaceutical nanotechnology: More than size: Ten topics for research. International Journal of Pharmaceutics, 339, 1–2.
Kong, M., Park, H., Cheng, X., & Chen, X. (2013). Spatial–temporal event adaptive characteristics of nanocarrier drug delivery in cancer therapy. Journal of Controlled Release, 172, 281–291.
Kwon, I. K., Lee, S. C., Han, B., & Park, K. (2012). Analysis on the current status of targeted drug delivery to tumors. Journal of Controlled Release, 164, 108–114.
Yameen, B., Choi, W., Vilos, C., Swami, A., Shi, J., & Farokhzad, O. (2014). Insight into nanoparticle cellular uptake and intracellular targeting. Controlled Release, 190, 485–499.
Bae, Y. H., & Park, K. (2011). Targeted drug delivery to tumors: Myths, reality and possibility. Journal of Controlled Release, 153, 198–205.
Maeda, H., Bharate, G. Y., & Daruwalla, J. (2009). Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European Journal of Pharmaceutics and Biopharmaceutics, 71, 409–419.
Hudson, D., & Margaritis, A. (2013). Biopolymer nanoparticle production for controlled release of biopharmaceuticals. Critical Reviews in Biotechnology, 0, 1–19.
Kumari, A., Yadav, S. K., & Yadav, S. C. (2010). Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B, 75, 1–18.
Duceppe, N., & Tabrizian, M. (2010). Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Expert opinion on drug delivery, 7, 1191–1207.
Rai, P., Mallidi, S., Zheng, X., Rahmanzadeh, R., Mir, Y., Elrington, S., et al. (2010). Development and applications of photo-triggered theranostic agents. Advanced Drug Delivery Reviews, 62, 1094–1124.
Gulbake, A., & Jain, S. K. (2012). Chitosan: A potential polymer for colon-specific drug delivery system. Expert opinion on drug delivery, 9, 713–729.
Ashkenazi, A. (2002). Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nature Reviews Cancer, 2, 420–430.
Riva, R., Ragelle, H., Rieux, A., Duhem, N., Jérôme, C., & Préat, V. (2011). Chitosan and chitosan derivatives in drug delivery and tissue engineering. In R. Jayakumar, M. Prabaharan, & R. A. A. Muzzarelli (Eds.), Chitosan for biomaterials II, vol. 244: Advances in polymer science (pp. 19–44). Berlin: Springer.
Liu, J., Li, H., Jiang, X., Zhang, C., & Ping, Q. (2010). Novel pH-sensitive chitosan-derived micelles loaded with paclitaxel. Carbohydrate Polymers, 82, 432–439.
Nogueira, D. R., Tavano, L., Mitjans, M., Pérez, L., Infante, M. R., & Vinardell, M. P. (2013). In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials, 34, 2758–2772.
Derakhshandeh, K., & Fathi, S. (2012). Role of chitosan nanoparticles in the oral absorption of Gemcitabine. International Journal of Pharmaceutics, 437, 172–177.
Garg, N. K., Dwivedi, P., Campbell, C., & Tyagi, R. K. (2012). Site specific/targeted delivery of gemcitabine through anisamide anchored chitosan/poly ethylene glycol nanoparticles: An improved understanding of lung cancer therapeutic intervention. European Journal of Pharmaceutical Sciences, 47, 1006–1014.
Arya, G., Vandana, M., Acharya, S., & Sahoo, S. K. (2011). Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine, 7, 859–870.
Anitha, A., Maya, S., Deepa, N., Chennazhi, K. P., Nair, S. V., Tamura, H., et al. (2011). Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydrate Polymers, 83, 452–461.
Zhang, C., Qu, G., Sun, Y., Yang, T., Yao, Z., Shen, W., et al. (2008). Biological evaluation of N-octyl-O-sulfate chitosan as a new nano-carrier of intravenous drugs. European Journal of Pharmaceutical Sciences, 33, 415–423.
Zhang, C., Qineng, P., & Zhang, H. (2004). Self-assembly and characterization of paclitaxel-loaded N-octyl-O-sulfate chitosan micellar system. Colloids and Surfaces B, 39, 69–75.
Deckert, P. (2009). Current constructs and targets in clinical development for antibody-based cancer therapy. Current Drug Targets, 10, 158–175.
Malmiri, H. J., Jahanian, M. A. G., & Berenjian, A. (2012). Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. American Journal of Biochemistry & Biotechnology, 8, 203–219.
Folkes, L. K., & Wardman, P. (2003). Enhancing the efficacy of photodynamic cancer therapy by radicals from plant auxin (indole-3-acetic acid). Cancer Research, 63, 776–779.
Yuan, Q., Venkatasubramanian, R., Hein, S., & Misra, R. D. K. (2008). A stimulus-responsive magnetic nanoparticle drug carrier: Magnetite encapsulated by chitosan-grafted-copolymer. Acta Biomaterialia, 4, 1024–1037.
Razmi, M., Divsalar, A., Saboury, A. A., Izadi, Z., Haertlé, T., & Mansuri-Torshizi, H. (2013). Beta-casein and its complexes with chitosan as nanovehicles for delivery of a platinum anticancer drug. Colloids and Surfaces B, 112, 362–367.
Arias, J. L., López-Viota, M., Sáez-Fernández, E., Ruiz, M. A., & Delgado, Á. V. (2011). Engineering of an antitumor (core/shell) magnetic nanoformulation based on the chemotherapy agent ftorafur. Colloids and Surfaces A, 384, 157–163.
Unsoy, G., Yalcin, S., Khodadust, R., Mutlu, P., Onguru, O., & Gunduz, U. (2014). Chitosan magnetic nanoparticles for pH responsive Bortezomib release in cancer therapy. Biomedicine & Pharmacotherapy. doi:10.1016/j.biopha.2014.04.003.
Feng, C., Wang, Z., Jiang, C., Kong, M., Zhou, X., Li, Y., et al. (2013). Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. International Journal of Pharmaceutics, 457, 158–167.
Mura, S., Nicolas, J., & Couvreur, P. (2013). Stimuli-responsive nanocarriers for drug delivery. Nature Materials, 12, 991–1003.
Zhou, N., Zan, X., Wang, Z., Wu, H., Yin, D., Liao, C., et al. (2013). Galactosylated chitosan–polycaprolactone nanoparticles for hepatocyte-targeted delivery of curcumin. Carbohydrate Polymers, 94, 420–429.
Huo, M., Zou, A., Yao, C., Zhang, Y., Zhou, J., Wang, J., et al. (2012). Somatostatin receptor-mediated tumor-targeting drug delivery using octreotide-PEG-deoxycholic acid conjugate-modified N-deoxycholic acid-O, N-hydroxyethylation chitosan micelles. Biomaterials, 33, 6393–6407.
Park, E. K., Lee, S. B., & Lee, Y. M. (2005). Preparation and characterization of methoxy poly(ethylene glycol)/poly(ε-caprolactone) amphiphilic block copolymeric nanospheres for tumor-specific folate-mediated targeting of anticancer drugs. Biomaterials, 26, 1053–1061.
Chan, P., Kurisawa, M., Chung, J. E., & Yang, Y.-Y. (2007). Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials, 28, 540–549.
Yang, S.-J., Lin, F.-H., Tsai, K.-C., Wei, M.-F., Tsai, H.-M., Wong, J.-M., et al. (2010). Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjugate Chemistry, 21, 679–689.
Guillermet-Guibert, J., Lahlou, H., Cordelier, P., Bousquet, C., Pyronnet, S., & Susini, C. (2005). Physiology of somatostatin receptors. Journal of Endocrinological Investigation, 28, 5.
Mariniello, B., Finco, I., Sartorato, P., Patalano, A., Iacobone, M., Guzzardo, V., et al. (2011). Somatostatin receptor expression in adrenocortical tumors and effect of a new somatostatin analog SOM230 on hormone secretion in vitro and in ex vivo adrenal cells. Journal of Endocrinological Investigation, 34, e131–e138.
Guan, M., Zhou, Y., Zhu, Q.-L., Liu, Y., Bei, Y.-Y., Zhang, X.-N., et al. (2012). N-trimethyl chitosan nanoparticle-encapsulated lactosyl-norcantharidin for liver cancer therapy with high targeting efficacy. Nanomedicine, 8, 1172–1181.
Yang, K., Kong, M., Wei, Y., Liu, Y., Cheng, X., Li, J., et al. (2013). Folate-modified–chitosan-coated liposomes for tumor-targeted drug delivery. Journal of Materials Science, 48, 1717–1728.
Fukumura, D., & Jain, R. K. (2007). Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvascular Research, 74, 72–84.
Pouyssegur, J., Dayan, F., & Mazure, N. M. (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature, 441, 437–443.
Deng, Z., Zhen, Z., Hu, X., Wu, S., Xu, Z., & Chu, P. K. (2011). Hollow chitosan–silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials, 32, 4976–4986.
Wu, W., Shen, J., Banerjee, P., & Zhou, S. (2010). Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging and controlled drug delivery. Biomaterials, 31, 8371–8381.
Sun, G., Zhang, X.-Z., & Chu, C.-C. (2007). Formulation and characterization of chitosan-based hydrogel films having both temperature and pH sensitivity. Journal of Materials Science. Materials in Medicine, 18, 1563–1577.
Danhier, F., Feron, O., & Préat, V. (2010). To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. Journal of Controlled Release, 148, 135–146.
Alarcon, C.d. l. H., Pennadam, S., & Alexander, C. (2005). Stimuli responsive polymers for biomedical applications. Chemical Society Reviews, 34, 276–285.
Twaites, B., de las Heras Alarcon, C., & Alexander, C. (2005). Synthetic polymers as drugs and therapeutics. Journal of Materials Chemistry, 15, 441–455.
Rejinold, N. S., Chennazhi, K. P., Nair, S. V., Tamura, H., & Jayakumar, R. (2011). Biodegradable and thermo-sensitive chitosan-g-poly(N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydrate Polymers, 83, 776–786.
Hilger, I., Hiergeist, R., Hergt, R., Winnefeld, K., Schubert, H., & Kaiser, W. A. (2002). Thermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility study. Investigative Radiology, 37, 580–586.
Pradhan, P., Giri, J., Rieken, F., Koch, C., Mykhaylyk, O., Döblinger, M., et al. (2010). Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. Journal of Controlled Release, 142, 108–121.
Hassan, E. E., & Gallo, J. M. (1993). Targeting anticancer drugs to the brain. I: Enhanced brain delivery of oxantrazole following administration in magnetic cationic microspheres. Journal of Drug Targeting, 1, 7–14.
Klostergaard, J., & Seeney, C. E. (2012). Magnetic nanovectors for drug delivery. Maturitas, 73, 33–44.
Lee, S. J., Koo, H., Lee, D.-E., Min, S., Lee, S., Chen, X., et al. (2011). Tumor-homing photosensitizer-conjugated glycol chitosan nanoparticles for synchronous photodynamic imaging and therapy based on cellular on/off system. Biomaterials, 32, 4021–4029.
Oh, I.-H., Min, H. S., Li, L., Tran, T. H., Lee, Y.-K., Kwon, I. C., et al. (2013). Cancer cell-specific photoactivity of pheophorbide a–glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials, 34, 6454–6463.
Goodwin, A. P., Mynar, J. L., Ma, Y., Fleming, G. R., & Fréchet, J. M. J. (2005). Synthetic micelle sensitive to IR light via a two-photon process. Journal of the American Chemical Society, 127, 9952–9953.
Jiang, J., Tong, X., Morris, D., & Zhao, Y. (2006). Toward photocontrolled release using light-dissociable block copolymer micelles. Macromolecules, 39, 4633–4640.
Chin, W. W. L., Heng, P. W. S., Thong, P. S. P., Bhuvaneswari, R., Hirt, W., Kuenzel, S., et al. (2008). Improved formulation of photosensitizer chlorin e6 polyvinylpyrrolidone for fluorescence diagnostic imaging and photodynamic therapy of human cancer. European Journal of Pharmaceutics and Biopharmaceutics, 69, 1083–1093.
Lovell, J. F., Chen, J., Jarvi, M. T., Cao, W.-G., Allen, A. D., Liu, Y., et al. (2009). FRET quenching of photosensitizer singlet oxygen generation. The Journal of Physical Chemistry B, 113, 3203–3211.
Zhang, D., Sun, P., Li, P., Xue, A., Zhang, X., Zhang, H., et al. (2013). A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette–Guérin in the treatment of bladder cancer. Biomaterials, 34, 10258–10266.
Chang, Y.-C., Shieh, D.-B., Chang, C.-H., & Chen, D.-H. (2005). Conjugation of monodisperse chitosan-bound magnetic nanocarrier with epirubicin for targeted cancer therapy. Journal of Biomedical Nanotechnology, 1, 196–201.
Rajan, M., Raj, V., Al-Arfaj, A. A., & Murugan, A. M. (2013). Hyaluronidase enzyme core-5-fluorouracil-loaded chitosan-PEG-gelatin polymer nanocomposites as targeted and controlled drug delivery vehicles. International Journal of Pharmaceutics, 453, 514–522.
Puga, A. M., Lima, A. C., Mano, J. F., Concheiro, A., & Alvarez-Lorenzo, C. (2013). Pectin-coated chitosan microgels crosslinked on superhydrophobic surfaces for 5-fluorouracil encapsulation. Carbohydrate Polymers, 98, 331–340.
Vega-Villa, K. R., Takemoto, J. K., Yáñez, J. A., Remsberg, C. M., Forrest, M. L., & Davies, N. M. (2008). Clinical toxicities of nanocarrier systems. Advanced Drug Delivery Reviews, 60, 929–938.
Gupta, A. K., & Gupta, M. (2005). Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials, 26, 3995–4021.
Jokerst, J. V., Lobovkina, T., Zare, R. N., & Gambhir, S. S. (2011). Nanoparticle PEGylation for imaging and therapy. Nanomedicine, 6, 715–728.
Mebius, R. E., & Kraal, G. (2005). Structure and function of the spleen. Nature Reviews Immunology, 5, 606–616.
Peer, D., Karp, J. M., Hong, S., Farokhzad, O. C., Margalit, R., & Langer, R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology, 2, 751–760.
Berenjian, A., Ghasemi, M. R., & Zarghi, A. (2011). Preparation of barium sulfate nanoparticles using semi-batch precipitation. Asian Journal of Chemistry, 23, 491–494.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ghaz-Jahanian, M.A., Abbaspour-Aghdam, F., Anarjan, N. et al. Application of Chitosan-Based Nanocarriers in Tumor-Targeted Drug Delivery. Mol Biotechnol 57, 201–218 (2015). https://doi.org/10.1007/s12033-014-9816-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9816-3