Abstract
Moscatilin, a bibenzyl derivative from the Dendrobium genus, has been traditionally used in Chinese medicine. Recent studies suggest its potential as a powerful anticancer agent due to its diverse pharmacological properties.This review aims to consolidate current research on moscatilin’s anticancer mechanisms, structure–activity relationships, and therapeutic potential to assess its viability for clinical use. A literature search was performed in PubMed/MedLine, Scopus, and Web of Science.The search focused on “cancer,” “moscatilin,” “anticancer,” “bioactivity,” “dendrobium,” and “pharmacological properties.” Relevant studies on molecular mechanisms, preclinical and clinical efficacy, and bioavailability were reviewed. Moscatilin exhibits significant anticancer effects in lung, breast, colorectal, and pancreatic cancers. It induces apoptosis via the JNK/SAPK pathway, inhibits cell proliferation, and suppresses metastasis. Structure–activity relationship studies reveal that phenolic groups and a two-carbon bridge are crucial for its efficacy. Additionally, moscatilin shows good bioavailability and a favorable safety profile, with low toxicity to healthy cells. Moscatilin demonstrates considerable potential as an anticancer agent, targeting multiple cancer progression pathways. Further clinical trials are essential to confirm its therapeutic efficacy and safety in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second leading cause of death worldwide, with an estimated 18.1 million new cases and 9.6 million deaths in 2018; according to GLOBOCAN, there will be a significant increase in both cancer incidence and mortality between 2020 and 2030, reaching 24.6 million new cases and 12.9 million deaths, respectively [1]. Until the year 2020, the most diagnosed cancers were breast cancer (2.3 million cases), lung cancer (2.2 million cases), and colorectal cancer (1.8 million cases) [1]. Some risk factors for cancer can include age, lifestyle, family history, environmental factors, and genetics [2]. This condition is characterized by the cancer cells’ ability to invade adjacent tissues, spread to distant regions of the body, and proliferate in an uncontrolled way. In this sense, it is important to continue exploring other potential adjuvant therapies for cancer [3, 4].
Moscatilin, a bibenzyl derivative common in the genus Dendrobium has been used in traditional Chinese medicine for decades due to its immunomodulatory, antioxidant and anti-aging potential [5]. Furthermore, it has been the subject of several investigations due to its potential use in cancer therapy [6]. In this regard, distinct authors have demonstrated that moscatilin has the potential to inhibit the growth of several cancers, including colon [7], breast [8], pancreatic [9], and lung cancer [10]. The antitumor potential of this compound has been validated through several mechanisms, including induction of apoptosis, inhibition of cell proliferation and tumor invasion and migration [10,11,12,13]. Besides, several moscatilin derivatives also exhibit antiproliferative activity in cancer cell lines, the ability to inhibit the cell cycle in the G2/M phase in HCT116 cells and anti-angiogenic effects in vivo zebrafish model [14]. These findings suggest that moscatilin has the potential to be used as an anticancer therapeutic.
In addition to its potential anticancer effects, moscatilin has been demonstrated to have several pharmacological properties, including anti-inflammatory [15, 16], antioxidant [17], antiplatelet aggregation [18], anti-bacterial, anti-fungal [19], and neuroprotective [20]. Indeed, this was reported to inhibit the pro-inflammatory enzymes cyclooxygenase-2 and iNOS after lipopolysaccharide treatment in RAW264.7 cells [21]. Also, it was demonstrated to possess neuroprotective benefits against Alzheimer’s disease, by improving learning and memory deficits in mice [17]. However, only a few pharmaceutical manufacturers in China sell moscatilin, which has a current price of about $ 649 (10 mg), despite having a wide range of therapeutic benefits [22]. Thus, more clinical research should be done in this field, to better understand how it affects human health.
This review highlights the novel aspects of moscatilin, providing a comprehensive understanding of its anticancer mechanisms, including apoptosis induction, cell cycle arrest, and anti-metastatic activities. It delves into the structure–activity relationships, identifying critical functional groups for efficacy. Additionally, this review addresses gaps in current knowledge by compiling recent findings on moscatilin’s metabolism, bioavailability, and pharmacokinetics, paving the way for future clinical applications. By incorporating the most recent research, this review offers a comprehensive and reliable overview of moscatilin’s anticancer properties.
Review methodology
A comprehensive literature search was conducted across three electronic bibliographic databases: PubMed/MedLine, Scopus, and Web of Science. The search strategy employed both Medical Subject Headings (MeSH) and relevant keywords to identify pertinent studies. The specific MeSH terms and keywords used included “Cancer,” “Moscatilin,” “Anticancer,” “Bioactivity,” “Dendrobium,” “Drug metabolism,” “Moscatilin derivatives,” “Anticancer mechanisms,” “Pharmacological properties,” “Bioavailability,” “Drug safety,” and “Toxicity.” These terms were used individually and in various combinations with Boolean operators (AND, OR, NOT) to refine the search results. The inclusion criteria for this review were articles published in English, studies focusing on the anticancer properties and mechanisms of moscatilin, research on the bioactivity, pharmacokinetics, and bioavailability of moscatilin, and papers discussing the structure–activity relationships of moscatilin and its derivatives. Studies not related to moscatilin or its derivatives, non-English articles, and papers lacking significant data on anticancer mechanisms or pharmacological properties were excluded from this review. To ensure accurate species identification, the taxonomy of Dendrobium species was validated using the World Flora Online (WFO) database. Additionally, chemical structures and related data were verified using PubChem to confirm the identity and properties of moscatilin and its derivatives. The most important data were summarized in tables and figures.
Sources, phytochemistry, and chemical structure
The use of orchids in herbal medicine has a very long history. Like many other plants, orchids, produce many phytochemicals and secondary metabolites, such as phenanthrenes, bibenzyls, fluorenones, sesquiterpenes, and alkaloids, which are responsible for their wide variety of medicinal properties [23, 24]. Moscatilin (1) is a natural product isolated, for the first time, from the orchid Dendrobium moscatum [25].
Chemically, it corresponds to the 4,4’-dihydroxy-3,3’,5-trimethoxybibenzyl and can be classified as a bibenzyl or as a dihydrostilbene (Fig. 1).
It has been extensively reported in Dendrobium plants, including for example D. rotundatum [26], D. nobile [27], D. amoenum [28], D. plicatile [29], D. densiflorum [30], D. loddigesii [18, 31,32,33], D. polyanthum [34], D. secundum [35, 36], D. capillipes [36], D. oficinalle [37], D. pulchellum [38], D. williamsonii [39], D. trigonopus [40], D. ellipsophyllum [41], D. fimbriatum Hook [42], D. brymerianum [43], D. aurantiacum var. denneanum [44],D. moniliforme [45], D. formosum [46], D. wardianum Warner [47], D. infundibulum [48], D. palpebrae [49], D. christyanum [50], D. chrysanthum [51] among approximately 52 species [52, 53].
The occurrence of the dihydrostilbenes in nature in limited amounts and inaccessible plant species has driven the search for synthetic methods for their preparation. Consequently, many synthetic methodologies have been developed to produce these compounds, but most of them rely on the hydrogenation of stilbene derivatives which were obtained by means of Wittig-Horner olefination [54, 55]. Claisen–Schmidt condensation [54], or Mizoroki–Heck cross-coupling reactions, and the Ramberg-Bäcklund rearrangement that consists in the conversion of readily available α- and α’-hydrogen bearing substituted dibenzyl sulfones into the corresponding stilbenes, followed by the double bond hydrogenation in the presence of 10% Pd/C to afford the bibenzyl derivatives [56].
Semi-synthetic derivatives and chemical structureanticancer activity relationship
Moscatilin and other related natural antimitotic compounds are capable to interact with tubulin and inhibit microtubule assembly at nanomolar concentration. Structure–activity relationship studies have shown that phenolic functional groups are essential for anticancer activity. In addition, the length of the bridge that links the two aryl rings is strongly correlated with the potency of the compound; the two-carbon bridge analogs show the maximum activity, while the one-carbon and three-carbon bridged analogs are less potent (Fig. 2) [56].
At present, nearly 90 bibenzyl analogs of moscatilin have been extracted and tested from Dendrobium species, some of which showed various pharmacological activities such as, antioxidant, anti-inflammatory, neuroprotective, antidiabetic, and antitumor. Additionally, with the development of novel synthetic routes for the synthesis of bibenzyls, several synthetic derivatives have been produced and tested widening the SAR (Structure–Activity Relationship studies). For example, in 2019, Guan et al. synthesized a series of substituted moscatilin derivatives (2) and evaluated their antitumor and angiogenesis activity [14, 52]. They observed that the same compound has distinct potency for different cancer cell lines. Nonetheless, important effects of substituent groups on phenyl rings of compound 2 were observed and a compound was selected for further molecular docking studies. Among the compounds studied, five compounds monosubstituted with chloro- or aza- groups on the B ring showed IC50 below the micromolar level in HCT116 cells. For A549 cells, the most potent compound presented the methoxy group in the ortho-position of the Y ring, being more active than moscatilin (1). For compounds with R3 = 2-chloro or R3 = 2-fluoro, the activity was close to that of 1. However, these two compounds were more active than 1 against HepG2 cells. Compounds 3 were less active than 2, which suggests that 4-hydroxy and 5-methoxy are important active groups. For compounds 3, all compounds assessed were active against HepG2 cell with exception of that with R3 = 4-fluoro. Surprisingly, when R3 = 4-chloro, the compound was fivefold more active than 1. Moscatilin was the best drug against MDA-MB-231 cells, since all the other compounds had weaker activity, with exception of the compound with R3 = 3-hydroxy-4-methoxy that showed similar activity to 1. For MKN-45 cells, some halogenated compounds showed good activity, especially the compound 2, characterized by the 2,3-dichloro substitution pattern in the B ring, which showed 300-fold higher activity than 1.
Overall, this study revealed that the anticancer activity on different cancer cell lines is strongly dependent on the substitution pattern of these compounds. For example, for compounds 3, the derivative containing chloro group in the para-position is most active against HepG2 cells, while the derivative with the methoxy group in the para-position and a hydroxy group in the meta-position is more active against MDA-M-231 cells (Fig. 3).
Compound 2 (R3 = 2-Cl), which exhibited the best activity against five cell lines, was selected for molecular docking with tubulin, and it had a good affinity. The main interaction between this compound and tubulin was revealed to be Glu-200 (bond length: 1.8 Å), and the residue of Tyr-202 (length: 3.1 Å) forms two hydrogen bonds with the hydroxy group. In addition, the B-ring stretched into the hydrophobic pocket composed of Leu-255, Ala-316, Ala-317, and Thr-353, forming a stable hydrophobic bond. Ala-316 and Ala-317 residues form a CH-π interaction with the 2’-chlorophenyl group. Overall, this compound has a “V-shaped” conformation in the pocket of tubulin contributing to a strong activity on tumor cells [25, 26].
Other substituted bibenzyls related to moscatilin 4–9 (Fig. 4) have been isolated from D. williamsonii and were screened for cytotoxic activity against five human tumor lines (HL-60, SMMC-7721, A-549, MCF-7, and SW-480). All these compounds showed very weak cytotoxicity when compared with the positive control taxol. However, aloifol I (5) and moscatilin (1) displayed similar activity against HL-60 with IC50 values of 4.48 μM and 5.10 μM, respectively, followed by moniliformine (2) with IC50 of 10.69 μM [57].
Bioavailability and pharmacokinetics of moscatilin
Drug metabolism is the process by which medications are chemically changed within the body to allow for their removal. The process typically involves three phases: phase I, through which drug molecules are exposed to liver enzymes, changing their structure to make them more accessible for phase II metabolism; phase II, where the biotransformed drug molecules are attached to molecules known as conjugates, making them more water-soluble; and phase III, where the drugs are eliminated from the body [58]. To the best of our knowledge, just a few studies have been performed on moscatilin metabolism and only phases I and II were evaluated and proposed.
A study conducted by Zhang et al. determined the moscatilin metabolites by LC–MS (Liquid Chromatography-Mass Spectrometry) and suggested the activation routes of the metabolites, using rat, dog, monkey, and human liver microsomes. Six NADPH-dependent metabolites were identified in phase I, the majority of which were produced via hydroxylation and demethylation reactions (Fig. 5) [6]. Yet, the process of dehydrogenation also generated a metabolite. Moreover, a metabolite formed from demethylation and subsequent hydroxylation was found in monkey but not in rat, dog, and human liver microsomes, suggesting that different species have different metabolic pathways. In phase II, eight metabolites conjugated with glutathione (GSH) were detected, producing reactive metabolites. In the liver hepatocytes of the aforementioned species as well as of mice, Hu et al. reported a total of 18 metabolites, with seven compounds related to phase I and 11 to phase II [59]. Glucuronidation and sulfation were observed in phase II, in addition to the metabolic pathways reported by Zhang et al. [6]. Five of the detected metabolites were not recognized in human hepatocytes, which is a significant point to make because the success of the treatment must always be adjusted to the species. Dealkylation in conjunction with GSH was also observed in moscatilin metabolism. Both experiments suggested that moscatilin can be transformed into ortho-quinone and quinone-methide intermediates, which can interact with cytochrome P450 enzymes and result in inhibition, leading to pharmacological interactions. In fact, ortho-quinone methide species are reactive metabolites that can show antitumor activity when they are activated in the body through oxidation reactions [60]. These findings can contribute for a better understanding of moscatilin bioavailability and pharmacokinetics in the future.
Mechanism of antitumor action of moscatilin
The existing data indicate that moscatilin exhibits encouraging anticancer properties in vitro and in vivo against different forms of cancer cells including: lung, prostate, breast, cervical, ovarian, gastrointestinal (colorectal, gastric, oesophageal), hepatocellular cancers, sarcoma and melanoma—Tables 1 and 2 [7, 9, 13, 14, 38, 41, 43, 61, 62].
Moscatilin has been extensively studied in vitro using diverse cancer cell lines. The data reveal that lung cancer is one of the most frequently investigated cancer types with moscatilin, highlighting its significant cytotoxic effects. For instance, in H292 lung cancer cells, moscatilin demonstrates an IC50 of 226 ± 6 μM, inducing apoptosis and reducing metastasis [41]. Similarly, in H23 lung adenocarcinoma cells, moscatilin shows an IC50 in the range of 0–5 μM, effectively reducing metastasis and cancer cell migration by decreasing endogenous hydroxyl radical levels and inhibiting the phosphorylation of FAK and Akt [10]. In melanoma, the A375 cell line exhibits sensitivity to moscatilin, with IC50 values between 6.25 and 50 μM. The compound induces apoptosis, modulates Hsp70 and caspase-3 activities, and decreases cell viability in a concentration-dependent manner [13]. Colorectal cancer cell lines, including HCT-116 and HT-29, also respond significantly to moscatilin, with IC50 values of 4.48 μM and 4.4 μM, respectively. The mechanisms involve tubulin depolymerization, DNA damage, and JNK1/2 phosphorylation, leading to cell cycle arrest and increased apoptosis [7].Breast cancer cells, notably the MDA-MB-231 and MCF-7 cell lines, show varying responses to moscatilin. In MDA-MB-231 cells, moscatilin reduces cell migration and proliferation by inhibiting HDAC3 and increasing the acetylation status of histones H3 and H4 [62]. Prostate cancer cells, such as the PC-3 cell line, are also notably affected, with IC50 values around 4.95 μM, although specific mechanistic details are less extensively documented [63].
In vivo studies further validate the antitumor efficacy of moscatilin. For example, in an A549 xenograft lung cancer mouse model, moscatilin administered at 100 mg/kg intraperitoneally 3 times a week significantly reduces tumor growth and vasculature without impacting body weight [32]. In breast cancer models, nude mice treated with 100 mg/kg/day of moscatilin show a dramatic decrease in HDAC3 protein levels, reduced tumor growth, and prolonged survival [62]. Similar antitumor effects are observed in pancreatic and colorectal cancer models, with significant reductions in tumor weight and volume, highlighting the compound’s potential for broad-spectrum anticancer activity without apparent toxicity [7, 9].
The studies summarized in the attached tables underscore the ongoing research into moscatilin’s potential as a potent anticancer agent. The compound is primarily derived from extracts of the Dendrobium genus, although several derivatives have been synthesized to enhance its bioactivity and therapeutic potential. The recent studies focus on elucidating the multifaceted mechanisms of moscatilin, including apoptosis induction, inhibition of cell migration and metastasis, and antiangiogenic activities. These findings position moscatilin as a promising candidate for further clinical investigation and potential therapeutic application.
The specific mechanisms by which moscatilin exhibits its anticancer activity may vary depending on the type of cancer cell being studied.
Apoptosis induction
Moscatilin is found to induce G2/M phase arrest in placenta and stomach cancer cells that were treated with a lethal dose. However, there is no detectable inhibitory effect of moscatilin on the activity of cyclin B-cdc-2 kinase [65]. Cell cycle arrest at G2/M phase is also observed in colorectal, esophageal, ovarian, and lung cancer cells [7, 12, 14, 61]. The apoptosis is a result of inhibition of tubulin polymerization, and activation of the intrinsic apoptosis pathway [7].
In paper by Zhang et al. [16], an in vitro study conducted on several cancer cell lines demonstrated that the apoptosis of cancer cells is triggered through the activation of the c-Jun N-terminal kinase/Stress-activated protein kinase (JNK/SAPK) pathway and the generation of reactive oxygen species (ROS) with an increase in apoptotic DNA fragmentation. It also modulates the ratio of pro-apoptotic Bcl-2-associated X protein (Bax) to anti-apoptotic B-cell lymphoma 2 protein (Bcl2), thereby leading to the caspase-dependent mitochondrial apoptosis pathway [9]. JNK activation with subsequent increase in apoptosis-related protein expression (e.g., cleaved caspase-3, cleaved caspase-7, cleaved caspase-8, cytochrome c, cleaved caspase-9, and Poly (ADP-ribose) Polymerase [PARP]) is observed also in head and neck squamous cell carcinoma or colorectal cancer cells [7, 64]. The apoptotic response to moscatilin treatment is also linked to ROS generation, as well as to increased activity of Phosphatase and Tensin homolog (PTEN) and suppressed Heat shock protein 70 (Hsp70) expression in melanoma cells [13]. PTEN is known to inhibit Phosphatidylinositol-3 kinase/Protein kinase B (PI3K/Akt) pathway influencing cells survival [67]. There are data showing that moscatilin can induce apoptosis through epigenetic modifications as well [62].
Anoikis-sensitizing effect
Anoikis-sensitizing agents enhance cells’ susceptibility to programmed cell death following detachment or loss of contact with cells’ matrix. These agents may be effective in promoting the death of improperly attached cancer cells, which are often resistant to anoikis and prone to metastasize. This effect is a promising therapeutic strategy in cancer treatment [68]. In vitro studies on lung cancer cell lines showed ability to support anoikis effect by moscatilin [38, 41].
Inhibition of migration and invasion, antimetastatic activity
Metastasis is a multistep process that involves the dissemination of cancer cells from the primary site to distant organs, involving several key steps such as invasion, survival in the circulation or migration [69]. Moscatilin is observed to inhibit the migration and invasion of lung cancer cells in vitro, possibly by suppressing filopodia formation via decreased activity of phosphorylated Focal adhesion kinase (FAK) and phosphorylated Akt, as well as via endogenous ROS [10]. A separate investigation conducted on lung cancer cells demonstrated that either the caveolin-1 or the Akt signaling pathway can regulate cell migration [43]. Breast cancer has been identified as another malignancy that is affected by aberrations in the Akt pathway. Moscatilin inhibits Akt and Twist components, which are involved in cell migration, both in vitro and in vivo. Additionally, it reduces the expression of N-cadherin, which is known to play a role in epithelial-mesenchymal transition (EMT) that supports cancer cell migration [12]. The Akt/ Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway is implicated in the invasion of hepatocellular carcinoma cell lines as well. Moscatilin has been found to inhibit this pathway, resulting in a reduction in cell line invasion and expression of urokinase-type plasminogen activator (uPA), a protein that breaks down extracellular matrix proteins.
Suppression of cancer growth/proliferation
Decreased cancer cell proliferation was seen in in vitro studies on lung and esophageal cancer cell lines [12, 14]. These results were confirmed by in vivo data: the growth of human esophageal xenografts in mice was significantly inhibited by moscatilin, without any observable impact on body weight, liver, renal, or bone marrow function [63]. Similar results were confirmed for ovarian cancer studies [61]. Cell cycle arrest described previously can be one of the mechanisms responsible for that proliferation inhibition.
Antiangiogenic activity
In both in vitro and in vivo studies, moscatilin inhibits the growth of lung cancer cells and neovascularization, as well as the proliferation, migration, and tube formation of human umbilical vein endothelial cells (HUVECs) in response to Vascular endothelial growth factor (VEGF) and basic Fibroblast growth factor (bFGF). It achieves this by blocking essential signaling pathways in HUVECs, such as Extracellular signal-regulated kinase 1/2 (ERK1/2)/Akt, which suggest that moscatilin may be a promising therapy for cancer by inhibiting angiogenesis [32]. In a dose-dependent manner, moscatilin was observed to hinder the development of capillary-like tubes in HUVECs that were induced by VEGF. Additionally, it blocked the activation of VEGF Receptor 2, ERK1/2, proto-oncogene c-Raf, and mitogen-activated protein kinase 1/2 (MEK1/2), all of which are involved in the VEGF signaling pathway [70].
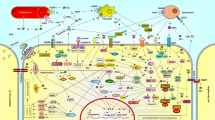
Moscatilin is a promising candidate for further exploration as a cancer treatment. An overview of its activity is provided in Fig. 6. In addition, there are other mechanisms proposed in the literature, such as its potential to enhance the sensitivity of cancer cells to radiation therapy [63, 71], or its immunomodulating activity [16].
Diagram with mechanisms underlying moscatilin’s anticancer effects. The figure delineates four primary mechanisms: (1) Apoptosis Induction: Moscatilin promotes apoptosis through enhanced PTEN activity, increased reactive oxygen species (ROS) production, activation of the caspase cascade, cell cycle arrest, microtubule depolymerization, and DNA damage. Additionally, moscatilin sensitizes cancer cells to anoikis, a form of programmed cell death. (2) Suppression of Proliferation: Moscatilin inhibits tumor growth by inducing cell cycle arrest at the G2/M phase and modulating the Akt and MEK1/2 signaling pathways. (3) Antimetastatic Activity: Moscatilin reduces cell migration and invasion by inhibiting the Akt pathway, focal adhesion kinase (FAK) activity, and epithelial-mesenchymal transition (EMT), and downregulates the expression of metastasis-related genes such as urokinase plasminogen activator (uPA) and N-cadherin. (4) Antiangiogenic Activity: Moscatilin blocks angiogenesis by interfering with VEGF signaling pathways in human umbilical vein endothelial cells (HUVECs), targeting VEGFR2, ERK1/2, Akt, and MEK1/2 pathways. Akt protein kinase B, EMT epithelial-mesenchymal transition, ERK1/2 extracellular signal-regulated kinase ½, FAK focal adhesion kinase, HUVEC human umbilical vein endothelial cells, MEK1/2 mitogen-activated protein kinase ½, PTEN phosphatase and tensin homolog, ROS reactive oxygen species, uPA urokinase plasminogen activator, VEGF vascular endothelial growth factor, VEGFR2 vascular endothelial growth factor receptor 2
Toxicity, side effects and safety
In terms of this medication’s safety, moscatilin does not cause cytotoxicity in healthy cells and may precisely and successfully act on cancer cells at low concentrations [71]. In reality, the compound was found to be a much more targeted medication for cancer cell lines and less hazardous for healthy cells by Pujari et al. [22], using the MTT cell proliferation assay. Despite the lack of information about the safety of this bibenzyl derivative, long-term consumption of dendrobium was shown to be safe in several studies, disclosing its potential as a supplement or medicine [72]. The Meng et al. study further confirmed the security of dendrobium composition and showed that it had no adverse effects on skin irritability [73]. However, a better understanding of the performance of isolated compounds is required because this impact was only shown in the mixture of plant chemicals.
Limitations and clinical gaps
Bioactive compounds, such as moscatilin, often exhibit a wide range of pharmacological properties, including antiviral effects against various viruses, antioxidant activities, antibacterial effects, and other therapeutic benefits [74,75,76]. This versatility underscores moscatilin’s potential as a potential therapeutic agent for cancer treatment, with multifaceted molecular mechanisms and promising clinical prospects. Despite the promising anticancer potential of moscatilin, several limitations and clinical gaps need to be addressed to fully understand its therapeutic viability and facilitate its translation from bench to bedside. The clinical data on moscatilin’s safety and efficacy in humans remain limited, with most evidence derived from preclinical studies involving various cancer cell lines and animal models. Comprehensive data on moscatilin’s bioavailability, metabolism, and excretion in humans are lacking, hindering accurate predictions of its behavior in the human body. While the mechanisms of action, such as apoptosis induction, inhibition of proliferation, and suppression of metastasis, are well-documented, the precise molecular pathways involved require further elucidation to optimize therapeutic potential and minimize off-target effects. Additionally, potential mechanisms of resistance to moscatilin in cancer cells have not been thoroughly investigated, which is crucial for ensuring long-term efficacy. Furthermore, optimal dosing regimens for moscatilin are not yet established, necessitating the determination of appropriate doses that maximize therapeutic efficacy while minimizing toxicity. There is an urgent need for early-phase clinical trials (Phase I/II) to assess the safety, tolerability, and preliminary efficacy of moscatilin in cancer patients, providing critical data to inform subsequent larger-scale studies. Investigating the potential of moscatilin in combination with existing chemotherapeutics, targeted therapies, or immunotherapies could enhance its anticancer efficacy; thus, research into synergistic effects and optimal combination strategies is required. Studies exploring the specificity of moscatilin’s anticancer effects across different tumor types and genetic backgrounds are necessary to identify which patient populations may benefit the most from this treatment. Identifying biomarkers that predict response to moscatilin could enable personalized treatment approaches, improving outcomes and minimizing unnecessary exposure for non-responders. Lastly, long-term safety studies are needed to evaluate potential adverse effects and ensure that prolonged use of moscatilin does not lead to unacceptable toxicities or secondary malignancies.
Conclusion and prospects
Moscatilin and related bibenzyl analogues present significant development prospects due to their broad pharmacological effects, particularly their potent anticancer activity, and their extensive natural sources. These compounds can be synthesized efficiently, facilitating large-scale production. Although only a limited number of studies have explored the structure–activity relationships of these compounds, critical functional groups essential for anticancer efficacy have been identified, with the presence of phenolic groups and a two-carbon linker between aryl rings being particularly favorable. Moscatilin has demonstrated substantial anticancer properties, including the inhibition of cell proliferation, induction of apoptosis, and suppression of metastasis across various studies, underscoring its potential as a robust candidate for cancer therapy. Despite these promising findings, comprehensive and extensive clinical investigations into moscatilin’s pharmacokinetics, bioavailability, and metabolism in humans are crucial. These studies are necessary to ensure patient safety, determine therapeutic efficacy, and optimize dosing regimens. Moreover, rigorous clinical trials are essential to confirm the preliminary findings and to explore potential synergistic effects when combined with existing cancer treatments. Identifying predictive biomarkers for moscatilin response will enhance its therapeutic application by enabling personalized treatment strategies. Addressing these research gaps will be critical in advancing moscatilin from a promising natural compound to a clinically viable anticancer therapy, ensuring its safe and effective use in cancer prevention and treatment.
Data availability
No datasets were generated or analyzed during the current study.
Abbreviations
- ADC:
-
Adenocarcinoma
- Akt:
-
Protein kinase B
- Bax:
-
Bcl2-associated X protein
- Bcl2:
-
B-cell lymphoma 2
- EMT:
-
Epithelial-mesenchymal transition
- ERK1/2:
-
Extracellular signal-regulated kinase ½
- FAK:
-
Focal adhesion kinase
- Glu A:
-
Glucuronic acid
- GSH:
-
Glutathione
- HDAC3:
-
Histone deacetylase 3
- HSP70:
-
Heat shock protein 70
- HUVEC:
-
Human umbilical vein endothelial cells
- IC50:
-
Half maximal inhibitory concentration
- JNK:
-
C-Jun NH2-terminal protein kinase
- LD50:
-
Lethal dose 50
- Me:
-
Methyl
- MEK1/2:
-
Mitogen-activated protein kinase ½
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PARP:
-
Poly (ADP-ribose) polymerase
- PI3K:
-
Phosphoinositide 3-kinase
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome 10
- ROS:
-
Reactive oxygen species
- SAPK:
-
Stress-activated protein kinases
- SCC:
-
Squamous cell carcinoma
- SO3:
-
Sulfur trioxide
- uPA:
-
Urokinase plasminogen activator
- VEGF:
-
Vascular endothelial growth factor
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
References
Deo SVS, Sharma J, Kumar S. GLOBOCAN 2020 report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol. 2022;29(11):6497–500. https://doi.org/10.1245/s10434-022-12151-6.
Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, et al. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol. 2021. https://doi.org/10.1155/2021/5905357.
Motyka S, Jafernik K, Ekiert H, Sharifi-Rad J, Calina D, Al-Omari B, et al. Podophyllotoxin and its derivatives: potential anticancer agents of natural origin in cancer chemotherapy. Biomed Pharmacother. 2023;158:114145. https://doi.org/10.1016/j.biopha.2022.114145.
Pezzani R, Jimenez-Garcia M, Capó X, Gürer ES, Sharopov F, Rachel TYL, et al. Anticancer properties of bromelain: state-of-the-art and recent trends. Front Oncol. 2023. https://doi.org/10.3389/fonc.2022.1068778.
Xiong L, Cao ZX, Peng C, Li XH, Xie XF, Zhang TM, et al. Phenolic glucosides from Dendrobium aurantiacum var. denneanum and their bioactivities. Molecules. 2013;18(6):6153–60. https://doi.org/10.3390/molecules18066153.
Zhang R, Wu Q, Gao H, Li Y, Zhang P. Rapid separation and characterization of the in vitro metabolites of moscatilin by ultra-high performance liquid chromatography coupled to hybrid quadrupole orbitrap tandem mass spectrometry. J Sep Sci. 2022;45(23):4167–75. https://doi.org/10.1002/jssc.202200617.
Chen TH, Pan SL, Guh JH, Liao CH, Huang DY, Chen CC, et al. Moscatilin induces apoptosis in human colorectal cancer cells: a crucial role of c-Jun NH2-terminal protein kinase activation caused by tubulin depolymerization and DNA damage. Clin Cancer Res. 2008;14(13):4250–8. https://doi.org/10.1158/1078-0432.CCR-07-4578.
Su W, Zeng L, Chen W. Moscatilin suppresses the breast cancer both in vitro and in vivo by inhibiting HDAC3. Dose Response. 2021;19(1):15593258211001252. https://doi.org/10.1177/15593258211001251.
Zhang L, Fang Y, Xu XF, Jin DY. Moscatilin induces apoptosis of pancreatic cancer cells via reactive oxygen species and the JNK/SAPK pathway. Mol Med Rep. 2017;15(3):1195–203. https://doi.org/10.3892/mmr.2017.6144.
Kowitdamrong A, Chanvorachote P, Sritularak B, Pongrakhananon V. Moscatilin inhibits lung cancer cell motility and invasion via suppression of endogenous reactive oxygen species. Biomed Res Int. 2013;2013:765894. https://doi.org/10.1155/2013/765894.
Busaranon K, Plaimee P, Sritularak B, Chanvorachote P. Moscatilin inhibits epithelial-to-mesenchymal transition and sensitizes anoikis in human lung cancer H460 cells. J Nat Med. 2016;70(1):18–27. https://doi.org/10.1007/s11418-015-0931-7.
Pai HC, Chang LH, Peng CY, Chang YL, Chen CC, Shen CC, et al. Moscatilin inhibits migration and metastasis of human breast cancer MDA-MB-231 cells through inhibition of Akt and twist signaling pathway. J Mol Med (Berl). 2013;91(3):347–56. https://doi.org/10.1007/s00109-012-0945-5.
Cardile V, Avola R, Graziano ACE, Russo A. Moscatilin, a bibenzyl derivative from the orchid Dendrobium loddigesii, induces apoptosis in melanoma cells. Chem Biol Interact. 2020;323:109075. https://doi.org/10.1016/j.cbi.2020.109075.
Guan L, Zhou J, Lin Q, Zhu H, Liu W, Liu B, et al. Design, synthesis and antitumour and anti-angiogenesis evaluation of 22 moscatilin derivatives. Bioorg Med Chem. 2019;27(12):2657–65. https://doi.org/10.1016/j.bmc.2019.04.027.
Hwang JS, Lee SA, Hong SS, Han XH, Lee C, Kang SJ, et al. Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Bioorg Med Chem Lett. 2010;20(12):3785–7. https://doi.org/10.1016/j.bmcl.2010.04.054.
Zhang Y, Xu Y, Jing X, Lu W, Zhang F, Qin C. Moscatilin suppresses the inflammation from macrophages and T cells. Open Med (Wars). 2022;17(1):756–67. https://doi.org/10.1515/med-2022-0456.
Huang JM, Huang FI, Yang CR. Moscatilin ameliorates tau phosphorylation and cognitive deficits in Alzheimer’s disease models. J Nat Prod. 2019;82(7):1979–88. https://doi.org/10.1021/acs.jnatprod.9b00375.
Chen CC, Wu LG, Ko FN, Teng CM. Antiplatelet aggregation principles of Dendrobium loddigesii. J Nat Prod. 1994;57(9):1271–4. https://doi.org/10.1021/np50111a014.
Pujari I, Babu VS. Rhizobium rhizogenes infection in threatened Indian orchid Dendrobium ovatum mobilises ‘Moscatilin’ to enhance plant defensins. 3 Biotech. 2022;12(5):119. https://doi.org/10.1007/s13205-022-03180-9.
Lai MC, Liu WY, Liou SS, Liu IM. A bibenzyl component moscatilin mitigates glycation-mediated damages in an SH-SY5Y cell model of neurodegenerative diseases through AMPK activation and RAGE/NF-kappaB pathway suppression. Molecules. 2020. https://doi.org/10.3390/molecules25194574.
Liu YN, Pan SL, Peng CY, Huang DY, Guh JH, Chen CC, et al. Moscatilin repressed lipopolysaccharide-induced HIF-1alpha accumulation and NF-kappaB activation in murine RAW264.7 cells. Shock. 2010;33(1):70–5. https://doi.org/10.1097/SHK.0b013e3181a7ff4a.
Pujari I, Sengupta R, Babu VS. Docking and ADMET studies for investigating the anticancer potency of Moscatilin on APC10/DOC1 and PKM2 against five clinical drugs. J Genet Eng Biotechnol. 2021;19(1):161. https://doi.org/10.1186/s43141-021-00256-6.
Hossain MM. Therapeutic orchids: traditional uses and recent advances—an overview. Fitoterapia. 2011;82(2):102–40. https://doi.org/10.1016/j.fitote.2010.09.007.
Ng TB, Liu J, Wong JH, Ye X, Wing Sze SC, Tong Y, et al. Review of research on Dendrobium, a prized folk medicine. Appl Microbiol Biotechnol. 2012;93(5):1795–803. https://doi.org/10.1007/s00253-011-3829-7.
Majumder PL, Sen RC. Moscatilin, a bibenzyl derivative from the orchid Dendrobium moscatum. Phytochemistry. 1987;26(7):2121–4. https://doi.org/10.1016/s0031-9422(00)81777-x.
Majumder PL, Pal S. Rotundatin, a new 9,10-didydrophenanthrene derivative from Dendrobium rotundatum. Phytochemistry. 1992;31(9):3225–8. https://doi.org/10.1016/0031-9422(92)83480-m.
Miyazawa M, Shimamura H, Nakamura S, Sugiura W, Kosaka H, Kameoka H. Moscatilin from Dendrobium nobile, a naturally occurring bibenzyl compound with potential antimutagenic activity. J Agric Food Chem. 1999;47(5):2163–7. https://doi.org/10.1021/jf970930a.
Majumder PL, Guha S, Sen S. Bibenzyl derivatives from the orchid Dendrobium amoenum. Phytochemistry. 1999;52(7):1365–9. https://doi.org/10.1016/s0031-9422(99)00370-2.
Honda C, Yamaki M. Phenanthrenes from Dendrobium plicatile. Phytochemistry. 2000;53(8):987–90. https://doi.org/10.1016/s0031-9422(99)00497-5.
Fan C, Wang W, Wang Y, Qin G, Zhao W. Chemical constituents from Dendrobium densiflorum. Phytochemistry. 2001;57(8):1255–8. https://doi.org/10.1016/s0031-9422(01)00168-6.
Li CY, Lu Y, Chen Y, Zheng JW, Wang J. Chemical components of Dendrobium loddigesii. Acta Sci Nat Univ Sunyatseni. 2013;52(3):73–6.
Tsai AC, Pan SL, Liao CH, Guh JH, Wang SW, Sun HL, et al. Moscatilin, a bibenzyl derivative from the India orchid Dendrobrium loddigesii, suppresses tumor angiogenesis and growth in vitro and in vivo. Cancer Lett. 2010;292(2):163–70. https://doi.org/10.1016/j.canlet.2009.11.020.
Ho CK, Chen CC. Moscatilin from the orchid Dendrobrium loddigesii is a potential anticancer agent. Cancer Invest. 2003;21(5):729–36. https://doi.org/10.1081/cnv-120023771.
Hu JM, Zhao YX, Miao ZH, Zhou J. Chemical components of Dendrobium polyanthum. B Korean Chem Soc. 2009;30(9):2098–100. https://doi.org/10.5012/bkcs.2009.30.9.2098.
Sritularak B, Duangrak N, Likhitwitayawuid K. A new bibenzyl from Dendrobium secundum. Z Naturforsch C J Biosci. 2011;66(5–6):205–8. https://doi.org/10.1515/znc-2011-5-602.
Phechrmeekha T, Sritularak B, Likhitwitayawuid K. New phenolic compounds from Dendrobium capillipes and Dendrobium secundum. J Asian Nat Prod Res. 2012;14(8):748–54. https://doi.org/10.1080/10286020.2012.689979.
Chen X, Wang F, Wang Y, Li X, Wang A, Wang C, et al. Discrimination of the rare medicinal plant Dendrobium officinale based on naringenin, bibenzyl, and polysaccharides. Sci China Life Sci. 2012;55(12):1092–9. https://doi.org/10.1007/s11427-012-4419-3.
Chanvorachote P, Kowitdamrong A, Ruanghirun T, Sritularak B, Mungmee C, Likhitwitayawuid K. Anti-metastatic activities of bibenzyls from Dendrobium pulchellum. Natural Product Commun. 2013. https://doi.org/10.1177/1934578x1300800127.
Rungwichaniwat P, Sritularak B, Likhitwitayawuid K. Chemical constituents of Dendrobium williamsonii. Pharmacognosy Journal. 2014;6(3):36–41. https://doi.org/10.5530/pj.2014.3.6.
Hu JM, Chen JJ, Yu H, Zhao YX, Zhou J. Two novel bibenzyls from Dendrobium trigonopus. J Asian Nat Prod Res. 2008;10(7–8):653–7. https://doi.org/10.1080/10286020802133605.
Tanagornmeatar K, Chaotham C, Sritularak B, Likhitwitayawuid K, Chanvorachote P. Cytotoxic and anti-metastatic activities of phenolic compounds from Dendrobium ellipsophyllum. Anticancer Res. 2014;34(11):6573–9.
Xu FQ, Xu FC, Hou B, Fan WW, Zi CT, Li Y, et al. Cytotoxic bibenzyl dimers from the stems of Dendrobium fimbriatum hook. Bioorg Med Chem Lett. 2014;24(22):5268–73. https://doi.org/10.1016/j.bmcl.2014.09.052.
Klongkumnuankarn P, Busaranon K, Chanvorachote P, Sritularak B, Jongbunprasert V, Likhitwitayawuid K. Cytotoxic and antimigratory activities of phenolic compounds from Dendrobium brymerianum. Evid Based Complement Alternat Med. 2015;2015:350410. https://doi.org/10.1155/2015/350410.
Ying L, Jin-He J, Yan Z, Ye-Gao C. Chemical constituents of Dendrobium aurantiacum var. denneanum. Chem Nat Compd. 2009;45(4):525–7. https://doi.org/10.1007/s10600-009-9393-z.
Zhao N, Yang G, Zhang Y, Chen L, Chen Y. A new 9,10-dihydrophenanthrene from Dendrobium moniliforme. Nat Prod Res. 2016;30(2):174–9. https://doi.org/10.1080/14786419.2015.1046379.
Inthongkaew P, Chatsumpun N, Supasuteekul C, Kitisripanya T, Putalun W, Likhitwitayawuid K, et al. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev Bras. 2017;27(4):480–7. https://doi.org/10.1016/j.bjp.2017.05.005.
Zhang C, Liu SJ, Yang L, Yuan MY, Li JY, Hou B, et al. Sesquiterpene amino ether and cytotoxic phenols from Dendrobium wardianum Warner. Fitoterapia. 2017;122:76–9. https://doi.org/10.1016/j.fitote.2017.08.015.
Na Ranong S, Likhitwitayawuid K, Mekboonsonglarp W, Sritularak B. New dihydrophenanthrenes from Dendrobium infundibulum. Nat Prod Res. 2019;33(3):420–6. https://doi.org/10.1080/14786419.2018.1455050.
Kyokong N, Muangnoi C, Thaweesest W, Kongkatitham V, Likhitwitayawuid K, Rojsitthisak P, et al. A new phenanthrene dimer from Dendrobium palpebrae. J Asian Nat Prod Res. 2019;21(4):391–7. https://doi.org/10.1080/10286020.2018.1429416.
San HT, Boonsnongcheep P, Putalun W, Mekboonsonglarp W, Sritularak B, Likhitwitayawuid K. α-Glucosidase inhibitory and glucose uptake stimulatory effects of phenolic compounds from Dendrobium christyanum. Nat Prod Commun. 2020. https://doi.org/10.1177/1934578x20913453.
Zhang Z-M, Liu S, Yang H, Wang N, Zou Y-H, Zhuang P-Y, et al. Chemical constituents from Dendrobium chrysanthum and their chemotaxonomic significance. Biochem Syst Ecol. 2022. https://doi.org/10.1016/j.bse.2022.104522.
He L, Su Q, Bai L, Li M, Liu J, Liu X, et al. Recent research progress on natural small molecule bibenzyls and its derivatives in Dendrobium species. Eur J Med Chem. 2020;204:112530. https://doi.org/10.1016/j.ejmech.2020.112530.
Zhai D, Lv X, Chen J, Peng M, Cai J. Recent research progress on natural stilbenes in Dendrobium species. Molecules. 2022. https://doi.org/10.3390/molecules27217233.
Jang HY, Park HJ, Damodar K, Kim JK, Jun JG. Dihydrostilbenes and diarylpropanes: synthesis and in vitro pharmacological evaluation as potent nitric oxide production inhibition agents. Bioorg Med Chem Lett. 2016;26(22):5438–43. https://doi.org/10.1016/j.bmcl.2016.10.034.
Chien-Chih Chen Y-LH, Shiao Y-J, Huang R-L, Tai-Ti Fu, Shen C-C. Synthesis of antioxidative moscatilin and its bibenzyl derivatives. Chin Pharm J. 2003;55:381–90.
Wang X-L, Liu D, Xia Y-M, Cao X-P, Pan X-F. Ramberg-bäcklund rearrangement approaches to the synthesis of natural bibenzyls. Chin J Chem. 2010;22(5):467–72. https://doi.org/10.1002/cjoc.20040220515.
Yang M, Zhang Y, Chen L, Chen Y. A new (propylphenyl)bibenzyl derivative from Dendrobium williamsonii. Nat Prod Res. 2018;32(14):1699–705. https://doi.org/10.1080/14786419.2017.1396599.
Stanley LA. Drug metabolism. In: Pharmacognosy. Amsterdam: Elsevier; 2017. p. 527–45.
Hu A, Liu Q, Ouyang J. Identification and characterization of the metabolites of moscatilin in mouse, rat, dog, monkey and human hepatocytes by LC-Orbitrap-MS/MS combined with diagnostic fragment ions and accurate mass measurements. Biomed Chromatogr. 2023;37(4):e5573. https://doi.org/10.1002/bmc.5573.
Uyanik M, Nishioka K, Kondo R, Ishihara K. Chemoselective oxidative generation of ortho-quinone methides and tandem transformations. Nat Chem. 2020;12(4):353–62. https://doi.org/10.1038/s41557-020-0433-4.
Chen M-C, Kuo Y-C, Hsu C-M, Chen Y-L, Shen C-C, Teng C-M, et al. The apoptotic mechanisms of MT-6, a mitotic arrest inducer, in human ovarian cancer cells. Sci Rep. 2017;7(1):46149. https://doi.org/10.1038/srep46149.
Su W, Zeng L, Chen W. Moscatilin suppresses the breast cancer both in vitro and in vivo by inhibiting HDAC3. Dose-Response. 2021;19(1):15593258211001252. https://doi.org/10.1177/15593258211001251.
Chen W-K, Chen C-A, Chi C-W, Li L-H, Lin C-P, Shieh H-R, et al. Moscatilin inhibits growth of human esophageal cancer xenograft and sensitizes cancer cells to radiotherapy. J Clin Med. 2019;8(2):187.
Lee E, Han A-R, Nam B, Kim Y-R, Jin CH, Kim J-B, et al. Moscatilin induces apoptosis in human head and neck squamous cell carcinoma cells via JNK signaling pathway. Molecules. 2020;25(4):901.
Ho C-K, Chen C-C. Moscatilin from the orchid Dendrobrium loddigesii is a potential anticancer agent. Cancer Invest. 2003;21(5):729–36.
Chen D-N, Wang Y-Y, Liu W-J, Chen Y-J, Wu Y-P, Wang J-X, et al. Stilbenoids from aerial parts of Dendrobium plicatile. Nat Prod Res. 2020;34(3):323–8.
Lu X-X, Cao L-Y, Chen X, Xiao J, Zou Y, Chen Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res Int. 2016;2016(1):2476842.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226(2):380–93.
Ajithkumar P, Vasantharajan SS, Pattison S, McCall JL, Rodger EJ, Chatterjee A. Exploring potential epigenetic biomarkers for colorectal cancer metastasis. Int J Mol Sci. 2024. https://doi.org/10.3390/ijms25020874.
Gong C-Y, Lu B, Yang L, Wang L, Ji L-L. Bibenzyl from Dendrobium inhibits angiogenesis and its underlying mechanism. Yao xue xue bao= Acta Pharmaceutica Sinica. 2013;48(3):337–42.
Pujari I, Thomas A, Thomas J, Jhawar N, Guruprasad KP, Rai PS, et al. Cytotoxicity and radiosensitizing potency of moscatilin in cancer cells at low radiation doses of X-ray and UV-C. 3 Biotech. 2021;11(6):281. https://doi.org/10.1007/s13205-021-02827-3.
Fu X, Chen S, Xian S, Wu Q, Shi J, Zhou S. Dendrobium and its active ingredients: emerging role in liver protection. Biomed Pharmacother. 2023;157:114043. https://doi.org/10.1016/j.biopha.2022.114043.
Meng H, Li J, Dong Y, He Y, Ren H, Liu Y, et al. Poly traditional Chinese medicine formulation prepared with skin moisturizing properties. Dermatol Ther. 2020;33(6):e14105. https://doi.org/10.1111/dth.14105.
Aljeldah MM. Evaluation of the anticancer and antibacterial activities of moscatilin. Heliyon. 2024;10(10):e31131. https://doi.org/10.1016/j.heliyon.2024.e31131.
Udriștoiu AL, Ghenea AE, Udriștoiu Ș, Neaga M, Zlatian OM, Vasile CM, et al. COVID-19 and artificial intelligence: an approach to forecast the severity of diagnosis. Life. 2021;11(11):1281.
Popescu M, Terzea DC, Carsote M, Ghenea AE, Costache A, Popescu IAS, et al. COVID-19 infection: from stress-related cortisol levels to adrenal glands infarction. Rom J Morphol Embryol. 2022;63(1):39–48. https://doi.org/10.47162/rjme.63.1.03.
Acknowledgements
The authors would like to express their gratitude to Dr. Irina Zamfir, MD, RCP London, Basildon University Hospital UK, for providing professional English editing of this manuscript and for editorial support.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas. That is revising or critically reviewing the article, giving final approval of the version to be published, agreeing on the journal to which the article has been submitted, and confirming accountabilities for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors wish to confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Ethical approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva-Reis, R., Silva, V.L.M., Cardoso, S.M. et al. Moscatilin, a potential therapeutic agent for cancer treatment: insights into molecular mechanisms and clinical prospects. Med Oncol 41, 228 (2024). https://doi.org/10.1007/s12032-024-02467-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02467-6