Abstract
Neoadjuvant chemotherapy (NAC) improves overall survival in muscle-invasive bladder cancer (MIBC). Older patients often do not receive NAC due to its potential toxicities. We examined treatment patterns of elderly MIBC patients as well as impact of NAC on survival in this population. The National Cancer Database was queried from 2006 to 2019 for stage T2-T4a MIBC patients ≥ 80 years old. Treatment exposures (extirpative surgery; chemotherapy; radiation) were ascertained. Kaplan–Meier survival curves were generated based on treatment modalities (no treatment; radiation only; chemotherapy only; chemoradiation; surgery only; NAC with surgery). Multivariable Cox proportional hazards regression assessed associations with overall survival (OS). The cohort included 16,391 patients (mean age 86 years); 51% received treatment. MIBC treatment was less common with advancing age; patients receiving NAC then surgery were younger and had lower comorbidity scores. From 2006 to 2019, more patients received chemoradiation, while rates of NAC rose modestly. Median OS for the NAC with surgery group was 48 months versus 9 months for the no treatment group. Log-rank tests showed significantly improved survival in the NAC with surgery group compared to the surgery only group, while Cox proportional hazards regression analysis showed highest survival benefit in the NAC with surgery group. Only half of elderly MIBC patients received treatment, with fewer undergoing curative intent. NAC with surgery was associated with the greatest survival benefit. While our findings should be taken in the context of potential selection bias and patient preferences, they support NAC as part of shared-decision making regardless of age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer continues to inflict significant morbidity and mortality. In the United States, this malignancy is the sixth most common cancer and is the 10th most common cause of death by cancer [1]. Multiple phase III randomized controlled trials have shown that cisplatin-based neoadjuvant chemotherapy (NAC) improves overall survival in patients with muscle-invasive bladder cancer (MIBC). However, the median age of patients in these studies have generally been in the early to mid-sixties [2, 3]. In contrast, the average age at diagnosis for bladder cancer in the United States is about 73 years [4]. Although rates of NAC administration are increasing, there remains disparities in its administration, with older patients being less likely to receive this therapy [5]. We thus sought to evaluate treatment patterns for patients ≥ 80 years with MIBC, with a particular focus on NAC, as well as associations with survival outcomes based on treatment.
Methods
Data source
We used the National Cancer Database (NCDB) to identify cases of MIBC. The NCDB reports cancer cases from member facilities of the Commission on Cancer and captures more than 70% of all cancer diagnoses within the United States. Member facilities include a combination of academic centers and community cancer programs. Given that this study was conducted with deidentified data, it was determined to be exempt from review by the Oregon Health & Science University Institutional Review Board. The study was conducted in a manner consistent with STROBE reporting guidelines.
Study population and definitions of variables
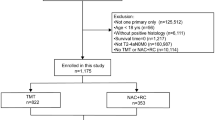
Using data from January 1, 2006 to December 31, 2019, we identified patients ≥ 80 years with clinical stage T2, T3, and T4a bladder cancer. We restricted eligible patients to those with urothelial cell carcinoma histology, those with bladder cancer as their sole cancer diagnosis or the first of multiple cancer diagnoses, as well as those who received at least part of their treatment at the reporting facility. Detailed exclusions are available in Fig. S1.
Demographic data collected included patient age, distance from reporting facility, insurance, income, education, facility data (academic versus non-academic), facility geographic location, year of diagnosis, Charlson-Deyo score, clinical T stage, and vital status. Of note, there was a very high proportion of missing values for income and educational status (> 60%), limiting its utility as a covariable. Treatment strategies identified included radiation, chemotherapy, chemoradiation, extirpative surgery, as well as NAC with surgery. The primary endpoint was overall survival (OS).
Statistical methods
Standard descriptive statistics were presented as frequencies and percentages for categorical variables, whereas means with standard deviations were obtained for continuous variables. The Kruskal–Wallis test was used to assess differences in distribution of continuous variables by treatment group, whereas the chi-square test was used for comparison of categorical variables.
Kaplan–Meier survival curves were generated to visualize survival estimates between different treatment groups. Log-rank tests were applied to compare survival distributions across treatment groups. Multivariable Cox proportional hazards regression analyses were conducted to assess associations with overall survivals. The criterion for statistical significance was p < 0.05. Data were analyzed using Statistical Analysis System version 9.4.
Results
The overall cohort included 16,391 patients, with a mean age of 86 years (Table S1). Fifty-one percent of patients were recorded as receiving treatment, and 3% underwent NAC with surgery. Significant differences were seen across treatment groups. The NAC with surgery group had a lower mean age of 83 years, were more likely to be treated at an academic facility and tended to have lower Charlson-Deyo scores.
Over the 13 year period of interest, surgery remained the dominant treatment type (Fig. S2). Chemoradiation showed a substantial increase in proportion, whereas NAC with surgery exhibited a very modest increase.
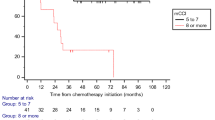
Median survival time was significantly higher for the NAC with surgery group at 48 months, compared to 9 months for those who received no treatment (Table 1). Kaplan–Meier curves also demonstrated significantly improved OS for the NAC with surgery group compared to the surgery only group (Fig. 1). Interestingly, the chemoradiation group had a similar median survival time with the surgery group.
On multivariable Cox regression analyses, significantly decreased hazards for mortality for the NAC with surgery group was demonstrated even after adjusting for patient factors, facility characteristics, and clinical T stage (Table 2). Compared to patients who received no subsequent treatment, the NAC with surgery group had reduced hazards for mortality by 58%, compared to 43% in the surgery only group.
Discussion
High-level data have consistently shown that NAC for patients with MIBC has a significant survival benefit. However, most of these data have been performed in patients younger than the average person with MIBC. Thus, we sought to evaluate survival outcomes in patients of advanced age. We found that only a small proportion of patients ≥ 80 years of age receive treatment for their MIBC with curative intent. NAC with surgery was associated with the greatest survival benefit.
Approximately 30–50% of patients with MIBC are thought to be eligible for cisplatin-based NAC [6], but recent estimates of NAC administration are about 21–32% [7]. These findings highlight that a substantial proportion of patients who are eligible for NAC may not be receiving this therapy. A prior study by Reardon and colleagues in 2015 showed that uptake of NAC is fortunately increasing, with 10% of patients who underwent radical cystectomy receiving it in 2006 per NCDB data compared to 21% in 2010. In that same study, factors associated with lower rates of NAC included advanced age, increasing comorbidity, lack of insurance, increased travel distance, as well as lower income.
More recently, a study by Andino and colleagues in 2021 found that 85% of patients at their institution (median age 66 years) were referred to medical oncology and 63% (212/339) ultimately received NAC [8]. Factors associated with decreased odds of receiving NAC included renal insufficiency, hearing loss, congestive heart failure, older age, and poorer functional status. Importantly, the authors did note that half of patients not referred to medical oncology lacked obvious medical contraindications for NAC.
Cisplatin ineligibility criteria proposed in 2011 by Galsky and colleagues in the setting of metastatic bladder cancer included: poor performance status; creatinine clearance < 60 ml/min; significant peripheral neuropathy; significant hearing loss; heart failure [9]. Advanced age is likely associated with impaired renal function could be strongly associated with advanced age, with estimates for the proportion of patients ≥ 85 years of age with creatinine clearance < 60 ml/min ranging from 55–90% depending on the equation used. We did not have data for renal function in this NCDB cohort, an important limitation. However, there are increasing arguments for use of cisplatin-based NAC in patients with baseline creatinine clearance between 40–60 ml/min [10].
Moreover, while age is certainly associated with poorer health and performance status, it should not disqualify a patient alone from treatment for MIBC. A prior study examining data from the Surveillance, Epidemiology, and End Results (SEER) registry showed significant reduction in cancer-specific and all-cause mortality in octogenarians who underwent surgery, a finding that was replicated in our study.
Although not a primary question our study attempted to address, our results interestingly suggested equivalence between surgery alone and chemoradiation. Retrospective data suggest that in select patients, trimodal therapy provides similar oncological outcomes to radical cystectomy [11].
Our study does have important limitations. The NCDB lacks cancer-specific survival data and granularity regarding chemotherapy regimens, such as use of gemcitabine-cisplatin versus MVAC regimens, as well as the number of cycles received. However, this could have potentially limited the proportion of patients receiving full cisplatin-based NAC and thus diluted survival benefit in the NAC with surgery group. Additionally, a small proportion of patents were clinically node positive and thus may have been receiving induction chemotherapy as opposed to true NAC. The NCDB also excludes patients who were not treated at a Commission on Cancer-accredited facility, potentially limited generalizability nationally. Patient selection bias is also a concern, as we could not ascertain potential contraindications for NAC as mentioned above, such as renal insufficiency, significant hearing loss or peripheral neuropathy, and poor functional status.
We did attempt to balance for this using Charlson-Deyo scores, but this is a weighted aggregate of comorbidities as opposed to a specific measure for NAC eligibility. Finally, our study did not capture treatment-associated comorbidities and patient preferences that may have been shaped by these adverse effects. Cisplatin-based NAC is not without potential significant adverse effects, notably irreversible nephrotoxicity, cumulative peripheral neuropathy, ototoxicity, as well as myelosuppression.
Overall though, our study demonstrates several interesting findings using NCDB data that encompasses over 70% of cancer diagnoses within the United States. Only about half of patients ≥ 80 years receive some form of treatment for MIBC, and even fewer receive treatment with curative intent. NAC with surgery was associated with the highest survival benefit, and chemoradiation was associated with similar survival outcomes with those who only received extirpative surgery. These findings expand on prior research demonstrating absolute survival benefit from NAC and support offering this therapy to eligible patients regardless of age. Underutilization of NAC may stem from urologists who act as “gatekeepers” for medical oncology referrals [12], but has been shown to be mitigated through standardized referral algorithms requiring patients to be seen by both surgical and medical oncology services [13]. Further research will be needed to analyze drivers of low administration rates of NAC in this patient population.
Data availability
Data are publicly available through the NCDB.
References
Common cancer sites–cancer stat facts. SEER. [Online]. https://seer.cancer.gov/statfacts/html/common.html. Accessed 24 Mar 2024.
Sherif A, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45(3):297–303. https://doi.org/10.1016/j.eururo.2003.09.019.
Grossman HB, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. https://doi.org/10.1056/NEJMoa022148.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551.
Reardon ZD, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol. 2015;67(1):165–70. https://doi.org/10.1016/j.eururo.2014.01.009.
Dash A, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107(3):506–13. https://doi.org/10.1002/cncr.22031.
Booth CM, Karim S, Brennan K, Siemens DR, Peng Y, Mackillop WJ. Perioperative chemotherapy for bladder cancer in the general population: are practice patterns finally changing? Urol Oncol. 2018;36(3):89.e13-89.e20. https://doi.org/10.1016/j.urolonc.2017.11.015.
Andino JJ, et al. Understanding the barriers to neoadjuvant chemotherapy in patients with muscle invasive bladder cancer: a quality improvement initiative. Urol Pract. 2021;8(2):217–25. https://doi.org/10.1097/UPJ.0000000000000200.
Galsky MD, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011;12(3):211–4. https://doi.org/10.1016/S1470-2045(10)70275-8.
Jiang DM, et al. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat Rev Urol. 2021;18(2):104–14. https://doi.org/10.1038/s41585-020-00404-6.
Zlotta AR, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023;24(6):669–81. https://doi.org/10.1016/S1470-2045(23)00170-5.
Patafio FM, Mackillop WJ, Feldman-Stewart D, Siemens DR, Booth CM. Why is perioperative chemotherapy for bladder cancer underutilized? Urol Oncol. 2014;32(4):391–5. https://doi.org/10.1016/j.urolonc.2013.11.003.
Rehman S, et al. Understanding avoidance, refusal, and abandonment of chemotherapy before and after cystectomy for bladder cancer. Urology. 2013;82(6):1370–5. https://doi.org/10.1016/j.urology.2013.07.055.
Funding
No funding was received to assist with the preparation of this study.
Author information
Authors and Affiliations
Contributions
WH Chou: project development, data analysis, manuscript writing/editing. A Wang: project development, data analysis, manuscript writing/editing. S Bassale: data collection, data analysis. E Latour: data collection, data analysis. S Isharwal: project development, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chou, W.H., Wang, A., Bassale, S. et al. Utilization of neoadjuvant chemotherapy for muscle-invasive bladder cancer in elderly patients: a retrospective cohort study. Med Oncol 41, 197 (2024). https://doi.org/10.1007/s12032-024-02430-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02430-5