Abstract
The immune system plays a pivotal role in the battle against cancer, serving as a formidable guardian in the ongoing fight against malignant cells. To combat these malignant cells, immunotherapy has emerged as a prevalent approach leveraging antibodies and peptides such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 to inhibit immune checkpoints and activate T lymphocytes. The optimization of gut microbiota plays a significant role in modulating the defense system in the body. This study explores the potential of certain gut-resident bacteria to amplify the impact of immunotherapy. Contemporary antibiotic treatments, which can impair gut flora, may diminish the efficacy of immune checkpoint blockers. Conversely, probiotics or fecal microbiota transplantation can help re-establish intestinal microflora equilibrium. Additionally, the gut microbiome has been implicated in various strategies to counteract immune resistance, thereby enhancing the success of cancer immunotherapy. This paper also acknowledges cutting-edge technologies such as nanotechnology, CAR-T therapy, ACT therapy, and oncolytic viruses in modulating gut microbiota. Thus, an exhaustive review of literature was performed to uncover the elusive link that could potentiate the gut microbiome’s role in augmenting the success of cancer immunotherapy.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A healthy human gut serves as a cocoon to multiple microorganisms and helps to thrive and replenish the ecosystem by creating a symbiotic relationship with the host. This has a significant impact on how diseases and human health are modulated. The intestinal epithelial layer acts as a home to a diverse range of microorganisms, which includes bacteria, fungus, viruses, archaea, and protozoa [1, 2]. The epithelial layer consists of mucosal immune cells which is responsible for maintaining the integrity of this barrier. The normal gut microbiota fulfills unique functions in the host, including processing nutrients, metabolizing foreign compounds and medications, maintaining the structural integrity of the intestinal mucosal barrier, regulating the immune system, and protecting against harmful pathogens [3, 4]. When the intestinal ecosystem is slightly modified, some commensal bacteria like Clostridium difficile or vancomycin-resistant enterococcus rapidly proliferate and gather pathogenic features. These are known as pathobionts [5, 6]. Within the gastrointestinal realm, the gut microbiome forms intricate partnerships with epithelial and stromal cells, demonstrating a diverse array of essential regulatory tasks. These include upholding the integrity of protective barriers, ensuring a harmonious balance in mucosal immune activity and the coexistence of host and microbes, defeating potential pathogenic invasions, curbing the proliferation of detrimental organisms, and regulating metabolic functions [7,8,9,10,11]. The gut microbiota is also responsible for metabolism of various nutrients, lipids, and xenobiotic drugs. The gut microbiota helps to synthesize vitamin K and multiple components of vitamin B. Recent studies have shown that diet plays a very important role to prevent cancer. Organic diets contain inactive polyphenols. Many plants, fruits, and plant-based items contain polyphenolic secondary compounds, such as flavanols, flavanones, flavan-3-ols, isoflavones, anthocyanidins, flavones, tannins, lignans, and chlorogenic acids. These inactive polyphenols are converted to their active form by biotransformation of the sugar moiety with the help of gut microbiota and are absorbed in the small intestine [12]. These flavonoids have the efficacy to scavenge free radicals, regulate cellular metabolism, and prevent oxidative stress thus participating in cell cycle arrest, inducing cell cycle and apoptosis and autophagy, and suppressing cancer cell proliferation and invasiveness [13].
The latest treatment for cancer in this century that has become a boon to the mankind is cancer immunotherapy. The immune system is utilized to provide a cytotoxic impact. The most novel immunotherapeutic agents for cancer immunotherapy is the utilization of immune checkpoint inhibitors (ICIs). These ICIs function using antibodies to block cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein 1/programmed cell death ligand 1 (PD1/PD-L1). Though ICI therapy has reached heights in cases of advanced hematologic malignancies but the main limitation of this therapy is that it causes subjects to inherit acquired or primary resistance, which restricts the broad clinical use of ICIs [14, 15].
In this context, we dive into the intricate interplay between the gut microbiome, dietary factors, cancer, immunology, and cancer immunotherapy, while also providing a concise overview of the relevant obstacles influencing treatment effectiveness and potential remedies.
Gut structure and composition of gut microbiota
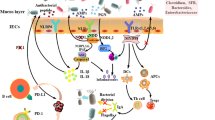
The human body serves as a home to several trillions of microbes [16]. These microbes constantly interact with the host by the symbiotic relationship they withhold with the multiple parts of human body, like the skin and the interior surfaces of mucosa [12]. The intestinal mucosa is the innermost layer of the intestine and is made up of three layers. The inner layer of intestinal mucosa is the epithelium where most the processes like digestion, absorption, and secretion occurs. It comprises intraepithelial lymphocytes and intestinal epithelial cells (IECs). Within the IECs, Paneth cells and goblet cells secrete mucus and antimicrobial peptides, respectively. A layer of connective tissue is also present within the mucosa which is called lamina propria [17]. This specialized structure helps to interact with the immune cells. Aggregated lymphoid nodules and numerous immune cells such as T, B, innate lymphoid cells, and antigen-presenting cells (APCs) are found in this layer at higher proportions [18]. This tissue found in the intestine is a prime example of the most substantial element within a living immune system, as it holds a pivotal function in both local and systemic immune responses. Apart from this, there is the muscularis mucosae, a thin layer of smooth muscle outside the lamina propria that facilitates the passage of body fluids and enhances peristalsis and agitation to improve the interaction between the lumen’s contents and the epithelial layer (Fig. 1) [19].
Structure and composition of gut microbiota illustrating the submucosal plexus (SMP), circular muscle (CM), myenteric plexus (MP), and longitudinal layer of muscle (LM). Within this layer, the enteroendocrine cells are present which are responsible for secretion of multiple hormones and peptides that regulate digestion, metabolism and absorption. The paneth cells and tuft cells also located in this layer secretes antimicrobial mediators that prevent the growth of microorganisms and prevent parasitic infections. The goblet cells and stem cells help in mucus secretion and helps to repair the damaged cells
The gut microbiota is composed of three “enterotypes” [20]. Bacteroides, Prevotella, or Ruminococcus are mainly prevalent in this region, without any biasness over nationality, age, or body mass [21,22,23]. Populations that consume high-fat diets, large amounts of animal protein, a sufficient quantity of amino acids, and saturated fats, while consuming very little fiber are more likely to have dominant Bacteroides and Bifidobacteriales. People consuming high quantity of carbohydrates and simple sugar and having low quantity of aforementioned products are more prone to be dominant to Prevotella [22]. As a result, Prevotella is more common in vegetarians, whereas people who are vegan have high prevalence of F. prausnitzii. Based on the available data, it appears that dietary habits have a significant influence on the enterotype level of gut microbiota [22,23,24]. Based on gut environment, an intriguing theory of high gene count (HGC) and low gene count (LGC) was identified from a Danish study, which involved multiple non-obese and obese individuals and both of them had separate impacts on health and disease (Fig. 2) [12].
Arrangement of microbes on the basis of multiple classification systems. A Arrangement of microbes on the basis of body levels. B Types of microbes on the basis of enterotypes. C Types of microbes on the basis of Danish study. The above figure demonstrates the location of numerous microbes at different pHs in the gastrointestinal tracts which are responsible for synthesis of multiple forms of vitamins and short chain fatty acids that helps in proper functioning of our body. HGC high gene count, LGC low gene count
Dietary influence on gut microbiota
To decode immunotherapy, the proper understanding between dietary factors and its impact on an individual’s gut flora is essential. Patients may experience varying responses to immunotherapy based on the makeup of their gut flora [25,26,27,28]. The constructiveness of immunotherapy can be directly impacted by the intestinal microbiota through interactions with drugs, or indirectly with the host’s natural immune system responds to the treatment [25, 26, 29, 30]. The impact of the side effects to the treatment can also be altered by gut bacteria [25]. Hence, the diet significantly influences the makeup of the gut microbiota. [22, 26, 29, 31,32,33,34,35].
Animal-based diet in contrast to a plant-based diet
According to a study conducted by David and his researchers performed a clinical trial between the two groups where one group consumed a heavy diet including meat and dairy products and the other group that consumed only plant-based foods. According to this study, the conformation of the gut microbiota was found to be significantly more impacted by an animal-based diet as they produced more fermented products of amino acids and fewer fermented products of carbohydrates in comparison to a diet gathered from plants (Fig. 3).
Multiple diets and its effects on various gut microbes. Diets are responsible for the modulation of both commensal bacteria and the harmful bacteria. The arrows pointing upward depicts the increase in commensal bacteria on consumption of that particular diet, whereas the arrows pointing downward depicts the reduction of microorganisms that may be harmful or destructive for our gut
The quantity of putrefactive, bile-tolerant bacteria like Bacteroides and Clostridia correlated favorably with the amount of amino acid fermentation products [21, 22]; conversely, the numbers of friendly bacteria like Bifidobacteria and Eubacteria [21, 22, 36] had a negative correlation with the amount of amino acid fermentation products. Furthermore, a diet high in saturated fats increased the populations of Bacteroides and anaerobic bacteria [21, 22, 36, 37]. Consuming protein-rich diet broadens the variety of gut flora, but the benefits vary depending on the source. Consuming whey and pea protein raises Bifidobacterium and Lactobacillus levels, whereas it restricts the growth of Clostridium perfringens and Bacteroides fragilis [34]. Pea protein also raises the levels of gastrointestinal short chain fatty acids (SCFAs). A high-fiber diet promotes the growth of Firmicutes and Proteobacteria, which are typically reduced in patients who consume a high-fat diet [22]. Ruminococcus development in the gut was facilitated by high polyunsaturated fat intake, whereas Bacteroides proliferated quickly, when diet rich in carbohydrates and simple sugars were provided [36].
Different kinds of cancer that may result from alterations in the gut microbiome
Before examining the special combination of interactions between immune system and the gut microbiota, it is important to recognize about the connections between the immune system, the microbiota, and cancer that are connected to the alimentary tract.
Colorectal cancer (CRC)
Florencia McAllister and his research team demonstrated the interaction between the immune system and the microbiota in case of colorectal cancer [38]. Nevertheless, more recent research has revealed other host microbiome relationships that may contribute to the development of CRC. Scientists have established that in the APC-mutated model for CRC, cell type-specific IL-1 responses can lead the disease to an advanced stage [39]. IL-1 when uncontrolled can lead to development of cancer. In addition, researchers observed that IL-1 activation in the myeloid cells released neutrophils that halted the course of colorectal cancer by preventing bacterial invasion in the tumor. IL-1 activation in IEC and T cells thus leads to carcinogenesis in a cell autonomous manner [39]. This emphasizes the importance of accounting for cell-specific reactions when developing and implementing IL-1 receptor agonists and antagonists.
Activating transcription factor (ATF6), a modulator of endoplasmic reticulum stress, is an essential component for the onset of CRC. When the ATF6 expression in CRC patients increases the disease-free survival time of patient decreases [40]. The mice harboring triggered form of ATF6 (nATF6IEC) in the IEC develop colonic adenomas on their own intestines. Particularly, these mice developed changes in the makeup of their microbiomes, and the application of antibiotics or germ-free housing markedly reduced or stopped the growth of tumors. Subsequently, the genetic removal of MyD88/TRIF, which are essential molecules for detecting bacteria, in nATF6IEC mice notably decreased tumor formation and occurrence [40]. Therefore, this study highlighted ATF6 as a key player in the complex interactions among the immune system, the microbiota, and colorectal cancer.
Chemokines, including CCL5, CXCL9, and CXCL10, are linked to the infiltration of cytotoxic T cells into tumors. Notably, the expression of these chemokines was correlated with specific bacterial taxa, such as proteobacteria, particularly methylobacteriaceae [41]. Additionally recent research has demonstrated that in both CRC patients and mouse models of the disease, the ileal microbiota, in particular Bacteroides fragilis and Erysipelotrichaceae, can induce ICD in ileal intestinal epithelial cells. Irrespective of the stability or instability of the microsatellite, this sort of cell death produces PD-1+follicular helper T cells that are interleukin-1 receptor 1-dependent and interleukin-12-dependent that ultimately impacts the effectiveness of chemotherapy and PD-1 restriction in colon carcinoma [42]. These results indicate the potential of employing microbial-based therapies or probiotics to increase the infiltration of beneficial T cell into tumors and extending patient’s life.
Liver cancer
Hepatocellular cancer (HCC) ranks third among all cancers in the world. Ten-year cycles of liver injury and inflammation are a major cause of hepatocellular carcinoma. Leaky gut and microbial translocation to the liver constitute the primary cause of HCC development. These variables trigger ongoing immunological responses and simultaneously, emphasizing how the microbiota affects this illness [43].
Advanced-stage HCC has been managed using the multi-kinase inhibitor Sorafenib, which demonstrates an objective response rate (ORR) of less than 5% [44]. Multiple studies demonstrated the effectiveness of immune checkpoint blockers (ICBs) on microbiota in individuals with numerous cancers, such as melanoma, lung cancer, and renal cell carcinoma [18, 45, 46]. This may also reflect to HCC as Zhang et al. demonstrated that non-responders had an enriched environment of proteobacteria and HCC patients who responded well to anti-pd-1 therapy had an enriched environment of Akkermansia muciniphila and Ruminococcaceae spp. [44]. This demonstrates that patients responded well to ICB therapy when the gut microbiome is enriched with certain microbial species.
Recent research indicates that there is a complex link between the development of hepatocellular carcinoma, bile acid metabolism, and the gut microbiota. Secondary bile acids that develop when certain colonic bacteria, such as Clostridium scindens, metabolize primary bile acids and the increment of the secondary bile acids have a tremendous impact on the immune system of liver [47]. Specifically in the liver, they can lead to a decline in availability of natural killer T (NKT) Cell [47]. These NKT cells are vital for halting cancer cells from spreading to the liver. Therefore, a reduction in NKT cells due to increased secondary bile acids could potentially contribute to the development and progression of HCC.
Pancreatic cancer
Understanding the relationship between the microbiome and pancreatic ductal adenocarcinoma (PDAC) is still developing, while there have been multiple reports that associates between certain bacteria, such as Porphyromonas gingivalis or Fusobacterium spp., and PDAC [48]. Justification of such microorganisms and microbial profiles linked to long-term survival (LTS) in PDAC in comparison to short-term survival (STS) is currently being worked upon. Pseudoxanthomonas, Streptomyces, Saccharopolyspora, and Bacillus Clausii were among the distinct bacterial signatures identified during the examination of the tumor microbiome in PDAC patients. It was discovered that patients with LTS had an abundance of these bacteria. Mice with PDAC tumors that received fecal microbial transfer (FMT) from LTS patients not only had a decreased tumor burden but also had an increase in intratumoral CD8+IFN-g+T cells, which are immune cells linked to antitumor immunity. Comparing donor samples revealed that 40% of the transplanted microbiome engrafted into the mice, reflected only 25% of the tumor microbiome in the fecal microbiome [49]. This suggests that in PDAC, antitumor immunity may be induced by a small number of bacteria from the tumor microbiome. Certainly, the interactions between the microbiome associated with tumors in the pancreas and its influence on PDAC development is evident. However, a thorough understanding of the specific mechanisms underlying these host–microbiome interactions will require further investigation and research.
Microbiome modulation improves cancer immunotherapy responses
Modulation of gut microbiota by utilizing antibiotics
It is broadly acknowledged that antibiotics possess the ability to instigate transformations in the intestinal microbiota, prompting the emergence of transiently stable or alternative equilibrium conditions. These newly established states may, in turn, develop resistance to external influences. The post-antibiotic dysbiosis generally causes loss of variation in flora, loss of some important taxa, shift in metabolism rate and increased susceptibility against invading pathogens [50]. In mice exposed to antibiotics or raised in a germ-free (GF) environment, the ability of tumor-infiltrating lymphocytes (TILs) to stimulate inflammatory cytokines like TNF and IL-12 in correspondence to CpG-oligodeoxynucleotides is diminished. Conversely, administering oral gavage with lipopolysaccharide (LPS) has been detected to superficially restore this reduced response. TNF-alpha, renowned for its versatile cytotoxic properties, collaborates with its receptors (TNFR-1/TNFR-2) to activate various signal transduction pathways, leading to varied functions such as inducing apoptosis and necrosis in tumor cells, promoting the release of other cytokines, and activating or recruiting immune cells to the infection site [52].
Reduction in bacterial variations
Use of antibiotics leads to reduced diversity of microbiota and research shows that restoration of microbial diversity for post-antibiotic treatment takes about one month in children [53]. Using a combination of gentamicin, vancomycin, and meropenem as treatment in adults induces a drop in butyrate-producing species and a rise in Enterobacteriaceae and other pathobionts [54]. The gut microbiota’s initial composition is effectively re-established in 1.5 months. Antibiotics often destroy the normal balance between pathogenic and non-pathogenic bacteria and causes overgrowth of hazardous C. difficile [55].
A decrease in variation does not imply a decrease in the total number of bacteria. Antibiotic-resistant microorganisms proliferate, replicate, and replace those microbes that were formerly susceptible to antibiotics. This could result in a decrease in species diversity and an increase in the microbial load. In a study, patients receiving wide spectrum antibiotics recognized a twofold increase in the bacteria load in their stool specimens after receiving β-lactam treatment for seven days and the investigation also revealed that there was an increase in the proportions of Bacteroidetes to Firmicutes [56].
Alternation in the metabolome
The term “metabolome” refers to the entire collection of small molecules of the biological system that have a mass of less than 1500 Da [57]. Young mice were used in the investigation, and the results showed that small dose of antibiotics caused obesity and elevated hormone levels that were directly related to the metabolism of fats, carbs, and cholesterol [2]. A different study revealed that administering imipenem and vancomycin together raised the amounts of sugars and arabinitol in the feces [58]. Arabinitol could not be converted to pentose sugar due to the reduction of Ruminococcaceae and Lachnospiraceae to their relative proportions by the use of vancomycin or imipenem. As soon as the vancomycin/imipenem treatment was restricted, there was a significant drop in arginine levels. This drop was correlated with a higher abundance of Escherichia and Shigella species and a lower abundance of Ruminococcaceae and Bacteroides in the gut microbiota. On the other hand, at the end of the 9 days of vancomycin/imipenem therapy, there was an increase in arginine levels that was associated with a higher frequency of Enterobacter genus organisms and a lower frequency of Alistipes. Notably, arginine functions as a predecessor for a number of immunoregulating substances [58].
Fecal microbiota transplantation
Willaim Coley, the father of cancer immunotherapy used a mixture of specific species like streptococcus and serratia to treat tumors [59, 60]. Today, bacterial treatments for cancer are proof based, targeted, and individualized due to our expanding understanding of the microbiome. Fecal microbiota transplantation was among the initial attempts to adjust the microbiome for the treatment of cancer (FMT). In simple terms, this means taking the stool from people who responded well to cancer immunotherapy and giving it to those who did not respond as effectively. This was originally demonstrated in tumor-bearing [MCA-205 or BRAFV600E/PTEN-/- (BP) syngeneic tumor cell line] GF mice that obtained FMT from immunotherapy-responsive cancer patients. The tumors in these mice were smaller, and the tumor-infiltrating CD8+ T cells were more prevalent within the tumors [18, 46]. This concept was then tested in two clinical trials involving melanoma patients who were not responding well to immunotherapy. The results were very much positive. The combination of FMT and immunotherapy are safe and, importantly, it helped reverse resistance to immunotherapy in some melanoma patients [61, 62]. These patients had more activated immune cells in their tumors and fewer myeloid cells producing a substance called IL-8, which is often linked to poor responses to immunotherapy [62, 63]. Comparing responding patients to non-responding ones, the number of intestinal bacterial taxa associated with the ICI response, such as Ruminococcaceae and Lachnospiraceae, was considerably higher in the responding patients [62]. Regardless of these encouraging results, questions remain regarding the reliability and possible side effects of FMT therapy, such as the emergence of drug-resistant infections. Researchers are working to address these concerns as they continue to explore the potential of bacterial treatments for cancer [64].
Live biotherapeutic products
In response to the demand for a more trustworthy, safer, and simpler-to-develop substitute for FMT, researchers have proposed the concept of “well-defined bacterial therapeutics”. These therapies may involve the administration of either a single strain of bacteria or a combination of various bacterial species, typically sourced from individuals in good health. The biggest benefit of these treatments is that, in order to alleviate safety worries, they can be examined for pathogenicity, virulence, or antibacterial resistance components. Additionally, scientists have created dependable and effective techniques for producing huge amounts of live biotherapeutic products (LBPs), which facilitates the production of LBPs in batches for therapeutic use (Fig. 4) [65]. The selection of these bacterial therapies can be dependent on how well they influence the immune system of individuals. For instance, in one study, researchers dosed germ-free mice with the feces of healthy individuals in order to investigate several bacterial strains. They were looking for strains that could stimulate the production of a substance called IFN-γ by CD8+ T cells. For the immune system to effectively combat cancer, this component is crucial. Using MC38 adenocarcinoma-engrafted GF or antibiotic-treated specific pathogen-free (SPF) mice as a consortium, they were able to identify 11 bacterial strains that significantly increased the effectiveness of anti-PD-1 antibody treatment [66]. Comparing these animals to those who did not get this treatment, the tumors were smaller and the number of CD8+ T cells producing IFN-γ inside the tumors was higher. Experimental and clinical studies are also being used to produce simple LBP therapies with a single species of bacteria. For example, they found that certain strains of Bifidobacterium were linked to better responses against tumors, when engrafted with B16.SIY tumors in wild-type C57BL/6 mice [67] and also improved ICI responsiveness in patients suffering melanoma [45]. Analogous to this, patients with non-small cell lung cancer (NSCLC) who reacted effectively to anti-PD-L1 treatment had higher Bifidobacterium prevalence than non-responders [68]. When MC38 colon cancer was injected into wild-type C57BL/6 mice, certain strains of B. bifidum worked in collaboration with anti-PD-1 antibody treatment to repel the embedded tumor. This led to a reduction in the size of the tumor and an increase in the number of tumor-infiltrating CD8+ and CD4+ T cells that produce IFN-gamma and IL-2 when compared to anti-PD-1 antibody therapy alone [68].
Modulation of gut microbiome to improve responses to cancer immunotherapy. Multiple factors such as antibiotics, diet, bacterial consortia, and fecal microbiota transplantation (FMT) can significantly influence the gut microbiome. Negative modulators (red arrows) like antibiotics and high-fat diets lead to a decrease in bacterial diversity while increasing the population of antibiotic-resistant strains. Conversely, positive modulators (blue arrows) such as plant-based diets, FMT, and bacterial consortia promote the growth of beneficial commensal bacteria. This favorable modulation of the gut microbiome can enhance the efficacy of cancer immunotherapy treatments by improving the immune response against tumor cells
Influence of consumables high in fat and fructose content
Dietary habits, such as consuming high-fat, high-fructose diets, can significantly influence the onset of certain cancers [69, 70]. This implies that altering the composition of gut bacteria through diet may help increase the beneficial effects of cancer immunotherapies [71]. Administering specific dietary elements called prebiotics, such as inulin or mucin, altered the microbial composition of wild-type mice that received syngeneic melanoma tumor cell transplants (YUMM1.5 cells) [72]. Consequently, tumor-infiltrating effector CD8+ and CD4+ T cells that combat cancer were more prevalent within the tumors, which were smaller overall. This was verified by demonstrating synergy in wild-type mice injected with CT26 colon cancer cells when these dietary ingredients were coupled with anti-PD-1 antibody. In contrast to anti-PD-1 antibody treatment alone, the tumors vanished and the survival rate dramatically graduated upwards. A recent observational study on individuals with melanoma examined the influence of gut bacteria on the effectiveness of immune checkpoint blockade (ICB) treatment in cancer patients, an area where the effects of diet and supplements are not well understood. The study analyzed fecal microbiota profiles, dietary habits, and the use of commercially available probiotic supplements among melanoma patients, alongside conducting parallel preclinical studies. In 128 patients undergoing ICB, higher dietary fiber intake was significantly linked to improved progression-free survival, particularly in those who had sufficient dietary fiber intake and did not use probiotics. These findings were supported by preclinical models, which showed that a low-fiber diet or probiotic use impaired the response to anti-programmed cell death 1 (anti–PD-1) therapy in mice. This was indicated by a lower frequency of interferon-gamma-positive cytotoxic T cells in the tumor microenvironment. These results have significant clinical implications for cancer patients receiving ICB therapy [73]. While more research is needed to fully understand these findings, it is suggested to have a look on the patient’s diet before starting immunotherapy. It also shows that changing diets to influence the gut bacteria could potentially make immunotherapy treatments more effective against cancer.
Modulation of microbiota with probiotics, prebiotics, synbiotics, and physical exercise
When the beneficial bacteria get eliminated from our body because of the cancer treatment regimens, this may often lead to mucositis, diarrhea, vomiting, constipation, and abdominal pain [74]. Probiotics and prebiotics are frequently given to cancer patients to help diminish the side effects of chemotherapy and radiation on the gastrointestinal tract and oral toxicity. Probiotics are live bacteria that maximizes the healthy advantages of the host body when given in sufficient amounts [74]. A study found that only 8.5% of patients who took probiotic supplements experienced side effects such as diarrhea, vomiting, allergies, constipation, and flatulence after using probiotics during chemotherapy or chemoradiotherapy, compared to 28.5% of all patients [75]. Clinical research showed that probiotics are safe because it is uncommon for them to induce infections, bacteremia, or sepsis [76]. Probiotics are an inexpensive and safe way to help cancer patients with diarrhea and infections and that was established via a comprehensive meta-analysis study among the disease bearing individuals [77].
Probiotics
An important research field proposed and conceptualized by Metchnikoff was “probiotics” [74, 78]. These are live microorganisms which when administered in sufficient amount can bring health benefits to the host body. Few studies that have been done till date demonstrate the potential advantages of probiotics on cancer treatments and recent research has concentrated on the effects of probiotics to prevent the formation and metagenesis of tumors or on the toxicity connected to cancer therapy [79]. A placebo-controlled, double-blind study with 490 patients when administered with a probiotic preparation consisting of 8 strains of lactic acid producing bacteria (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. Bulgaricus) known as VSL#3, rescued the patients from diarrhea brought on by radiation [80]. Combining probiotics for preventing dysbiosis in patients receiving chemoradiotherapy for nasopharyngeal carcinoma in a randomized controlled clinical trial not only improved the immune response of the patients but also decreased the risk of radiation-related complications, especially oral mucositis. The probiotic combination reduced the risk of developing a severe form of oral mucositis in patients by increasing the quantity of CD4+ and CD3+ T cells [81]. CRC patients when administered perioperatively with a probiotic combination of Lactobacillus acidophilus, Enterococcus faecalis, and Bifidobacterium longum reduced the frequency of diarrhea and a reduced period of intestinal healing [82]. Fecal samples from 100 CRC patients receiving chemotherapy were supplemented with Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus tablets to restore the optimal microbiota composition. Patients who developed toxicity in the gastrointestinal tract, when were administered with probiotics had a diminished toxicity than to the placebo group [83].
Tanoue and colleagues extracted 11 commensal strains from the feces of healthy volunteers in their study. It was identified that in the intestines of germ-free (GF) mice, these strains were strong inducers of interferon-releasing CD8+ T cells. The identified strains, which include Ruthenibacterium lactatiformans, Eubacterium limosum, Fusobacterium ulcerans, Phascolarctobacterium succinatutens, Bacteroides uniformis, Bacteroides dorei, Paraprevotellaxylaniphila, Parabacteroides distasonis, Parabacteroides johnsonii, Parabacteroides gordonii, and Alistipes senegalensis, are elements of the human microbiome that are comparatively uncommon and low in quantity [66] (Table 1). Furthermore, in mice-bearing MC38 tumors and residing in specific pathogen-free (SPF) conditions, the response to anti-PD1 or anti-CTLA-4 antibodies were notably heightened when the mice were colonized with these 11 strains. These findings from translational research imply that the administration of these bacteria could potentially enhance the effectiveness of immune checkpoint inhibitor therapy and chemotherapy in various rodent tumor models. This enhancement appears to occur by instigating dendritic cells to release interleukin-12 (IL-12) and simultaneously promote the replication of killer T lymphocytes that target the tumor [84].
In summary, these findings provide essential insights into the selection of specific strains for enhancing the effectiveness of cancer therapy. Nevertheless, delving deeper into the molecular mechanisms responsible for the individual impacts of commensal or probiotic strains appears to be logical for the next phase in optimizing the utilization of probiotics in conjunction with cancer treatment.
Prebiotics and synbiotics
Numerous components found in our food supply serve as essential nutrients for gut bacteria and in return these gut bacteria can metabolize these compounds into substances that help suppress tumor growth [85]. Prebiotics are indigestible or non-absorbable dietary fibers that are especially used by gut microbes [86]. They may help certain beneficial bacteria and the metabolites to thrive and populate the gut. These metabolites, in turn, may play a positive role to enhance the therapies that prevent tumor metastasis. Prebiotics are substances that allow bacteria such as Lactobacilli and Bifidobacteria to flourish in the small intestine by remaining unabsorbed, indigestible, and non-viable [87]. In addition, a prebiotic need to have the ability to restore the gut microbiota to a healthy state and be fermented by advantageous bacteria in the colon [76]. This ultimately leads to the production of short-chain fatty acids or SCFAs in the colon. Prebiotics have the ability to enhance immune system and bowel function in addition to modulating gut microbiota in an in vitro setting by fostering Lactobacillus plantarum L12 and Bifidobacterium pseudocatenulatum B7003 [88]. Prebiotics, however, are only as useful as long as these good bacteria are present in the host’s digestive system. Thus, using probiotics and prebiotics together, sometimes known as a “synbiotic”, shows a lot of potential in this area.
In individuals with advanced melanoma, inadequate responses to immunotherapy have been solely associated with a restricted diversity of the gut microbiota [18]. Additionally, poor results from allogeneic stem cell transplant recipients have been linked to this decreased variety [89]. Taking into considerations these findings, the use of pre- and synbiotic therapies becomes an important consideration both before and alongside cancer treatment, with the aim of preserving microbiome diversity and enhancing treatment efficacy [90]. In a study, including patients with periampullary tumors undergoing palliative or curative therapy, researchers from Brazil assessed postoperative infection rates and mortality rates (NCT0146877). The results of this study showed that patients treated with a combination of fructo-oligosaccharides and Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, and Bifidobacterium bifidum both before and after surgery had lower postoperative mortality and complications [91]. In addition, a phase II randomized study was started in 2019 with the goal of improving treatment efficacy and clinical outcomes for individuals with anal canal squamous cell cancer by evaluating the effects of probiotics as well as prebiotics during the last stage of chemotherapy-radiotherapy (NCT03870607). Prebiotics and synbiotics have the capability to enhance patient outcomes in oncological environments, opening up new ways to boost the effectiveness of anticancer therapy.
Physical activity
Numerous pieces of data indicate that lifestyle choices are extremely important when it comes to cancer prognosis, especially for people with colorectal cancer (CRC) [92]. Dysbiosis, an imbalance in the gut microbiota, can have a negative impact on skeletal muscle integrity, leading to muscle atrophy [93,94,95,96]. Muscle atrophy is an essential indicator of life expectancy in patients, particularly those with colorectal cancer (CRC) [97,98,99,100,101], as it plays a major role in the morbidity and mortality of many cancer types [102]. Recently, a new concept has emerged, emphasizing the potential interplay between the gut microbiome and skeletal muscle, termed the ‛gut-muscle’ axis [93]. The gut-muscle axis explains the potential effects of gut bacteria on muscle mass, quality, and function. For example, the reinstatement of commensal E. coli levels was found to hinder muscle atrophy in a mouse model of chronic gastrointestinal inflammation [103]. For instance, in case of colorectal cancer (CRC), a number of observational and experimental research have suggested exercise as a useful strategy for preventing CRC and reducing the side effects of disease and its treatment [104, 105]. However, the precise chemical process responsible for exercise’s protective effects against cancer have yet to be fully defined. Exercise may have positive impacts on immune system function, oxidative stress, inflammation, metabolism and hormone balance, obesity, gene regulation, and mitochondrial integrity [92, 104, 106] (Fig. 5). Exercise may also improve the body’s anticancer immune response and alter the structural microenvironment of tumors, according to certain research [44, 107]. All these effects of exercise may collectively influence tumor growth kinetics and metabolism [108, 109].
Gut microbiome modulation by prebiotics, probiotics and moderate exercise. Obesity plays a major role in dysbiosis and inflammation as they lead to the release of LPS which are very strong stimulators of our innate immunity that leads to inflammation and metabolic disturbances. Obesity and processed food are also responsible for reduction in SCFAs-producing bacteria which are beneficial for DNA repair and multiplication. Whereas, exercise and a diet rich in polyphenols help to proliferate beneficial bacteria thus helps in regulation of T-regulatory cells. These processed foods not only increase the TMAO levels in the blood plasma but also increases the H2S levels in the blood which has been associated with atherosclerosis and ultimately causing metabolic disturbances. LPA lipopolysaccharides, SCFAs short chain fatty acids, H2S hydrogen sulfide, TMA trimethylamine, TMAO triethylamine N-oxide
Given the ideal significance of the ‛gut-muscle’ configuration, the concept of using physical exercise to modify the microbiome is novel and has significant ramifications for science and society. This interplay needs further investigation in the context of cancer to strengthen the case for the inclusion of physical activity in the treatment of cancer patients.
The gut microbiota and the effectiveness of PD-1 and CTLA-4 inhibitors
In 2015, the relationship between the ICI efficacy in preclinical mice models and the gut microbiota was unwrapped by two researchers that were published in science. It was shown that in mice harboring commensal intestinal flora, such as Bifidobacterium species and bacteroides, CTLA-4 and PD-1 inhibition could inhibit the growth of tumors [67, 110].
Impact of gut microbiota on the effectiveness of anti-CTLA-4 therapy
The importance of the presence of certain particular commensals in toxicity and clinical manifestations was confirmed by Chaput et al. A study with a cohort of 26 patients treated with ipilimumab for metastatic melanoma demonstrated that those with a rich microbiome of Faecalibacterium had better PFS and OS than those with a low diversity or had a shortage of Firmicutes [111]. However, the patients who had a great diversity of this commensals demonstrated frequent occurrence of ipilimumab-induced colitis.
When anti-CTLA-4 antibodies are given, the bacterial population of clostridiales bacteria increases, whereas bacteroidales, and Burkholderiales decreases in the gut and furthermore dysbiosis is fostered. Nevertheless, even after anti-CTLA-4 antibody treatment, the amount of Bacteroides fragilis in the intestine stays unchanged [110, 112, 113]. B. fragilis acts in the GI tract and helps in prevention and curing inflammation [114]. Bacteroides fragilis has an immunomodulatory role and helps to boost the production of polysaccharide A in the CTLA-4 pathway and helps in upregulation of IL-10 and leads to reduced inflammation [115]. When given orally to germ-free mice, B. fragilis, Bacteroides thetaiotaomicron, or a combination of B. fragilis and Burkholderia cepacia, exhibit antitumor immune response comparable to that of mice with normal microbiota, resulting in a reduction in tumor growth [110]. The bacterial ability to cause dendritic cell maturation and concurrent IL-12 synthesis in the lamina propria is what restores the response. When an antigen is processed and presented to T lymphocytes for destruction, dendritic cells serve as antigen-presenting cells or APCs. This reaction involves the surface molecule CD11b, which is frequently seen on DCs [116]. Th1 cells, or T helper cells, are activated and stimulated to aid in the antitumor immune response by the secretion of IL-12 from dendritic cells [67, 110]. Vancomycin antibiotics typically reduce the presence of gram-positive bacteria in the gut while preserving gram-negative species, like Bacteroidales and Burkholderiales. Pre-treatment with vancomycin before anti-CTLA-4 therapy holds promise for cancer treatment. Nevertheless, antibiotic use can disrupt the microbiome, potentially leading to challenges such as allowing harmful bacteria to thrive in the human colon and eliminating bacteria essential for drug metabolism [117].
Impact of gut microbiota on the effectiveness of anti-PD-1 therapy
Unlike CTLA-4 inhibition, the PD-1/PD-L1 pathway does not require gut microbiota in an absolute sense. The presence of bacteria belonging to the genus Bifidobacterium, specifically B. breve and B. longum, is highly associated with a definitive response to anti-PD-1 therapy, but no specific species of bacteria is necessary for the efficacy of PD-1 blockage [67]. Furthermore, positive reactions to PD-1 therapy have been associated with elevated levels of Faecalibacterium prausnitzii and Akkermansia muciniphila in the gastrointestinal tract.
In mice with melanoma, oral treatment of B. breve and B. longum independently enhanced tumor proliferation to the same degree as PD-1 inhibition [46]. Complete reduction in tumor growth was achieved by combination therapy of anti-PD-1 antibodies and oral B. breve and B. longum [67]. Patients treated with anti-PD-1 antibodies having Bifidobacterium in GI tract helped to instigate the immune system to deliberately strike tumor cells [67].
An anaerobic bacteria called Akkermansia muciniphila is found in healthy people which plays a critical role in mucous catabolism [46]. According to Routy and colleagues, individuals with RCC or NSCLC who had more A. muciniphilia in their microbiome than non-responders responded better to anti-PD-1 therapy [45]. The complete reaction to PD-1 therapy is attributed to the increased generation of memory T cells, which trigger the release of IFN-gamma, ultimately resulting in the destruction of tumor cells. Thus, patients receiving PD-1 therapy responds to those who have abundance of A. muciniphilia [46].
F. prausnitzii, an obligate anaerobe present in the gut, helps in the prevention of colonic mucosa [46]. In case of melanoma, according to research by Gopalkrishnan et al. patients with a more diversified gut microbiome had extended progression-free survival than those with fewer bacterial species present in their GI tract. This discovery indicates that multiple kinds of gut bacteria, rather not just one, are necessary to boost the effectiveness of anti-PD-1 therapy survival [18].
Interaction between gut microbes and immune cells
At local and systemic levels, certain components of microbiota have the unique capability to generate a favorable clinical impact on the microbiome to activate innate and adaptive immune cells that can overcome the constraints of tumor micro-environment. Below, the possible potential mechanisms are demonstrated that may be helpful to understand the events.
Patterns associated with microbial molecules
Friendly gut bacteria or commensal bacteria immediately trigger the immune system upon noticing any threat. They recognize certain patterns on harmful microorganisms known as microbial-associated molecular patterns (MAMPs). These patterns act as red signals to our immune system. This MAMPs associate or engage themselves to pattern recognition receptors which are expressed on TLRs and nucleotide oligomerization domain (NOD)-like receptors (NLRs) of our innate immune cells [118]. For example, there are specific red flags called unmethylated CpG DNA motifs which act as immunostimulants. Numerous bacterial phyla, including actinobacteria, bacteroidetes, and proteobacteria [119], showcase them on their DNA. When these motifs attach to TLR9, our immune system recognizes them and as a result, myeloid cells release cytokines that promote inflammation, such as type I IFN (Fig. 6A) [120]. Preclinical research has demonstrated that when CpG motifs and ICIs are engrafted in mice with multiple tumor cell lines cultures like CT26, MCA238, or NHRI-HN1, they indicated smaller tumors and decrease in mortality rates [121, 122]. This combination causes polyfunctional CD8+ and CD4+ T lymphocytes [121] to accumulate intratumorally and synergize with the intratumoral production of inflammatory mediators, such as IFN-Gamma, IL-12, and tumor necrosis factor (TNF-α) [122].
Mechanism of action of gut microbiome. a Patterns associated with microbial molecules. The gut commensal bacteria interact with the harmful bacteria that leads to the release of MAMPs. These MAMPs binds to TLRs which triggers the TLR signaling pathway which activates the MyD88 and TRIF proteins which causes the activation of type I interferon genes (by Interferon Regulatory Factors). This results into the release of interferons that stimulates the myeloid cells to release cytokines and activation of immune system. b Antigen mimicry. The beneficial bacteria exert its epitopes to which the cancer cell producing specific codes gets attached as a result CD8+ T cells are activated which ultimately proliferate to activate the immune system. c Immunomodulatory metabolites. Beneficial gut microbes releasing SCFAs and inosine synergize with the anti-pd1 antibody thus prevents the interaction between PD1 and PD-L1 of the T cells and antigen-presenting cells (APCs) and simultaneously activating the immune system. MAMPs microbial-associated molecular patterns, TLRs toll-like receptors, MyD88 Myeloid differentiation primary response 88, TRIF TIR-domain-containing adapter-inducing interferon-β
Flagellin developed from a bacteria called Enterococcus gallinarum which is a strong immunostimulant, activate our immune system that produce substances like IL-12, IL-23, TNF-α, and IL-6 from monocyte-derived DCs [123]. These substances are good at fighting off cancer. Presence of this bacterium in the body can help to slow down the growth of tumors in mice with breast, renal, and lung cancers [124]. Another bacterium called A. muciniphila which is found abundantly in the intestine has robust response to ICIs [46, 125]. The response is caused due to the secretion of immunogenic phospholipid, which may stimulate DCs via the TLR1/TLR2 heterodimer. This results in the production of distinct cytokines such as IL-6 and TNF-α by DCs generated from mouse bone marrow (BMDCs), but IL-2 and IL-10 were not present [126]. In a mouse model of B16 melanoma implanted with ICIs, peptidoglycans molecules produced from enterococcus species enhances the response, lowering tumor growth and stimulating CD8+ T cells that leads to reactive mechanisms activated by NOD-2 signaling [127].
Antigenic imitation
Friendly bacteria or commensal bacteria can affect the anticancer responses by antigenic imitation or antigen mimicry. The term antigen mimicry refers to a process in which the bacteria or the microbes act like a key which is a secret code and the cancer cells behaves like a lock. These certain strains of bacteria acting like keys are similar to the pattern of codes found on cancer cells fits properly, it triggers a powerful response in our immune system. Here, the keys are referred to as the epitopes and the tumor derived epitopes are referred to as codes found on the cancer cells. As a result, some commensal bacterial strains carry epitopes that are analogous to MHC-restricted tumor-derived epitopes (Fig. 6B) [127, 128]. These epitopes stimulate CD8+ T lymphocytes, which are able to identify and interact with tumor cells, eliminate tumor cells, and decrease tumor growth [128, 129].
For example, one type of bacteria, Bifidobacterium breve has a key (SVY epitope) that is similar to a code (SIY neoantigen) which are demonstrated by the melanoma B16.SIY cell line (Bessell et al. 2020). Mice with wild-type DNA and devoid of B. breve demonstrated a lower quantity of tumor-infiltrating SVY-specific CD8 + T cells compared to mice infested with this bacterial species which led to a more rapid development of the tumor and a shorter longevity [128].
Cyclophosphamide and anti-PD-1 antibody were given to wild-type mice carrying the PSMB4-expressing MCA-205 sarcoma cell line, TMP1(key), another MHC-I binding prophage epitope, which is found on Enterococcus hirae, that is similar to a tumor antigen (PSMB4), resulted in diminution in tumor size and elevated infiltrating of CD8+ T Cells [129]. The reported antitumor response was not exhibited by E. hirae strains that either lacked or expressed mutant TMP1 [129].These findings are significant because they suggest a link between the presence of certain bacteria in the gut and how well cancer patients respond to treatments like anti-PD-1 antibodies. In simple terms, having the right bacteria with the right keys in the gut might help your body fight cancer better.
Immunomodulatory metabolites
The efficacy of ICI may be influenced by gut microbiota metabolites because they have the ability to alter systemic immune activities [130]. Short-chain fatty acids (SCFAs) produced by the microbiota, which include butyrate, propionate, kynurenines, and acetate, are engaged in a number of biological activities and have an effect on ICI therapy [131]. For instance, in patients with non-small cell lung cancer, elevated levels of propionate and butyrate in the stool sample have been linked to a superior response to anti-PD-1 antibody therapy. However, butyrate normally activates peroxisome proliferator-activated receptor gamma (PPAR-gamma), a nuclear receptor mostly produced in intestinal epithelial cells, with the purpose of maintaining homeostasis [132] and induces oxidative phosphorylation in colonocytes and stimulates the mitochondrial β-oxidation of SCFAs, hence preserving a local hypoxic microenvironment. In such environment the obligate anaerobic SCFAs-producing bacteria grow rapidly, while the facultative anaerobic enteric pathogens growth gets suppressed [132]. Higher levels of SCFAs in the feces were linked to patients having a longer time before their cancer worsened (progression-free survival) in cases of melanoma, renal cell carcinoma (RCC), and head and neck carcinoma when administered with anti-PD-1 antibodies (Fig. 6C) [133]. Similarly, when a specific gut bacteria called F. prausnitzii, which produces butyrate (a type of SCFA), was more abundant in the gut, melanoma patients treated with anti-PD-1 antibodies also demonstrated an extended progression-free survival [18]. However, patients suffering from melanoma having elevated levels of propionate and butyrate in the bloodstream when treated with a different type of immunotherapy such as anti-CTLA-4 antibody, did not produce a favorable clinical outcome [134]. These differences suggest that SCFAs might have varying effects depending on where in the body they are found and the specific type of cancer treatment used.
Multiple connections have been observed between the availability of particular metabolites, which are influenced by the microbiota, and the ICI response [131]. NSCLC patients comprising alanine and pyruvate in their blood serum responded well to anti-PD-1 antibody than to those who did not have these in their blood plasma [135].
Mechanisms associated with overcoming immune resistance by the gut microbiome
Several studies have indicated that bacteria may have varying effects on the T cell-mediated immune responses, possibly by transforming the tumor microenvironment (TME) from “cold” or suppressive to “hot” or responsive. To comprehensively address this issue, data from a range of microbial genera are needed to be analyzed, considering the multivariate nature of tumors and considering various inducing factors, for example, the TME and host features affecting the effectiveness of therapeutic index [51]. It is plausible that metabolic alterations triggered by the microbiome could orchestrate the TME, allowing T cell activities to be restored in order to combat immunotolerance brought on by tumors [136].
Enhancing the generation of cytokines
Recent research has shown a significant rise in IFN-gamma levels in the tumor-draining lymph nodes and the spleen after the introduction of commensal bacteria [67]. IFN-gamma is a vital cytokine in antitumor defense that helps not only to increase MHC expression not only on tumor cells but also in M1 macrophages. Thus, assisting in identifying and removing transformed cells [137]. IFN-gamma acts as a dimer, binding to specific receptor heterodimers to activate JAK1/2, which then controls STAT1 dimerization and genetic transcription initiation [138]. Studies on tumor-bearing mice undergoing CTLA-4 blocking therapy have also demonstrated improvements in IFN-gamma signaling in T cells [139]. The absence of IFN-gamma signaling has been associated with resistance to anti-CTLA-4 therapy [140]. Consistent with the above discoveries, microorganisms might have the ability to counteract resistance to immunotherapy by reactivating IFN-gamma signaling pathways.
Previous studies have consistently demonstrated that oral supplementation with beneficial microbiota following fecal microbiota transplantation (FMT) from non-responder (NR) donors can restore the effectiveness of PD-1 blockade. According to Routy and his colleagues, interleukin-12 may be necessary for this restoration and promote the engagement of CD4+ T lymphocytes to multiple tumor sites [46]. IL-12 serves as a flexible cytokine pivotal in coordinating the immune reaction against tumors through the activation of Th1 cells. It is chiefly generated by activated antigen-presenting cells, like dendritic cells (DCs) and hematopoietic phagocytes [141]. IL-12 is an intricate protein characterized by its heterodimeric structure, comprising covalently linked p35 and p40 subunits. The receptor for IL-12 is expressed on various immune cell types, including natural killer (NK) cells, T cells, and B lymphocytes. When ligands bind to the IL-12 receptor, it leads to sequential phosphorylation events on tyrosine residues, activating JAK2 and TYK2 kinases [52]. Recent studies indicate that inhibiting IL-12 using antibodies and IL-12 knockout (KO) mice effectively mitigates the exaggerated response observed in adoptive cell transfer (ACT). This confirms the reliance of the gut microbiome’s impact on adoptive transfer therapy on IL-12 [142]. Consequently, elevating IL-12 levels could improve the efficacy of immunotherapy by stimulating IFN-gamma production, fostering the generation and cytotoxicity of activated natural killer (NK) cells and T cells, and expediting the differentiation of CD4+ Th0 cells into the Th1 phenotype, thereby augmenting antibody-mediated cell cytotoxicity [143].
The biological impact of various cytokines demonstrates a synergistic interaction. Gut microbiota associated with significantly prolonged progression-free survival (PFS) maintain a robust response of cytokines to treatments targeting PD-1 [110]. Hence, cytokine production could serve as one of the mechanisms through which the gut microbiota influences resistance to cancer immunotherapy.
Fostering the stimulation of dendritic cells
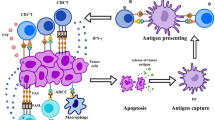
Recent studies conducted in animal models along with human patients suffering from advanced cancer have revealed that the gut microbiome regulates the initiation of tolerogenic macrophages and dendritic cells (DCs) during immunotherapy. In mice treated with Bifidobacterium, MHC-IIhi DCs were identified within tumors [144]. Furthermore, the oral delivery of B. fragilis initiated a Th1 immune reaction in the lymph nodes associated with the tumor and enhanced the development of DCs in the tumor microenvironment (TME). Consequently, this process aided in re-establishing the effectiveness of clinical treatments that inhibit CTLA-4 [145].
DCs play a pivotal role in antigen capture, transport, and processing. Upon receiving inflammatory signals, these cells transition into a mature state, during which they abandon their antigen uptake and processing functions. Concurrently, they upregulate chemokine receptor expression, which facilitates their relocation to sites of immune activity [52, 145]. The ability of DCs to initiate T cell responses is improved through several mechanisms, such as heightened surface expression of major histocompatibility complex (MHC) and costimulatory molecules, along with the elevation of soluble factors that impact the polarization of immune responses. PD-1 plays a crucial role in regulating host immunity by modulating the antigen response threshold and diminishing the cytotoxic function of CD8+ T cells [146]. Emerging studies have revealed that the expression of PD-1 on DCs markedly influences the secretion of IL-2 and IFN-gamma, which in turn attenuates the expansion of antigen-specific CD8+ T lymphocytes [147]. CTLA-4 expression decreases during differentiation into immature DCs but is substantially upregulated upon maturation. Elevated populations of CD8a+ dendritic cells in the spleens of mice treated with antibiotics and subjected to adoptive cell transfer exhibit improved cross-presentation of tumor antigens to CD8+ T lymphocytes. This enhancement is accompanied by an augmented release of Th1 cytokines, notably IL-12 and IFN-gamma, which are pivotal in the immune response [148].
Reducing the population of peripherally derived regulatory T cells (Tregs)
Regulatory T cells (Tregs), essential for preserving immunological self-tolerance, actively modulate immune responses by suppressing other immune cells. The transcription factor Foxp3, expressed by Tregs, is instrumental in curtailing cytokine production and preventing cellular interaction. Recent investigations have shown that when Tregs from germ-free mice are treated with propionate in a controlled environment, there is a significant upregulation of Foxp3 expression [149]. This indicates that SCFAs may have a selective role in promoting Treg induction. Additionally, the observed proliferation of Tregs after butyrate administration is linked to differentiation processes occurring in the body’s periphery. Moreover, propionate treatment has been associated with an increased formation of new Tregs in peripheral regions [52].
The blockade of the PD-1/PD-L1 pathway is known to potentiate the T cell response through the promotion of Treg differentiation. It is proposed that commensal bacteria and their metabolic by-products encourage the maturation and proliferation of Tregs, which leads to elevated CTLA-4 expression and an increased receptivity to CTLA-4 inhibition. This process potentially counteracts the immunosuppressive conditions within the tumor microenvironment (TME) [150]. Furthermore, short-chain fatty acids (SCFAs) are implicated in modulating both the build-up and function of Tregs in mice, thereby amplifying the efficacy of immunotherapeutic strategies [151].
Future prospects and innovative approaches
Modulation of gut microbiota by nanotechnology
Nanomaterials are broadly used in the last three decades for multiple cancer treatments. For clinical practice, nano-therapy system is rarely used and if ever used only first line therapy is applied like doxil or Abraxane [152]. Tissues having leaky endothelial wall contributes to the uptake and absorption of nanoparticles via the EPR effect through the porous capillaries and escape from opsonin proteins and being recognized from phagocytic cells [153]. These gets deposited at the tumor site to extend the penetrability and retention time [154] and manipulate the encapsulated drug discharge [155]. In order to improve their advanced functioning, such as impulse responsiveness, combined delivery and tissue targeting, the second-generation nanomaterials are currently undergoing clinical studies [156]. Third-generation nanomaterials possess sophisticated characteristics such as self-recognition [157], easy permeability across the biological barrier membrane [158], and immune system regulation [159].
In recent studies, the accumulation of copper nanoparticles (CuNPs) in the internal organs of Euodynerus crypticus (E. crypticus) was found to have a significance value of p < 0.05. Moreover, CuNPs were observed to bring alterations in the gut microbiota composition, particularly affecting bacterial species. Interestingly, CuNPs were also linked to a significant decrease (p < 0.05) in the variety and abundance of antibiotic-resistant genes in E. crypticus as elevation in Cu2+ concentrations can stop the horizontal gene transfer [160]. There are two distinct scenarios in which nanomaterials can be employed to influence the composition of the microbiota. Traditionally, antibiotics are used to treat infections while also changing the microbiota in the gut. However, the establishment of antibiotic resistance has been facilitated by the widespread implementation of broad-spectrum antibiotics. Wide range of nanomaterials have been used to target and eliminate specific bacterial species, including those that are known to cause cancer. For instance, nanomaterials possessing strong antibacterial properties have been used to compete against bacteria that causes cancer by producing reactive oxygen species [161,162,163,164]. It is essential to remember that these antimicrobial nanomaterials function as broad-spectrum antimicrobial agents rather than being specific to a particular kind of bacteria [165]. Consequently, targeted administration of antibacterial nanoparticles to particular bacterial species—like H. pylori—that are linked to stomach ulcers may improve the survival of advantageous microbial species that are normally negatively impacted by broad-spectrum antibiotics. Additionally, prebiotics can be carried by nanomaterials, which will boost the activity of specific bacterial species [166]. In the duodenum, for example, combination of curcumin and inulin as a nanoparticle formulate can release 90% of the curcumin and then synergize prebiotic aspects of inulin [167]. Furthermore, complex nanomaterial/prebiotic combinations can be delivered by nanomaterials, and this helps to control the metabolism of advantageous bacteria that have been shown to possess anticancer properties [168]. Through stimulus–response release mechanisms or by targeting certain microbial groups, the utilization of nanomaterial/prebiotic complexes is a unique method to tailor prebiotic delivery to specific microbial species within the gastrointestinal tract. The use of obsolete antibiotics has resulted in the development of antimicrobial resistance in bacteria that causes cancer, including F. nucleatum and C. butyricum. Therefore, nanomaterials present a viable remedy for this problem [169,170,171]. Various inorganic nanomaterials can also effectively eliminate bacteria by generating reactive oxygen species (ROS), which can synergize with antibiotics to enhance the eradication of toxic bacterial species that contributes to cancer [172]. This emphasizes how useful nanomaterials can be in combating antimicrobial resistance issues and in focusing on bacteria and other microorganisms linked to cancer.
Therapies involving chimeric antigen receptor T cells (CAR-Ts) and allogeneic hematopoietic cell transplantation (allo-HCT)
The use of ICI therapy has seen remarkable advancements in various solid tumor categories. Additionally, CAR-T cell therapy represents a groundbreaking advancement in cancer treatment, offering significant promise. While CAR-T cell treatment has yielded impressive clinical outcomes in specific types of B cell leukemia or lymphoma, numerous obstacles hinder its effectiveness in both solid tumors and hematological malignancies. Nonetheless, in the realm of hematological malignancies, CAR-T therapies directed at CD19 and allogeneic hematopoietic cell transplantation (allo-HCT) have emerged as pioneering and groundbreaking modalities in T cell-based cancer treatments [173]. Interest in the field of mucosal immunity, highlighting the impact of gut microbiota composition on the outcomes of allo-HCT and CAR-T immunotherapy have increased hastily in the recent times. Diverse communities of beneficial bacteria, particularly with a prevalence of Eubacterium limosum, have been linked to a reduced risk of progression and relapse following stem cell transplantation [174]. Conversely, an increase in enterococcus abundance due to broad-spectrum antibiotic usage has been associated with worsened graft-versus-host disease (GVHD) and poorer overall survival (OS) [175]. Smith and his coworkers found that variations in gut microbiota dominance prior to CD19-CAR-T therapy resulted in differing patient responses among those with B cell malignancies. Specifically, certain bacterial families such as oscillospiraceae, lachnospiraceae, and ruminococcaceae were enriched in patients who achieved complete responses (CR), whereas heightened levels of peptostreptococcaceae were correlated with resistance to anti-CD19 CAR-T cell treatment [176].
Another study conducted by Uribe-Herranz and collaborators explored the impact of vancomycin-induced gut microbiota imbalance on chimeric antigen receptor (CAR) T cell immunotherapy. In studies utilizing two mouse tumor models—CD19 ± A20 lymphoma and CD19 ± B16 melanoma—mice receiving a combination of vancomycin and CD19-specific chimeric antigen receptor T cell (CAR-T-19) therapy showed superior tumor suppression and increased cross-presentation of tumor-associated antigens (TAAs) relative to those treated with CAR-T-19 alone. Fecal microbiota transplants from healthy human donors into preconditioned mice resulted in outcomes akin to those in mice with intact gut microbiota. Furthermore, patients with B cell acute lymphoblastic leukemia undergoing CAR-T-19 therapy and given oral vancomycin experienced more pronounced CAR-T-19 cell expansion than those without vancomycin treatment [177].
These findings underscore the significance of gut microbiota in CAR-T cell therapy and propose that modifying the gut microbiota with vancomycin could potentially enhance outcomes following CAR-T cell therapy across various tumor types.
Gut microbiota and oncolytic virus
Emerging researches has highlighted the significant impact of gut microbiota on cancer therapy, including oncolytic virus therapy. Oncolytic viruses are a type of immunotherapy that utilizes viruses to selectively infect and kill cancer cells while sparing healthy cells. These viruses can be engineered or naturally occurring, and they exploit the unique vulnerabilities of cancer cells to replicate and spread within tumors, leading to their destruction. The interaction between gut microbiota and oncolytic virus therapy is multifaceted [178]. Studies have shown that the composition of the gut microbiota can influence the efficacy of oncolytic viruses by modulating the host immune response. Certain bacteria within the gut microbiota can enhance the antitumor immune response, thereby augmenting the therapeutic effects of oncolytic viruses. Conversely, dysbiosis or imbalance in the gut microbiota may impair the immune system’s ability to recognize and eliminate cancer cells, thereby limiting the effectiveness of oncolytic virus therapy. Additionally, the gut microbiota can influence the systemic immune response to oncolytic viruses, potentially impacting treatment outcomes. For example, specific bacterial species may regulate the production of pro-inflammatory cytokines or modulate the activity of immune cells involved in antitumor immunity [179].
Oncolytic viruses (OVs) predominantly serve as vectors for the delivery of specific checkpoint antibodies. These antibodies modulate immune checkpoints in the tumor microenvironment (TME), capitalizing on synergistic actions such as the secretion of cytokines and chemokines, oncolysis, and additional processes. This multifaceted approach aims to regulate the immune landscape within tumors effectively. Oncoytic viruses (OVs) that are genetically modified can autonomously synthesize checkpoint antibodies, which allows them to directly target and eradicate neoplastic cells within the tumor microenvironment (TME). As an illustration, an innovative recombinant myxoma virus, termed vPD-1, has been engineered to infect host cells and produce soluble PD-1. This mechanism activates and perpetuates anti-neoplastic responses, thereby providing a safety and efficacy profile that surpasses that of conventional PD-1 antibodies [180]. NK cells and CD8+ T cells rely on OV-mediated immune activation to amplify the effectiveness of antibodies that disrupt PD-1/PD-L1 or CTLA-4/B7 interactions [181]. OVs encoding specific protein genes, such as IFN-beta, can notably augment checkpoint blockade therapy. Vaccines based on oncolytic viruses like adenovirus and vaccinia virus are recognized for their robust oncolytic effects and their capacity to provoke immune reactions in cancer patients, especially in those with a sparse lymphocyte population in the TME [182]. The synergy between oncolytic viruses and gut microbiota is critical in modulating immune checkpoints in the TME, notably during colorectal cancer therapy involving anti-CTLA-4 and anti-PD-1 agents [179]. The gut microbiota and OVs synergistically enhance CRC therapy efficacy through several pathways. Microbial antigens from the gut, potential pathogenic entities, and OVs initiate pathogen-associated molecular patterns (PAMPs), which in turn activate signaling cascades, provoke inflammatory reactions, stimulate transcription factors, and lead to the secretion of pro-inflammatory cytokines and damage-associated molecular patterns (DAMPs). Simultaneously, the dense intestinal microbiota and OV infection generate molecules recognized by Toll-like receptors (TLRs), inducing inflammatory reactions and the release of IFN, other cytokines, and chemokines. Certain intestinal microorganisms, such as Bifidobacterium, can elicit immune responses via IFN gene stimulator (STING) and IFN-dependent pathways. OVs like HSV can inhibit the cGAS-STING pathway, thereby blocking IFN secretion and dampening the immune-inflammatory response elicited by intestinal bacteria [183]. Oncolytic viruses (OVs) are not only instrumental as gene carriers but also as stimulators of interferon (IFN) production. For example, herpes simplex virus 1 (HSV-1) activates plasmacytoid dendritic cells (pDCs) to produce IFN type I and prompts natural killer (NK) cells to secrete IFN type II through the TLR2/NF-kB pathway [184, 185]. In the context of gut microbiota, IFN type I signaling in dendritic cells is pivotal for enhancing the blockade of CD47, a process facilitated by Bifidobacterium. This signaling leads to increased cross-stimulation of DCs, thereby bolstering adaptive immune responses. Consequently, OVs may amplify the effectiveness of immunotherapies for colorectal cancer (CRC) by promoting IFN secretion in synergy with intestinal bacteria [186].
Adoptive cell transfer (ACT) therapy and gut microbiota
The efficacy of adoptive cell transfer (ACT) therapy after total body irradiation (TBI) in fighting cancer is significantly affected by the makeup of the gut microbiota. This is demonstrated by the noted reduction in therapeutic advantages when bacterial communities are diminished [112, 178, 187]. TBI stimulates the movement of gut microbes to secondary lymphoid organs, increases the levels of microbe-derived lipopolysaccharides (LPS) in circulation, and prompts dendritic cells (DCs) to generate pro-inflammatory cytokines. Conversely, the elimination of gut bacteria impedes microbial migration, reduces the activation of DCs, and weakens the antitumor immune response, thereby undermining the efficacy of ACT therapy. The interplay between the gut microbiota and ACT’s antitumor activity is mediated by the interaction of LPS with Toll-like receptor 4 (TLR4). Consequently, tumor-bearing mice lacking TLR4 fail to respond to ACT therapy, and the depletion of LPS diminishes the beneficial effects of TBI on tumor regression [188]. Additionally, studies conducted by Uribe-Herranz and colleagues revealed a significant impact of the native gut microbiome composition, antibiotic treatments, and heterologous fecal transfer on the efficacy of adoptive T cell therapy in tumor-bearing mice. Specifically, vancomycin-mediated depletion of bacteria led to a reduction in tumor growth rate in mice obtained from “The Jackson Laboratory” which were undergoing adoptive T cell therapy. Administering neomycin and metronidazole had no impact, emphasizing the significance of particular bacteria in regulating the host response. Treatment with vancomycin also led to a rise in systemic CD8α+ dendritic cells, supporting the systemic persistence of adoptively transferred antitumor T cells in an IL-12-dependent manner. Additionally, among individuals receiving allogeneic hematopoietic cell transplantation, oral vancomycin use was linked to increased IL-12 levels. These findings collectively underscore the crucial involvement of the gut microbiota in the antitumor efficacy of adoptive T cell therapy and suggest promising avenues for enhancing therapy response by modulating the gut microbiome [177, 189].
Conclusion
The era of understanding and controlling the gut microbiome not only depends upon animal studies and human trials but also have a substantial correlation with cancer immunotherapy. Previous findings portray a countless progress that has been made to improve the cancer therapy outcomes by discovering the influence of microbiome, but still the underlying mechanisms and the best ways to harness this knowledge is unexplored. One of the key prospects raised by this review is the need of microbiome profiling of cancer patients who are undergoing therapy. Furthermore, vast consideration is to be made on factors like mental health, obesity, and environmental effects and its impact on the gut microbiome and cancer therapy. There are some aspects that have also arises whether one should only monitor these factors or also modify these factors during the cancer treatment. As more and more delivery techniques based on nanoscience are getting established, the upcoming years should be focused and dedicated toward the subsequent effects and toxicity studies as they exhibit robust correlation with the commensal microbes which are specific for the treatment of cancer. Determining the optimal microbiome makeup to enhance anti-tumor immune responses, multiple methods are needed to be explored in modification of the gut microbiota to increase therapeutic responses. Yet, there are various ways to alter the microbiome, and careful testing of these approaches are needed to be done in clinical trials. In addition, multiple ways to maintain the intended alterations through dietary and prebiotic supplements, as well as proactive steps like administering antibiotics prior to microbiome modification are required. Ultimately, to fully grasp these complex interactions, extended comprehensive research in both animal models and clinical trials is necessary. In near future, there are certain avenues that needs to be explored to learn how to modify the gut microbiota and subsequently enhance immune function for effective and efficient cancer treatment. Further research is required to thoroughly map the profiles of beneficial commensal strains in different cancer types and monitor the evolving dynamics of gut microbiota during treatment. Additionally, there is a need to delve into the intricate role of gut microbiota in emerging forms of immunotherapy for future investigations. Simultaneously, the intricate interplay between the gut microbiota and emerging therapeutic modalities such as nanotechnology, CAR-T therapy, adoptive cell transfer therapy, and oncolytic viruses presents a fascinating frontier in cancer treatment. Understanding the dynamic relationship between the gut microbiota and these innovative approaches is crucial for optimizing therapeutic outcomes. Nanotechnology offers promising avenues for targeted drug delivery and precision medicine, while CAR-T therapy and adoptive cell transfer therapy harness the power of the immune system to combat cancer. Furthermore, the gut microbiota’s influence on systemic immune responses underscores its potential as a modifiable factor in enhancing treatment efficacy. Incorporating insights from microbiome research into the design and implementation of novel cancer therapies holds great promise for improving patient outcomes and advancing personalized medicine in oncology.
Data availability
Not Applicable.
Abbreviations
- ICIs:
-
Immune checkpoint inhibitors
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- PD1/PD-L1:
-
Programmed cell death protein 1/programmed cell death ligand 1
- IECS:
-
Intestinal epithelial cells
- APCs:
-
Antigen-presenting cells
- SMP:
-
Submucosal plexus
- CM:
-
Circular muscle
- MP:
-
Myenteric plexus
- LM:
-
Longitudinal layer of muscle
- HGC:
-
High gene count
- LGC:
-
Low gene count
- SCFAs:
-
Short chain fatty acids
- IL:
-
Interleukin
- CRC:
-
Colorectal cancer
- ATF6:
-
Activating transcription factor 6
- MyD88/TRIF:
-
Myeloid differentiation factor88/Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta
- CCL5:
-
CC-chemokine ligand 5
- CXCL9:
-
CXC chemokine ligand
- ICD:
-
Immunogenic cell death
- HCC:
-
Hepatocellular cancer
- ORR:
-
Objective response rate
- NKT:
-
Natural killer T cells
- PDAC:
-
Pancreatic ductal adenocarcinoma
- LTS:
-
Long-term survival
- STS:
-
Short-term survival
- FMT:
-
Fecal microbial transfer
- CD8:
-
Cluster of differentiation 8
- ESBL:
-
Extended-spectrum of beta-lactamase
- GVHD:
-
Graft versus host disease
- PAP2:
-
Phosphatic acid phosphatase type-2
- LBPs:
-
Live biotherapeutic products
- SPF:
-
Specific pathogen free
- NSCLC:
-
Non-small cell lung cancer
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- DCs:
-
Dendritic cells
- GI:
-
Gastrointestinal tract
- RCC:
-
Renal cell carcinoma
- MAMPs:
-
Microbial-associated molecular patterns
- TLRs:
-
Toll-like receptors
- NOD:
-
Nucleotide oligomerization domain
- NLRs:
-
NOD-like receptors
- PPAR:
-
Peroxisome proliferator-activated receptor gamma
- TNBC:
-
Triple-negative breast cancer
- AML:
-
Acute myeloid leukemia
- HCT:
-
Hematocrit test
References
Costello EK, Stagaman K, Dethlefsen L, et al. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–62.
Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–70.
Dzutsev A, Goldszmid RS, Viaud S, et al. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. 2015;45(1):17–31.
Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–28.
Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23(4):473–80.
Zechner EL. Inflammatory disease caused by intestinal pathobionts. Curr Opin Microbiol. 2017;35:64–9.
Chung WS, Walker AW, Louis P, et al. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016;14:3.
De Santis S, Cavalcanti E, Mastronardi M, et al. Nutritional keys for intestinal barrier modulation. Front Immunol. 2015;6:612.
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53.
Sommer F, Bäckhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–38.
Vaishnava S, Behrendt CL, Ismail AS, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105(52):20858–63.
Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–803.
Kopustinskiene DM, Jakstas V, Savickas A, et al. Flavonoids as anticancer agents. Nutrients. 2020;12(2):457.
O’Donnell JS, Long GV, Scolyer RA, et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71–81.
Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23.
Wang B, Yao M, Lv L, et al. The human microbiota in health and disease. Engineering. 2017;3(1):71–82.
Kong C, Faas MM, de Vos P, et al. Impact of dietary fibers in infant formulas on gut microbiota and the intestinal immune barrier. Food Funct. 2020;11(11):9445–67.
Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Ma J, Zhu W, Liu B. Role of gut microbiome in the outcome of cancer immunotherapy. In International Journal of Cancer. 2021;149(4):760–8.
Szczyrek M, Bitkowska P, Chunowski P, et al. Diet, microbiome, and cancer immunotherapy—a comprehensive review. Nutrients. 2021;13(7):2217.
David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
Bibbò S, Ianiro G, Giorgio V, et al. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;20(22):4742–9.
Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8.
Glick-Bauer M, Yeh MC. The health advantage of a vegan diet: exploring the gut microbiota connection. Nutrients. 2014;6(11):4822–38.
Villéger R, Lopès A, Carrier G, et al. Intestinal microbiota: a novel target to improve anti-tumor treatment? Int J Mol Sci. 2019;20(18):4584.
Brandi G, Frega G. Microbiota: overview and implication in immunotherapy-based cancer treatments. Int J Mol Sci. 2019;20(11):2699.
Shen H, Yang ES, Conry M, et al. Predictive biomarkers for immune checkpoint blockade and opportunities for combination therapies. Genes Dis. 2019;6(3):232–46.
Tarasiuk A, Fichna J. Gut microbiota: what is its place in pharmacology? Expert Rev Clin Pharmacol. 2019;12(10):921–30.
Klement RJ, Pazienza V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina (Kaunas). 2019;55(4):84.
Zhang C, Björkman A, Cai K, et al. Impact of a 3 months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908.
Bultman SJ. The microbiome and its potential as a cancer preventive intervention. Semin Oncol. 2016;43(1):97–106.
Zhang M, Yang XJ. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J Gastroenterol. 2016;22(40):8905–9.
Zhang N, Ju Z, Zuo T. Time for food: the impact of diet on gut microbiota and human health. Nutrition. 2018;51–52:80–5.
Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73.
Martinez KB, Leone V, Chang EB. Western diets, gut dysbiosis, and metabolic diseases: are they linked? Gut Microbes. 2017;8(2):130–42.
Soldati L, Di Renzo L, Jirillo E, et al. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16(1):75.
Hills RD Jr, Pontefract BA, Mishcon HR, et al. Gut Microbiome: profound implications for diet and disease. Nutrients. 2019;11(7):1613.
McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16(8):717–27.
Dmitrieva-Posocco O, Dzutsev A, Posocco DF, et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity. 2019;50(1):166-80.e7.
Coleman S, Smith IL, McGinnis E, et al. Clinical evaluation of a new pressure ulcer risk assessment instrument, the pressure ulcer risk primary or secondary evaluation tool (PURPOSE T). J Adv Nurs. 2018;74(2):407–24.
Cremonesi E, Governa V, Garzon JFG, et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67(11):1984–94.
Roberti MP, Yonekura S, Duong CPM, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020;26(6):919–31.
Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14(9):527–39.
Zhang X, Ashcraft KA, Betof Warner A, et al. Can exercise-induced modulation of the tumor physiologic microenvironment improve antitumor immunity? Cancer Res. 2019;79(10):2447–56.
Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–8.
Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018. https://doi.org/10.1126/science.aan5931.
Wei MY, Shi S, Liang C, et al. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol Cancer. 2019;18(1):97.
Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(4):795-806.e12.
Lange K, Buerger M, Stallmach A, et al. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34(3):260–8.
Marinelli L, Tenore GC, Novellino E. Probiotic species in the modulation of the anticancer immune response. Semin Cancer Biol. 2017;46:182–90. https://doi.org/10.1016/j.semcancer.2017.08.007.
Shui L, Yang X, Li J, et al. Gut microbiome as a potential factor for modulating resistance to cancer immunotherapy. Front Immunol. 2020;10:2989. https://doi.org/10.3389/fimmu.2019.02989.
Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016. https://doi.org/10.1126/scitranslmed.aad0917.
Palleja A, Mikkelsen KH, Forslund SK, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3(11):1255–65.
Ianiro G, Mullish BH, Kelly CR, et al. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut. 2020;69(9):1555–63.
Panda S, El Khader I, Casellas F, et al. Short-term effect of antibiotics on human gut microbiota. PloS ONE. 2014;9(4):e95476.
Lamichhane S, Sen P, Dickens AM, et al. Gut metabolome meets microbiome: A methodological perspective to understand the relationship between host and microbe. Methods. 2018;149:3–12.
Choo JM, Kanno T, Zain NM, et al. Divergent relationships between fecal microbiota and metabolome following distinct antibiotic-induced disruptions. mSphere. 2017. https://doi.org/10.1128/mSphere.00005-17.
Carlson RD, Flickinger JC JR, Snook AE. Talkin’ toxins: From coley’s to modern cancer immunotherapy. Toxins. 2020;12(4):241. https://doi.org/10.3390/toxins12040241
Sepich-Poore GD, Zitvogel L, Straussman R, et al. The microbiome and human cancer. Science. 2021. https://doi.org/10.1126/science.abc4552.
Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–9.
Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602.
Schalper KA, Carleton M, Zhou M, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune checkpoint inhibitors. Nat Med. 2020;26(5):688–92.
DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–50.
Rouanet A, Bolca S, Bru A, et al. Live biotherapeutic products, a road map for safety assessment. Front Med (Lausanne). 2020;7:237.
Tanoue T, Morita S, Plichta DR, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–5.
Sivan A, Corrales L, Hubert N, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Lee SH, Cho SY, Yoon Y, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021;6(3):277–88.
Kumar A, Lubet RA, Fox JT, et al. Effects of high-fructose diet vs. teklad diet in the mnu-induced rat mammary cancer model: altered tumorigenesis, metabolomics and tumor RNA expression. J Obes Chronic Dis. 2021;5(1):67–78.
Aardema ND, Rodriguez DM, Van Wettere AJ, et al. The western dietary pattern combined with vancomycin-mediated changes to the gut microbiome exacerbates colitis severity and colon tumorigenesis. Nutrients. 2021;13(3):881.
Li Y, Elmén L, Segota I, et al. Prebiotic-Induced anti-tumor immunity attenuates tumor growth. Cell Rep. 2020;30(6):1753-66.e6.
Han K, Nam J, Xu J, et al. Generation of systemic antitumour immunity via the in-situ modulation of the gut microbiome by an orally administered inulin gel. Nat Biomed Eng. 2021;5(11):1377–88.
Spencer CN, McQuade JL, Gopalakrishnan V, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–40.
Hill C, Guarner F, Reid G, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14.
Ciernikova S, Mego M, Semanova M, et al. Probiotic survey in cancer patients treated in the outpatient department in a comprehensive cancer center. Integr Cancer Ther. 2017;16(2):188–95.
Wang YH, Yao N, Wei KK, et al. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta-analysis. Eur J Clin Nutr. 2016;70(11):1246–53.
Hassan H, Rompola M, Glaser AW, et al. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Support Care Cancer. 2018;26(8):2503–9.
Food and Agriculture Organization of the United Nations; World Health Organization; Health Organization. Health and Nutritional Properties of Probiotics in Food including Powder Milk and Live Lactic Acid Bacteria. In Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO: Córdoba, Argentina, 2001.
Kristensen NB, Bryrup T, Allin KH, et al. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):52.
Delia P, Sansotta G, Donato V, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13(6):912–5.
Jiang C, Wang H, Xia C, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019;125(7):1081–90.
Yang Y, Xia Y, Chen H, et al. The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget. 2016;7(7):8432–40.
Huang F, Li S, Chen W, et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients. 2023;15(2):356.
Frankel AE, Deshmukh S, Reddy A, et al. Cancer immune checkpoint inhibitor therapy and the gut microbiota. Integr Cancer Ther. 2019;18:1534735419846379.
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72.
Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. https://doi.org/10.1038/nrgastro.2017.75.
Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr. 1999. https://doi.org/10.1093/jn/129.7.1438S.
Brownawell AM, Caers W, Gibson GR, et al. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr. 2012;142(5):962–74.
Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–82.
Scott AJ, Merrifield CA, Younes JA, et al. pre-, pro- and synbiotics in cancer prevention and treatment-a review of basic and clinical research. Ecancermedicalscience. 2018;12:869.
Sommacal HM, Bersch VP, Vitola SP, et al. Perioperative synbiotics decrease postoperative complications in periampullary neoplasms: a randomized, double-blind clinical trial. Nutr Cancer. 2015;67(3):457–62.
Song M, Chan AT. The potential role of exercise and nutrition in harnessing the immune system to improve colorectal cancer survival. Gastroenterology. 2018;155(3):596–600.
Lahiri S, Kim H, Garcia-Perez I, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aan5662.
Ticinesi A, Nouvenne A, Cerundolo N, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. 2019;11(7):1633.
Nay K, Jollet M, Goustard B, et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab. 2019;317(1):E158-71.
Yan H, Diao H, Xiao Y, et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci Rep. 2016;6:31786.
Chang KV, Chen JD, Wu WT, et al. Association between Loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. 2018;7(1):90–103.
Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–40.
van Vugt JLA, van den Braak RRJ, Lalmahomed ZS, et al. Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol. 2018;44(9):1354–60.
van der Kroft G, Bours DMJL, Janssen-Heijnen DM, et al. Value of sarcopenia assessed by computed tomography for the prediction of postoperative morbidity following oncological colorectal resection: a comparison with the malnutrition screening tool. Clin Nutr ESPEN. 2018;24:114–9.
Reisinger KW, Bosmans JW, Uittenbogaart M, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol. 2015;22(13):4445–52.
Qiu S, Jiang C, Zhou L. Physical activity and mortality in patients with colorectal cancer: a meta-analysis of prospective cohort studies. Eur J Cancer Prev. 2020;29(1):15–26.
Schieber AM, Lee YM, Chang MW, et al. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science. 2015;350(6260):558–63.
Oruç Z, Kaplan MA. Effect of exercise on colorectal cancer prevention and treatment. World J Gastrointest Oncol. 2019;11(5):348–66.
Courneya KS, Vardy JL, O’Callaghan CJ, et al. Effects of a structured exercise program on physical activity and fitness in colon cancer survivors: one year feasibility results from the CHALLENGE trial. Cancer Epidemiol Biomarkers Prev. 2016;25(6):969–77.
Ashcraft KA, Warner AB, Jones LW, et al. Exercise as adjunct therapy in cancer. Semin Radiat Oncol. 2019;29(1):16–24.
Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–62.
Hojman P, Gehl J, Christensen JF, et al. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10–21.
Idorn M, Thor SP. Exercise and cancer: from “healthy” to “therapeutic”? Cancer Immunol Immunother. 2017;66(5):667–71.
Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84.
Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–79.
Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–85.
Pitt JM, Vétizou M, Waldschmitt N, et al. Fine-tuning cancer immunotherapy: optimizing the gut microbiome. Cancer Res. 2016;76(16):4602–7.
Huang JY, Lee SM, Mazmanian SK. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe. 2011;17(4):137–41.
Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–5.
Sharpe AH, Abbas AK. T-cell costimulation–biology, therapeutic potential, and challenges. N Engl J Med. 2006;355(10):973–5.
Ma W, Mao Q, Xia W, Dong G, Yu C, Jiang F. Gut microbiota shapes the efficiency of cancer therapy. Front Microbiol. 2019;10:1050.
Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6(1):291.
Kant R, de Vos WM, Palva A, Satokari R. Immunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. J Med Microbiol. 2014;63(Pt 2):293–308.
Hemmi H, Kaisho T, Takeda K, et al. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol. 2003;170(6):3059–64.
Wang S, Campos J, Gallotta M, et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci USA. 2016;113(46):E7240-49.
Tseng JC, Yang JX, Liu YL, et al. Sharpening up tumor microenvironment to enhance the efficacy of immune checkpoint blockade on head and neck cancer using a CpG-oligodeoxynucleotide. Cancer Immunol Immunother. 2022;71(5):1115–28.
Lauté-Caly DL, Raftis EJ, Cowie P, et al. The flagellin of candidate live biotherapeutic Enterococcus gallinarum MRx0518 is a potent immunostimulant. Sci Rep. 2019;9(1):801.
Stevenson A, Panzica A, Holt A, et al. Host-microbe interactions mediating antitumorigenic effects of MRX0518, a gut microbiota-derived bacterial strain, in breast, renal and lung carcinoma. J Clin Oncol. 2018;36:e15006.
Derosa L, Routy B, Thomas AM, et al. Intestinal akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–24.
Bae M, Cassilly CD, Liu X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature. 2022;608(7921):168–73.
Griffin ME, Espinosa J, Becker JL, et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science. 2021;373(6558):1040–6.
Bessell CA, Isser A, Havel JJ, et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight. 2020;5(8):e135597.
Fluckiger A, Daillère R, Sassi M, et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 2020;369(6506):936–42.
Yang W, Cong Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol. 2021;18(4):866–77.
Botticelli A, Vernocchi P, Marini F, et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020;18(1):49.
Byndloss MX, Olsan EE, Rivera-Chávez F, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357(6351):570–5.
Nomura M, Nagatomo R, Doi K, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3(4):e202895.
Coutzac C, Jouniaux JM, Paci A, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020;11(1):2168.
Ghini V, Laera L, Fantechi B, et al. Metabolomics to assess response to immune checkpoint inhibitors in patients with non-small-cell lung cancer. Cancers (Basel). 2020;12(12):3574.
Viaud S, Daillère R, Boneca IG, et al. Gut microbiome and anticancer immune response: really hot Sh*t! Cell Death Differ. 2015;22(2):199–214. https://doi.org/10.1038/cdd.2014.56.
Kursunel MA, Esendagli G. The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev. 2016;31:73–81. https://doi.org/10.1016/j.cytogfr.2016.07.005.
Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. https://doi.org/10.1111/j.0105-2896.2004.00204.x.
Shi LZ, Fu T, Guan B, et al. Interdependent IL-7 and IFN-γ signalling in T-cell controls tumour eradication by combined α-CTLA-4+α-PD-1 therapy. Nat Commun. 2016;7:12335. https://doi.org/10.1038/ncomms12335.
Gao J, Shi LZ, Zhao H, et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167(2):397-404.e9. https://doi.org/10.1016/j.cell.2016.08.069.
Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunothe. 2014;63(5):419–35. https://doi.org/10.1007/s00262-014-1523-1.
Uribe-Herranz M, Bittinger K, Rafail S, Guedan S, et al. Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12. JCI insight. 2018;3(4):e94952. https://doi.org/10.1172/jci.insight.94952.
Luedke E, Jaime-Ramirez AC, Bhave N, et al. Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines. Surgery. 2012;152(3):431–40. https://doi.org/10.1016/j.surg.2012.05.035.
Kim YG, Udayanga KG, Totsuka N, et al. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe. 2014;15(1):95–102. https://doi.org/10.1016/j.chom.2013.12.010.
Hirvinen M, Rajecki M, Kapanen M, et al. Immunological effects of a tumor necrosis factor alpha-armed oncolytic adenovirus. Hum Gene Ther. 2015;26(3):134–44. https://doi.org/10.1089/hum.2014.069.
Sabado RL, Bhardwaj N. Dendritic cell immunotherapy. Ann NY Acad Sci. 2013;1284:31–45. https://doi.org/10.1111/nyas.12125.
Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–8. https://doi.org/10.1038/ni.2762.
Lim TS, Chew V, Sieow JL, et al. PD-1 expression on dendritic cells suppresses CD8+ T cell function and antitumor immunity. Oncoimmunology. 2015;5(3):e1085146. https://doi.org/10.1080/2162402X.2015.1085146.
Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. https://doi.org/10.1038/nature12726.
Chen X, Fosco D, Kline DE, et al. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol. 2014;44(9):2603–16. https://doi.org/10.1002/eji.201344423.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1(1):10–29.
Owens DE 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92.
Kamaly N, Yameen B, Wu J, et al. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–63.
Riaz Rajoka MS, Mehwish HM, Xiong Y, et al. Gut microbiota targeted nanomedicine for cancer therapy: challenges and future considerations. Trends Food Sci Technol. 2021;107:240–51.
Rodriguez PL, Harada T, Christian DA, et al. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–5.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv Mater. 2012;24(28):3724–346.
Zhang T, Hu W, Chen W. Plasma membrane integrates biophysical and biochemical regulation to trigger immune receptor functions. Front immunol. 2021;12:613185. https://doi.org/10.3389/fimmu.2021.613185
Ma J, Chen QL, O’Connor P, et al. Does soil CuO nanoparticles pollution alter the gut microbiota and resistome of enchytraeus crypticus? Environ Pollut. 2020;256:113463.
Vargas-Reus MA, Memarzadeh K, Huang J, et al. antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int J Antimicrob Agents. 2012;40(2):135–9.
Lu Z, Rong K, Li J, et al. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J Mater Sci Mater Med. 2013;24(6):1465–71.
Gao W, Thamphiwatana S, Angsantikul P, et al. Nanoparticle approaches against bacterial infections. WIREs Nanomed Nanobiotechnol. 2014;6(6):532–47.
Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511.
Cagno V, Andreozzi P, D’Alicarnasso M, et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater. 2018;17(2):195–203.
Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1-63.
Fares MM, Salem MS. Dissolution enhancement of curcumin via curcumin-prebiotic inulin nanoparticles. Drug Dev Ind Pharm. 2015;41(11):1785–92.
Wang T, Cai G, Qiu Y, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6(2):320–9.
Nyfors S, Könönen E, Syrjänen R, et al. Emergence of penicillin resistance among fusobacterium nucleatum populations of commensal oral flora during early childhood. J Antimicrobial Chemotherapy. 2003;51(1):107–12.
Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006. https://doi.org/10.1016/j.amjmed.2006.03.011.
Wu W, Yang Y, Sun G. Recent insights into antibiotic resistance in helicobacter pylori eradication. Gastroenterol Res Pract. 2012;2012:723183.
Li P, Li J, Wu C, et al. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnol. 2005;16:1912–7.
Schubert ML, Rohrbach R, Schmitt M, et al. The potential role of the intestinal micromilieu and individual microbes in the immunobiology of chimeric antigen receptor T-cell therapy. Front Immunol. 2021;12:670286. https://doi.org/10.3389/fimmu.2021.670286.
Peled JU, Devlin SM, Staffas A, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol. 2017;35(15):1650–9. https://doi.org/10.1200/JCO.2016.70.3348.
Stein-Thoeringer CK, Nichols KB, Lazrak A, et al. Lactose drives enterococcus expansion to promote graft-versus-host disease. Science. 2019. https://doi.org/10.1126/science.aax3760.
Smith M, Eric R, Littmann JB, Slingerland AC, Slingerland AE, Taur Y, Schluter J, et al. Intestinal microbiota composition prior to CAR T cell infusion correlates with efficacy and toxicity. Blood. 2018;132:3492. https://doi.org/10.1182/blood-2018-99-118628.
Uribe-Herranz M, Beghi S, Ruella M, et al. Modulation of the gut microbiota engages antigen cross-presentation to enhance antitumor effects of CAR T cell immunotherapy. Mol Ther. 2023;31(3):686–700. https://doi.org/10.1016/j.ymthe.2023.01.012.
Shi Y, Zheng W, Yang K, et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020;217(5):e20192282. https://doi.org/10.1084/jem.20192282.
Shi T, Ma Y, Yu L, et al. Cancer immunotherapy: a focus on the regulation of immune checkpoints. Int J Mol Sci. 2018;19(5):1389. https://doi.org/10.3390/ijms19051389.
Bartee MY, Dunlap KM, Bartee E. Tumor-localized secretion of soluble PD1 enhances oncolytic virotherapy. Can Res. 2017;77(11):2952–63. https://doi.org/10.1158/0008-5472.CAN-16-1638.
Rojas JJ, Sampath P, Hou W, et al. Defining effective combinations of immune checkpoint blockade and oncolytic virotherapy. Clin Cancer Res. 2015;21(24):5543–51. https://doi.org/10.1158/1078-0432.CCR-14-2009.
Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–9. https://doi.org/10.1158/1078-0432.CCR-13-0143.
Glorioso JC, Cohen JB, Goins WF, et al. Oncolytic HSV vectors and anti-tumor immunity. Curr Issues Mol biol. 2021;41:381–468. https://doi.org/10.21775/cimb.041.381.
Panagioti E, Kurokawa C, Viker K, et al. Immunostimulatory bacterial antigen-armed oncolytic measles virotherapy significantly increases the potency of anti-PD1 checkpoint therapy. J Clin Investig. 2021;131(13):e141614. https://doi.org/10.1172/JCI141614.
Wang Y, Jin J, Li Y, et al. NK cell tumor therapy modulated by UV-inactivated oncolytic herpes simplex virus type 2 and checkpoint inhibitors. Transl Res. 2022;240:64–86. https://doi.org/10.1016/j.trsl.2021.10.006.
Yi J, Lin P, Li Q, et al. A new strategy for treating colorectal cancer: regulating the influence of intestinal flora and oncolytic virus on interferon. Mol Ther Oncol. 2023;30:254–74. https://doi.org/10.1016/j.omto.2023.08.010.
Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13(18 Pt 1):5280–9. https://doi.org/10.1158/1078-0432.CCR-07-1378.
Huo S, Liu L, Liu J, et al. Modulation of cancer immunotherapy efficacy by gut microbiota. Discov Med. 2019;27(147):93–100.
Inamura K. Gut microbiota contributes towards immunomodulation against cancer: new frontiers in precision cancer therapeutics. Semin Cancer Biol. 2021;70:11–23. https://doi.org/10.1016/j.semcancer.2020.06.006.
Funding
This review received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RH conducted the literature search and selection and then wrote the manuscript; SC and AM designed the outline study, analyzed information, and then contributed to manuscript writing; SG designed and edited the figures for the manuscript. SR contributed to the study design and critically reviewed the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethical approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hazra, R., Chattopadhyay, S., Mallick, A. et al. Revealing the therapeutic properties of gut microbiota: transforming cancer immunotherapy from basic to clinical approaches. Med Oncol 41, 175 (2024). https://doi.org/10.1007/s12032-024-02416-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02416-3