Abstract
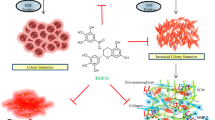
The handshake between the complex networks of matrix components in the tumor micro-environment (TME) is considered as a crucial event in the progression of several cancers including cervical carcinoma (CC). A number of studies report a connection between epidermal growth factor (EGF) and matrix component production. Studies demonstrate that the mechano-transduction trigger by collagen, influences the tumor cells to undergo epithelial–mesenchymal transition (EMT) and block the entry of drugs. We hypothesize that the intervention to prevent EGF triggered deposition of matrix components could sensitize several therapies for CC cells. We utilized morphological assessment, MTT assay, mitored tracking, acridine orange (AO)/ ethidium bromide (EtBr) staining and bromodeoxyuridine (BrdU) assay to measure the cell viability, mitochondrial activity, cellular apoptosis, and DNA synthesis. Clonogenic assay and scratch healing assay were executed to address the stemness and migratory potential. Detection of glycosaminoglycan’s (GAGs), collagen, matrix metalloproteinase (MMP)-2/9 secretion and calcium (Ca2+) ions were performed to assess the production of matrix components. Finally, the interaction between EGFR and plumbagin was evaluated by employing molecular dynamics (MD) simulation. Pre-treating the cells with plumbagin inhibited the EGF-induced EMT along with reduction in cell proliferation, migration, clonogenesis and depletion of matrix components. The actions of EGF and plumbagin were more pronounced in HPV-positive CC cells than HPV-negative CC cells. This study identified that increased matrix production triggered by EGF-rich milieu is inhibited by plumbagin in human papilloma viral (HPV) 68 positive ME180, HPV 16 positive SiHa and HPV-negative C33A cell lines. Delivery of plumbagin directly to TME would effectively accelerate the clearance of CC cells, reduce metastasis and matrix abundance by employing targeted delivery to minimize the undesired effects of plumbagin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of CC is raising at an alarming frequency due to limited awareness of vaccination and dysregulation of estrogen level. Considering an increase in CC, developed countries are insisting vaccination at an accelerated rate [1]. The virulence of HPV is greatly enhanced by EGF, which not only facilitates cellular transformation leading to increased migration and invasion, but also increases the deposition of matrix components that forms a barrier and prevents drugs access to CC site [2]. The bi-directional cross-talk between the CC cells and matrix components of TME is considered as the key feature for the initiation of EMT. ECM components during CC are known to exert mechanical force, alter the cellular stiffness and trigger EMT [3, 4]. To increase the access of therapeutic drugs, they are being conjugated with matrix degrading components such as hyaluronidase to improve the drug efficacy [5]. Failure to consider this fact during CC management would result in fibrosis and reduce the survival of CC patients. Tissue samples of locally advanced CC patients treated with chemo/radio-therapy showed aberrant protein expression of EGF receptor (EGFR), phosphorylated EGFR (pEGFR), phosphatase and tensin homolog (PTEN), phosphorylated AKR mouse strain thymoma (pAKT) and phosphorylated extracellular signal-regulated kinase (pERK). The increased translation of EGFR and pEGFR are highly allied with decline in patient survival along with the declined response to chemo/radio-therapy [6]. Presence of increased EGF could cause the residual cancer cells to rejuvenate and acquire stemness leading to tumor relapse. These observations collectively indicate EGF-rich milieu as highly responsible factor for CC progression and metastasis and tumor fibrosis [7].
ECM orchestrates a wide range of proteoglycan (PG) components like GAGs, heparan sulphate (HS), chondroitin sulfate (CS), dermatan sulfate (DS), keratin sulfate (KS), hyaluronic acid (HA), heparin, hyaletans, small leucine rich PGs (SLRPs) and proteinous components such as perlecan, argin, collagen, fibronectin, elastins, laminins glycol-conjugates, MMPs and cathepsins [3, 8]. Recently, we have shown that the presence of EGF micro-environment is associated with increased production of proteinous (collagenase) and non-proteinous matrix components (GAGs) [2, 9]. In addition, GAGs are utilized as HPV receptor (HS proteoglycan (HSPG)). Therefore, reduction of GAG levels could potentially reduce the HPV load [10, 11]. The sulfated region of HS and its core protein enables the binding ability of HSPG to ligands such as growth factors like EGF, cytokines, etc., and augment their function [12]. In order to overcome the matrix stiffness and rigidity, Kousar et al. [13] employed the chitosan nano-carrier coated with HA, for improved cisplatin release and CD44 targeted delivery. This approach increases the cellular uptake of the drug encapsulated in the nano-carrier along with increased bioavailability. Collagen, acts as a key player for the stabilization of the ECM and basement membrane of osseous tissue [14]. The increase in collagen expression aids in matrix rigidity, subsequent MMP synthesis and impair matrix turnover [15]. The transcriptional and translational levels of collagen are highly elevated by TGF-β signaling through EMT leading to CC progression [16]. These events thereby partake in the induction of cellular migration. Similarly, over-expression of collagen XVII is evident in CC tissues upon epigenetic hypomethylation in its promoter site leads to increased EMT and CC invasiveness [17]. Type IV collagen present in the basement membrane is an essential substrate for the MMP-2, whose expression was found to be elevated in EGF-induced CC cells [18]. Structural alterations in collagen are linked to increased biosynthesis of lysyl oxidase which triggers cyto-skeletal movements through mechano-receptor mediated process [19].
A comparative study conducted using 20 promising lead phyto-components in CC cells, identified plumbagin to reduce CC proliferation at much lower concentration [20]. Also, we found plumbagin to bind effectively to different domains of EGFR and attenuate its functions [9]. These observations prompted us to validate the utility of plumbagin, which effectively binds to EGFR under EGF-stimulated/unstimulated conditions in CC cells. This study is envisioned to address the efficacy of plumbagin during sustained EGF-stimulation in HPV-positive and HPV-negative cells.
Materials and methods
Cell culture and reagents
ME180, SiHa, and C33A cells were sourced from NCCS, Pune. The cells were cultured using DMEM media with 10% FBS in a 5% CO2 incubator at 37 °C. Plumbagin, Sirius Red (Sigma Aldrich); Mitored tracker (Thermo Fisher); Anti-BrdU primary antibody (Rockland); Alexa fluor-goat anti-rabbit secondary antibody (Invitrogen); Saffronin O (SRL) were utilized. AO, EtBr, alcian blue, alizarin red, and all supplementary cell culture reagents were obtained from Himedia.
Assessment of mitochondrial dehydrogenase activity
Exponentially growing ME180, SiHa and C33A cells at 1 X 104 cells per well were plated. After attachment, different concentration of plumbagin (0, 2, 4, 6, 8, and 10 µM) was added and incubated for 24 h to determine IC50. To address the ability of plumbagin in neutralizing the effects of EGF, IC50 concentration of plumbagin was used. The ability of plumbagin in EGF-unstimulated and stimulated conditions were assessed and the treatment paradigm is provided in Scheme 1. The cellular morphology was visualized and captured using ZOE™ Fluorescent Cell Imager after the incubation time, before the addition of MTT for three hours to assess mitochondrial dehydrogenase activity. The color generated was measured at 630 nm using BioRad multiplex ELISA reader. A graph was plotted to show the differences between the control and plumbagin treated cells.
Detection of mitochondrial membrane potential
1 × 104 cells per well in a 96 well plate was plated and treated as mentioned in Scheme 1. After 24 h, the mitored staining was accomplished as described previously [2].
Analysis of apoptotic index
1 × 104 cells per well in a 96 well plate was plated and treated with indicated group in Scheme 1 for 24 h. All cells tend to uptake AO and emit green fluorescence and the apoptotic or dead cells stain EtBr which can be measured using red fluorescence. The AO/EtBr staining was performed as priorly mentioned [9]. Representative fluorescent images were taken to compare the live/dead cell populations.
Determination of DNA synthesis
5 × 103 cells in a 96 well plate were seeded. After treatment for 24 h, briefly, the cells were pulsed with 10 µM of BrdU for 4 h at 37 °C. Then, the cells were then fixed using 10% formaldehyde, followed by permeabilization with 0.1% triton X-100 and subjected to acidic wash with 1 N and 2 N HCl. The cells were then incubated for 10 min with phosphate buffer (pH 7.4) and rinsed twice using permeabilization buffer for three minutes. It was then exposed to 1:500 dilution of anti-BrdU primary antibody and incubated overnight. Alexa fluor-goat anti-rabbit secondary antibody was added and incubated for one hour. The nucleus was counterstained using DAPI and the images were captured and merged using fluorescent microscopy.
Evaluation of clonogenesis
The 1 × 103 cells of ME180 and C33A were harvested from exponentially growing cells and were plated in a 12 well plate and treated as detailed in Scheme 1. After 24 h of treatment, the media were substituted with 2% FBS containing DMEM. The methodology was accomplished as formerly defined [2]. The visible colonies were photographed macroscopically and microscopically at 100× magnification using an inverted phase contrast microscope (Euromax, Holland). The colonies counted were represented graphically.
Measurement of cellular migration
Wound healing assay remains as an effective technique to visualize and measure the kinetics of migrating cells in a two-dimensional culture. Migration kinetics of cells was monitored using scratch healing assay as mentioned earlier [21]. The representative images were captured at 0, 6, 12, 24 h (ME180 cells) and 0, 12, 24, and 36 h (SiHa and C33A cells). The area covered is monitored and recorded by Biorad ZOE™ Fluorescent Cell Imager.
Evaluation of GAG sulphation by saffronin O staining
The ME180 and C33A cells were plated in a 12 well plate at a density of 1 × 105 cells per well and treated as mentioned in Scheme 1. The production of sulphated GAGs was determined using saffronin O staining. Briefly the cells were fixed using 0.1% glyceraldehyde for 30 min, subjected to PBS wash twice followed by 1% acetic acid wash and the cells were stained with 0.1% saffronin O for 10 min. The unbound dyes are removed by distilled water wash. The images were captured microscopically at 100X magnification using an inverted phase contrast microscope (Euromax, Holland).
Evaluation of GAG sulphation by alcian blue staining
1 × 105 cells per well was plated in a 12 well plate and treated as mentioned in Scheme 1. The production of sulphated GAGs was determined using alcian blue staining at pH 2.5. The intensity of sulphated GAGs was determined as described earlier [9].
Evaluation of collagen production
1 × 105 cells per well was plated in a 12 well plate and treated as detailed in Scheme 1. Sirius red is widely used to determine the presence of different collagen subtypes [22]. The collagen production was determined using sirius red staining as previously described [23].
Evaluation of Ca2+ deposition
1 × 105 cells per well was plated in a 12 well plate and treated as represented in Scheme 1. The cells were slightly rinsed with PBS and 1% alizarin red at pH 2.4 was added and incubated for 30 min in a rocker. The unbound dyes were removed upon thorough distilled water rinse and 70% ethanol was added and removed after two minutes for fixation. The cells were then replaced with distilled water and the microscopic images were captured at 100X magnification using an inverted phase contrast microscope (Euromax, Holland).
Detection of gelatinolytic activity
The conditioned media from ME180 and C33A cells were collected after treatment as detailed in Scheme 1 and spun at 1500 rpm to remove the cellular debris. The supernatant was concentrated 20 folds using BORG Lyo4 lyophilizer to perform gelatin zymography as detailed earlier [24].
MD simulation
MD simulation was performed to investigate the strength of binding constancy, conformational changes and peculiar inter-molecular relations of the EGFR–plumbagin complexes under the thermodynamics circumstance using Desmond. Initially, the EGFR–plumbagin complexes were imported into MD simulation panel and the system builder panel to solvate the EGFR–plumbagin complex with the help of simple point charge (SPC). Further, the orthorhombic water boundary box was generated for solvated system and the box size was set at X = 10 Å, Y = 10 Å, Z = 10 Å. Additionally, we added appropriate counter ions Na+ and Cl− to solvated system for the neutralization. Furthermore, for simulation, the isothermal-isobaric ensemble (NpT) group with continuous temperature at 300 k and pressure at 1 bar were applied to the system and OPLS3e force filed were applied to minimize the energy of the entire simulation system. Finally, MD simulation was performed for 100 ns for the complex and the trajectory interval of 100 ps and energy of 1.2 ps were recorded. After the simulation, the simulation event analysis panel was utilized to examine the constancy of the EGFR–plumbagin complexes using root mean square deviation (RMSD) followed by root mean square fluctuation (RMSF) and 2D diagram [25].
Statistical analysis
All the in vitro experiments were individually executed in triplicates and the Student’s ‘t’ test was employed to derive the statistical significance and the p value ≤ 0.05 was set as a limit.
Results
Plumbagin prevented morphological changes, EMT and cell population indicating its responsiveness towards EGF
A preliminary investigation spanning a wide range concentration (20–200 µM; 2–10 µM) of plumbagin identified 6 µM as its IC50 in ME180, SiHa, and C33A cells (Fig. 1A–D). The concentration of EGF at 50 ng/ml concentration is fixed based on previous work in order to create EGF micro-environment which is a causal factor for stemness, resistance, and relapse [21] in inducing EMT in ME180, SiHa, and C33A cells. EGF-stimulation in ME180 and SiHa cells showed EMT changes as evidenced by the morphological assessment indicate the features of mesenchymal stemness, as evidenced by spherical to spindle shaped morphology. Meanwhile, C33A cells showed minimal changes during morphological assessment upon EGF exposure. This indicates the increased propensity of HPV infected CC cells in responding to EGF than HPV negative CC cells. In order to understand the effect of plumbagin in CC cells under EGF-stimulated/unstimulated conditions, the cells were treated with plumbagin alone or pre-treated 30 min prior to EGF-stimulation for 24 h. Plumbagin decreased the cell population of ME180 (Fig. 1E), SiHa cells (Fig. 1F), and C33A cells (Fig. 1G) under EGF-stimulated/unstimulated conditions. The changes were converted into percentage viability and represented graphically (Fig. 1H). When compared with ME180 control cells, the EGF-stimulated cells exhibited 64% increase, the plumbagin treated cells showed 57.2% reduction and plumbagin pre-treated cells exhibited 40.3% reduction in ME180 cell population. Similarly, SiHa cells when stimulated with EGF showed 48% increase compared with control and exhibited 41% reduction in plumbagin treated cells and 23% in plumbagin pre-treated cells. Meanwhile, when compared with C33A control cells, the EGF-stimulated cells exhibited 30.6% increase, the plumbagin treated cells showed 45.2% reduction and plumbagin pre-treated cells exhibited 39.3% reduction in ME180 cell population.
The morphological representation of ME180 (A), SiHa (B) and C33A (C) cells treated for 24 h with different concentration of plumbagin (100 µm scale). The percentage cell population of plumbagin treated ME180, SiHa and C33A cells are indicated graphically (D). Alphabet ‘a’ indicates statistical significance at p value ≤ 0.05 when compared with control. The representative photo-micrographs of ME180 (E), SiHa (F) and C33A (G) cells depicts the morphological changes by plumbagin under EGF-stimulated/unstimulated conditions. The graphical depiction elucidates the percentage cell population of ME180, SiHa and C33A cells treated with plumbagin under EGF-stimulated/unstimulated conditions (H) for 24 h. Alphabet ‘a’ and ‘b’ indicates statistical significance at p value ≤ 0.05 when compared with control and EGF, respectively
Mitored tracking corroborates enhanced mitochondrial activity by EGF and its attenuation by plumbagin
To support the MTT assay based evaluation, the mitored tracking was performed, as the CC progression is highly dependent on its mitochondrial functional activities. The mitored dye diffuses passively into the plasma membrane and binds to the cysteine residues in the thiol group of active mitochondrial proteins. DAPI was used as a counterstain to witness the nuclear compartments of ME180 and C33A cells. ME180 and C33A cells stimulated with EGF showed higher mitochondrial activity as evidenced by intense staining by mitored. Plumbagin treated cells displayed dissipated mitochondrial membrane potential during EGF-stimulated/unstimulated conditions in ME180 (Fig. 2A) and C33A cells (Fig. 2B). The effects of plumbagin in reducing the mitochondrial membrane potential were found to be high in ME180 cells than C33A cells.
The representative fluorescent microscopy images at 100 µm scale depict the mitochondrial activity of ME180 (A) and C33A (B) cells through mitored tracking. The fluorescence intensity is indicative of mitochondrial membrane potential. EGF-stimulation increased the mitochondrial membrane potential and plumbagin inhibited the actions of EGF-induced mitochondrial membrane potential
Altered Mitochondrial Activity by EGF and Plumbagin Correlate with Regulated Apoptotic Index
The impairment in the activities of mitochondria by plumbagin could effectively lead to apoptosis through intrinsic/extrinsic pathways. To address this, we performed AO/EtBr staining to differentiate the proportion of live and apoptotic cells using fluorescence imaging. The control and EGF-stimulated CC cells displayed a typical nuclear chromatin emitting green fluorescence. Plumbagin treated cells under EGF-stimulated/unstimulated conditions showed increased red fluorescence indicating disrupted nuclear chromatin displaying the onset of late apoptosis in ME180 cells (Fig. 3A) and intense yellow indicates early apoptosis in C33A cells (Fig. 3B).
Plumbagin Mitigates DNA Synthesis in the EGF-Stimulated/Unstimulated Conditions upon BrdU Incorporation in HPV-Positive Cells
EGF is a known mitogen and has the ability to increase DNA synthesis in a number of cells in addition to differentiation. To understand the ability of plumbagin in regulating DNA synthesis under EGF-stimulated/unstimulated conditions, we utilized BrdU that is capable of incorporating in the new strands of DNA during S phase. To capture the incorporated BrdU, the primary BrdU antibody was used and the detection was performed by utilizing fluorescence tagged secondary antibody. DAPI was used as a counterstain to show the nuclear compartments of ME180 cells. The increased fluorescence signal indicates the quantum of BrdU incorporation, which in turn reflects enhanced DNA synthesis in EGF-stimulated ME180 cells. This increase in DNA after EGF-stimulation is not evident in plumbagin pre-treated cells indicates a key role for plumbagin. Representative fluorescence images indicating BrdU incorporated is captured in each group and shown in Fig. 4.
The fluorescent signals captured after BrdU pulsing depicts the enhanced DNA synthesis in EGF treated ME180 cells. Plumbagin treatment inhibited the DNA replication in EGF-stimulated and unstimulated conditions. The fluorescence images were visualized captured and documented using fluorescent microscopy
Plumbagin constrains the ability of colony formation in EGF-stimulated/unstimulated conditions
Another hallmark of EGF is its ability to influence a residual stem cell to develop into a clone, a cellular basis of tumor relapse condition. Therefore, we addressed the ability of EGF in augmenting clonogenesis as an index of relapse propensity. EGF was capable of promising clonogenesis in ME180 and C33A cells. Interestingly, plumbagin was able to arrest the clonogenesis in both cells under EGF-stimulated/unstimulated conditions and hints the drugability of this lead molecule in individuals with higher EGFR staining in fine needle aspiration cytology (FNAC) test. EGF-stimulated cells enhanced the quality of colonies in both the cell lines indicates the HPV independent actions for clonogenesis. To elaborate further, the quality of the colonies was macroscopically and microscopically compromised upon plumbagin treatment in ME180 and C33A cells (Fig. 5A, B) and the quantum of colonies were recorded graphically (Fig. 5C).
The macroscopic images of colonies formed in ME180 (A) and C33A (B) cells indicate enhanced clonogenesis by EGF and its inhibition by plumbagin. The microscopic images were captured using inverted phase contrast microscopy to assess the quality of clones. The quantum of colonies in ME180 and C33A cells were recorded graphically (C)
Kinetics of cellular migration is triggered by EGF-stimulation and inhibited by plumbagin
The actively proliferating and differentiating CC cells tend to acquire the ability to migrate. The cellular migration in healing the wound area was observed at different time elapse from 0, 6, 12, and 24 h for ME180 cells (Fig. 6A) and 0, 12, 24, and 48 h for SiHa (Fig. 6B) and C33A cells (Fig. 6C). The migratory ability of CC cells after EGF-stimulation was prominent in 24 h in ME180 cells and 36 h in SiHa cells whereas, the actions of EGF were not much pronounced in C33A cells. Plumbagin inhibited the migratory ability of EGF-stimulated/unstimulated conditions in both the cell lines tested. This observation is consistent with our earlier findings and indicates HPV infection as another key factor for migration of CC cells.
Plumbagin reduces EGF-induced matrix production which serves as scaffold for attachment of migrated cells
After ascertaining the enhanced cell migration, we determined the ability of CC cells to produce matrix components such as GAGs, collagen, Ca2+ and to reduce MMP-2/9. These components serve as scaffold and retain the migratory cells, provide appropriate mechanical support for attachment and to stretch to regain their cellular phenotype. The production of sulphated GAGs in both the cell lines were stained and visualized using saffronin O and alcian blue. EGF increased the stain intensity of saffronin O and alcian blue, whereas, plumbagin significantly reduced the sulphated GAG accumulation in ME180 (Fig. 7A, C) and C33A cells (Fig. 7B, D). The cells stained with alcian blue were quantified using guanidium HCl and are represented in Fig. 7E. An attempt was made to stain collagen using sirius red and the ability of plumbagin in inhibiting the cellular accumulation of collagen in EGF-stimulated/unstimulated conditions. CC cells stimulated with EGF were found with intense staining and plumbagin inhibited the collagen accumulation in ME180 (Fig. 7F) and C33A cells (Fig. 7G) and were represented graphically (Fig. 7H).
The microscopic images at 100X magnification denote the staining intensity of saffronin O specific for sulphated GAGs in ME180 (A) and C33A (B) cells. Similarly, the staining intensity of alcian blue specific for sulphated GAGs were represented in ME180 (C) and C33A (D) cells. The EGF treated cells show increased alcian blue stain intensity denoting abundance in GAG sulphation and its inhibition by plumbagin treatment (E). The staining by sirius red shows the collagen deposition. EGF treated ME180 (F) and C33A (G) cells display increased collagen deposition and its inhibition by plumbagin. The graphical representation denotes the sirius red stain intensity of sirius red which is directly proportional to collagen accumulation (H). Alphabet ‘a’ and ‘b’ indicates statistical significance at p value ≤ 0.05 when compared with control and EGF, respectively. Representative zymographic images show the activities of MMP-2/9 in ME180 (I) and C33A (J) cells were determined upon plumbagin treatment. The MMPs activity is determined by measuring the intensity of gelatin digestion, which is captured and documented using Biorad Chemidoc. An increase in MMP-9 activity correlates with the reduction in collagen deposition. The Ca2+ ion deposition in ME180 (K) and C33A (L) cells were determined using alizarin red. It showed increased staining in EGF treated cells and its reduction by plumbagin
To provide more insights on collagen accumulation, we evaluated the activity of MMP-2/9 in the conditioned media as an index to understand the matrix turnover. MMP-2/9 are the key enzymes that predominantly participate in altering matrix components. Plumbagin was concentration dependently treated in ME180 and C33A cells under EGF-unstimulated conditions and the conditioned media was assessed for gelatinolytic activity. Surprisingly, a concentration dependent increase in the levels of MMP-9 after stimulation with plumbagin was identified, which can be linked to reduced levels of collagen observed in the present study. However, plumbagin could not interfere with the activity of MMP-2 in both ME180 (Fig. 7I) and C33A cells (Fig. 7J). The relative levels of MMP-2/9 could determine the efficacy of drugs. EGF-stimulated cells were found with increased Ca2+ ion accumulation and these actions were inhibited by plumbagin alone and plumbagin pre-treated ME180 (Fig. 7K) and C33A cells (Fig. 7L). Collectively, plumbagin effectively blocked the ability of EGF to produce matrix components and can be considered to reduce tumor fibrosis.
MD simulation of EGFR–plumbagin complex
The binding stability of EGFR–plumbagin was studied using MD simulation analysis. The RMSD of EGFR–plumbagin was represented in Fig. 8A. We observed the major fluctuation for protein and ligand RMSD at 2 ns, with the RMSD 12.4 Å, 22.4 Å, respectively. It is followed by the increased stability of the complex till entire simulation with an observed RMSD value of 7.3 Å, 10.2 Å, respectively. The bar diagram represents EGFR–plumbagin interactions (Fig. 8B). Hydrogen bond interactions were observed with Met769 and the occupancy was noted 11.8%. The overall EGFR–plumbagin interactions were observed after the simulation (Fig. 8C). The amino acid residue Met769 was involved in the hydrogen bond formation with maximum occupancy 78%, interestingly, the Met769 was predominantly reported to enhance the binding constancy of the EGFR–plumbagin during the course of simulation.
The selected protein–ligand complex was verified using RMSD and RMSF to ascertain the stability and interaction. The RMSD plot of EGFR–plumbagin during 100 ns simulations is indicated (A). The RMSF bar diagram depicts the EGFR–plumbagin interactions during 100 ns simulation (B). The 2D diagram of EGFR–plumbagin complex at the termination of the 100 ns simulation (C) indicates a crucial role for Met769 in EGFR for binding with plumbagin
Discussion
Monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs) are usually regarded as sub-curative therapies when administrated individually. Thus, mAbs and TKIs are often prescribed in combination with chemo/radio-therapy to the patients with metastatic CC or tumor recurrence. Recently, the utilization of nanocarriers/liposomal mediated drug delivery system are being employed to improve the targeted therapy accompanied by increased bioavailability and reduced toxicity to the adjacent tissues [26]. However, these treatment approaches lack responsiveness in the host body because of resistance mechanism and complex matrix abundancy that block drug entry. Thus, it is crucial to identify a lead molecule that is capable of crossing the ECM and inhibit the CC progression and metastasis. An attempt was made to exogenously induce the stemness by creating an EGF-stimulated ambience and ascertain the efficacy of plumbagin in reducing the stemness properties viz., morphological assessment, inhibit clone formation, ability to migrate and lay down matrix component production. ME180 cells were chosen due to its increased receptivity towards EGF and increased EGFR expression when compared with SiHa and are considered as the best cell line model to study CC metastasis. HPV-negative C33A cells were simultaneously studied to compare the responsiveness of ME180, SiHa, and C33A cells towards EGF and the inhibitory role of plumbagin.
The alterations in the ECM are highly associated with the differentiation of CC cells and migration. The induction of matrix stiffness is a hallmark during CC progression, metastasis and tumor recurrence. Initially, the morphological evidences were corroborated by the cellular population and mitochondrial membrane potential. Similarly, a study conducted by Sidhu and Capalash [27] showed the inhibitory effects of plumbagin in decreasing the mitochondrial membrane potential in CC cells. Plumbagin exerted reduced growth of ME180 under EGF-stimulated/unstimulated conditions and it is supported by reduced BrdU incorporation. Jaiswal et al. [28] stated that plumbagin is capable of inducing the cell cycle arrest at S/G2 and G2 phase in HeLa and SiHa cells, respectively. This indicates plumbagin treated and pre-treated groups have impairment in DNA synthesis/S phase in ME180 cells. The observed reduction in the DNA synthesis by plumbagin may involve direct inhibition of DNA polymerase or attenuate its activation by impairing the components of mitogenic signaling. Upon modulating the cell cycle arrest, plumbagin is capable of inducing the apoptosis, as evidenced by AO/EtBr staining. Increased early and late apoptotic cells observed in the plumbagin treated group indicates its ability to mediate apoptosis. Plumbagin, in combination with 2 Gy radiation was previously identified to exert apoptosis by activating caspase dependent apoptotic pathway, altering the ratio between Bax, Bcl2 and decreasing the expression of surviving in SiHa, HeLa, and C33A cells [29]. Since, radio-resistance is linked to nuclear EGFR phosphorylation [21], the radio-sensitizing effects of plumbagin is supported by the aforementioned statement. Similarly, Jaiswal et al. [28] identified that plumbagin induced apoptosis by increasing the expression of Bax, decreasing the levels of Bcl2, and by activating caspase 3,9 and its downstream target poly (ADP-ribose) polymerase (PARP) in HeLa and SiHa cells. These apoptotic events are mediated by the release of cytochrome C, translocation of mitochondrial apoptosis inducible factor 1 (AIF1) into the nucleus leading to activation of caspase dependent apoptotic pathway, resulting in increased chromatin condensation, DNA fragmentation, and cellular apoptosis [29]. The apoptosis data obtained in the present study supports these observations and extends these effects under EGF-stimulated conditions. Therefore, it is plausible to suggest plumbagin to cause apoptosis of CC stem cells during relapse.
Migration is triggered by several factors such as matrix components like collagen, GAGs, integrins, and MMPs. Though, plumbagin is reported to reduce the CC cell migration, here, we showed its ability to reduce the migration of HPV-positive and HPV-negative cells under EGF-exposed conditions. The reduction in the migration of CC cells in plumbagin treated groups can be attributed to increased apoptosis as the apoptotic cells are deprived of energy and migrate at less pace even under stimulated conditions. Meanwhile, a reduction in the amplification of E6 DNA in the cells treated with plumbagin under EGF-stimulated/unstimulated conditions were observed (Data not shown). This suggests that plumbagin could exert its inhibitory actions via targeting HPV E6 oncoprotein in CC cells. These results were substantiated by the findings of Sidhu and Capalash [27] in exploring the migratory inhibition of CC cells by plumbagin.
Along with migration, we showed the formation of colonies from the single subset of cells. EGF exhibited increased clone forming ability as EGF-stimulated cells carries the properties of stemness, which are aligned with our earlier studies [2]. Most importantly, plumbagin treatment and pre-treatment in ME180 and C33A cells completely inhibited the clone formation. This shows that plumbagin can be a potential lead molecule to target the CC cells that escapes surgical resection and enters tumor recurrence.
In the pursuit, this study is also an attempt to understand the effect of plumbagin in altering the cellular deposition of proteinous and non-proteinous matrix components. Plumbagin was identified to reduce the production of collagen, sulphated GAGs and Ca2+ deposition. EGF-induced increase in the matrix component production attests its ability to cause resistance to several drugs as these matrix components can serve as a barrier. The presence of collagen in tumor stroma significantly cause resistance to EGFR TKIs and limits its utility [30]. Lin [31], reported the ability of gefitinib, a known EGFR TKI, to reduce collagen in endothelial cells. This study indicates the involvement of EGFR activation for the collagen production. The reduction in the deposition of these matrix components by plumbagin treatment indicates its ability to sensitize the resistance mechanisms driven by EGFR abundance as noticed during chemo/radio-resistance.
In order to understand the regulation of collagen, we performed gelatinolytic activity in plumbagin treated cells in EGF-unstimulated condition. Plumbagin potentially increased the production of MMP-9 which can be attributed to reduced levels of collagen observed in the present study. In contrary, our previous investigation reports that plumbagin decreases the activity of MMP-2 [9]. Thus, it is suspected that migration of these cells may be facilitated by other proteases apart from MMP-2/9. Ca2+ ions are obligatory to stabilize the network of matrix components in the extracellular space and it has the ability to manipulate the EMT during CC. Thus, it was intended to stain the cells for Ca2+ ions using alizarin red and plumbagin was capable of reducing the Ca2+ ion deposition in EGF-stimulated/unstimulated conditions. Thus, these events warrant the utility of plumbagin in crossing the EGF-induced complex ECM network during the treatment of CC and facilitate the ease of drug delivery and access to immune cells.
The ability of plumbagin in binding with different domains of EGFR by molecular docking was previously reported [9] and in the current study, MD simulation was utilized to understand the binding stability and interaction between EGFR–plumbagin complexes. MD studies showed an acceptable interaction between EGFR–plumbagin and a predominant interaction was witnessed between Met769 residue of EGFR and plumbagin with the maximum of 78% occupancy throughout the simulation. This interaction and stability between EGFR–plumbagin could be the potential causal factor for reduction in the EGF-stimulated survival of HPV-positive and HPV-negative cells, clonogenesis and matrix production. The observed absence in the key features during plumbagin treatment in EGF- stimulated cells indicate the observed interaction between EGFR–plumbagin attenuate the EGFR signaling. However, the alterations of the key components of EGF signaling needs to be studied in plumbagin pre-treated cells to understand the involvement of specific molecules that are regulated by EGF/plumbagin.
Conclusion
To the best of prevailing evidence, this is the first study to report the ability of plumbagin in attenuating EGF-stimulated matrix abundance, clonogenesis and migration in CC cells. These findings collectively indicate the possibility of utilizing plumbagin to sensitize chemo/radio-resistance in CC cells with ECM abundance driven by EGF. The current study found that increased matrix production triggered by EGF-rich milieu is inhibited by plumbagin in both the HPV-positive and HPV-negative cell lines. Targeted delivery of plumbagin to TME would effectively hasten the clearance of CC cells, reduce metastasis and matrix abundance. Liposomes containing plumbagin conjugated with HPV processed antigen would be very useful in specifically targeting HPV-infected cells and largely minimize the undesirable effects of plumbagin. Therefore, further studies are warranted to extend this finding using pre-clinical xenograft model to delineate the efficacy of plumbagin.
Data availability
Data generated during this study will be made available from the corresponding author upon reasonable request.
Abbreviations
- AIF2:
-
Apoptosis inducible factor 1
- AO:
-
Acridine orange
- BrdU:
-
Bromodeoxyuridine
- Ca2+ :
-
Calcium
- CC:
-
Cervical carcinoma
- CS:
-
Chondroitin sulfate
- DS:
-
Dermatan sulfate
- EGF:
-
Epidermal growth factor
- EGFR:
-
EGF receptor
- EMT:
-
Epithelial–mesenchymal transition
- EtBr:
-
Ethidium bromide
- GAG:
-
Glycosaminoglycan
- HA:
-
Hyaluronic acid
- HPV:
-
Human papilloma virus
- HS:
-
Heparan sulphate
- HSPG:
-
Heparan sulphate proteoglycan
- KS:
-
Keratin sulfate
- mAbs:
-
Monoclonal antibodies
- MD:
-
Molecular dynamics
- MMP:
-
Matrix metalloproteinase
- pAKT:
-
Phosphorylated AKR mouse strain thymoma
- PARP:
-
Poly (ADP-ribose) polymerase
- pEGFR:
-
Phosphorylated EGFR
- pERK:
-
Phosphorylated extracellular signal-regulated kinase
- PG:
-
Proteoglycan
- PTEN:
-
Phosphatase and tensin homolog
- SLRP:
-
Small leucine rich PGs
- TKIs:
-
Tyrosine kinase inhibitors
- TME:
-
Tumor micro-environment
References
Cuzick J, Adcock R, Kinney W, Castle PE, Robertson M, McDonald RM, Stoler MH, Du R, Wheeler CM, New Mexico HPV Pap Registry Steering Committee. Impact of HPV testing in opportunistic cervical screening: support for primary HPV screening in the United States. Int J Cancer. 2023;153(1):83–93. https://doi.org/10.1002/ijc.34519.
Sabanayagam R, Krishnamoorthy S, Gnanagurusamy J, Muruganatham B, Muthusami S. EGCG attenuate EGF triggered matrix abundance and migration in HPV positive and HPV negative cervical cancer cells. Med Oncol. 2023;40(9):261. https://doi.org/10.1007/s12032-023-02135-1.
Fullár A, Dudás J, Oláh L, Hollósi P, Papp Z, Sobel G, Karászi K, Paku S, Baghy K, Kovalszky I. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer. 2015;15:256. https://doi.org/10.1186/s12885-015-1272-3.
Piao J, You K, Guo Y, Zhang Y, Li Z, Geng L. Substrate stiffness affects epithelial–mesenchymal transition of cervical cancer cells through miR-106b and its target protein DAB2. Int J Oncol. 2017;50(6):2033–42. https://doi.org/10.3892/ijo.2017.3978.
Kim JH, Moon MJ, Kim DY, Heo SH, Jeong YY. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers (Basel). 2018;10(10):1133. https://doi.org/10.3390/polym10101133.
Noordhuis MG, Eijsink JJ, Ten Hoor KA, Roossink F, Hollema H, Arts HJ, Pras E, Maduro JH, Reyners AK, de Bock GH, Wisman GB, Schuuring E, van der Zee AG. Expression of epidermal growth factor receptor (EGFR) and activated EGFR predict poor response to (chemo)radiation and survival in cervical cancer. Clin Cancer Res. 2009;15(23):7389–97. https://doi.org/10.1158/1078-0432.CCR-09-1149.
Muthusami S, Sabanayagam R, Periyasamy L, Muruganantham B, Park WY. A review on the role of epidermal growth factor signaling in the development, progression and treatment of cervical cancer. Int J Biol Macromol. 2022;194:179–87. https://doi.org/10.1016/j.ijbiomac.2021.11.117.
Karamanos NK, Piperigkou Z, Passi A, Götte M, Rousselle P, Vlodavsky I. Extracellular matrix-based cancer targeting. Trends Mol Med. 2021;27(10):1000–13. https://doi.org/10.1016/j.molmed.2021.07.009.
Periyasamy L, Murugantham B, Muthusami S. Plumbagin binds to epidermal growth factor receptor and mitigate the effects of epidermal growth factor micro-environment in PANC-1 cells. Med Oncol. 2023;40(7):184. https://doi.org/10.1007/s12032-023-02048-z.
Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie. 2001;83(8):811–7. https://doi.org/10.1016/s0300-9084(01)01290-1.
De Pasquale V, Pavone LM. Heparan sulfate proteoglycans: the sweet side of development turns sour in mucopolysaccharidoses. Biochim Biophys Acta Mol Basis Dis. 2019;1865(11): 165539. https://doi.org/10.1016/j.bbadis.2019.165539.
Billings PC, Pacifici M. Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: mechanisms and mysteries. Connect Tissue Res. 2015;56(4):272–80. https://doi.org/10.3109/03008207.2015.1045066.
Kousar K, Naseer F, Abduh MS, Kakar S, Gul R, Anjum S, Ahmad T. Green synthesis of hyaluronic acid coated, thiolated chitosan nanoparticles for CD44 targeted delivery and sustained release of Cisplatin in cervical carcinoma. Front Pharmacol. 2023;13:1073004. https://doi.org/10.3389/fphar.2022.1073004.
Xu S, Xu H, Wang W, Li S, Li H, Li T, Zhang W, Yu X, Liu L. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309. https://doi.org/10.1186/s12967-019-2058-1.
Futyma K, Miotła P, Różyńska K, Zdunek M, Semczuk A, Rechberger T, Wojcierowski J. Expression of genes encoding extracellular matrix proteins: a macroarray study. Oncol Rep. 2014;32(6):2349–53. https://doi.org/10.3892/or.2014.3493.
Sun Y, Ling J, Liu L. Collagen type X alpha 1 promotes proliferation, invasion and epithelial–mesenchymal transition of cervical cancer through activation of TGF-β/Smad signaling. Physiol Int. 2022. https://doi.org/10.1556/2060.2022.00006.
Thangavelu PU, Krenács T, Dray E, Duijf PH. In epithelial cancers, aberrant COL17A1 promoter methylation predicts its misexpression and increased invasion. Clin Epigenetics. 2016;8:120. https://doi.org/10.1186/s13148-016-0290-6.
Monaco S, Sparano V, Gioia M, Sbardella D, Di Pierro D, Marini S, Coletta M. Enzymatic processing of collagen IV by MMP-2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci. 2006;15(12):2805–15. https://doi.org/10.1110/ps.062430706.
Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20(8):931–41. https://doi.org/10.1016/j.semcdb.2009.08.005.
Sabanayagam R, Krishnamoorthy S, Mohan A, Muruganatham B, Muthusami S. A comparative analysis of phyto-components on EGFR binding, viability and migration in HPV positive ME180 and HPV negative C33A cervical cancer cells. Med Oncol. 2023;40(12):357.
Muthusami S, Prabakaran DS, Yu JR, Park WY. EGF-induced expression of Fused Toes Homolog (FTS) facilitates epithelial–mesenchymal transition and promotes cell migration in ME180 cervical cancer cells. Cancer Lett. 2014;351(2):252–9. https://doi.org/10.1016/j.canlet.2014.06.007.
López De Padilla CM, Coenen MJ, Tovar A, De la Vega RE, Evans CH, Müller SA. Picrosirius red staining: revisiting its application to the qualitative and quantitative assessment of collagen type I and Type III in Tendon. J Histochem Cytochem. 2021;69(10):633–43. https://doi.org/10.1369/00221554211046777.
Muthusami S, Senthilkumar K, Vignesh C, Ilangovan R, Stanley J, Selvamurugan N, Srinivasan N. Effects of Cissus quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 cells. J Cell Biochem. 2011;112(4):1035–45. https://doi.org/10.1002/jcb.23016.
Periyasamy L, Murugantham B, Sundaraj R, Krishnamoorthi S, Muthusami S. Screening of MMP-2 inhibiting phytoconstituents for the development of newer pancreatic cancer treatment modalities. Protein Pept Lett. 2023;30(4):304–13.
Mohan A, Krishnamoorthy S, Sabanayagam R, Schwenk G, Feng E, Ji HF, Muthusami S. Pharmacophore based virtual screening for identification of effective inhibitors to combat HPV 16 E6 driven cervical cancer. Eur J Pharmacol. 2023;957:175961.
Cheng X, Yan H, Pang S, Ya M, Qiu F, Qin P, Zeng C, Lu Y. Liposomes as multifunctional nano-carriers for medicinal natural products. Front Chem. 2022;10: 963004. https://doi.org/10.3389/fchem.2022.963004.
Sidhu H, Capalash N. Plumbagin downregulates UHRF1, p-Akt, MMP-2 and suppresses survival, growth and migration of cervical cancer CaSki cells. Toxicol In Vitro. 2023;86: 105512. https://doi.org/10.1016/j.tiv.2022.105512.
Jaiswal A, Sabarwal A, Narayan Mishra JP, Singh RP. Plumbagin induces ROS-mediated apoptosis and cell cycle arrest and inhibits EMT in human cervical carcinoma cells. RSC Adv. 2018;8(56):32022–37. https://doi.org/10.1039/c8ra05339a.
Srinivas P, Gopinath G, Banerji A, Dinakar A, Srinivas G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog. 2004;40(4):201–11. https://doi.org/10.1002/mc.20031.
Yamazaki S, Higuchi Y, Ishibashi M, Hashimoto H, Yasunaga M, Matsumura Y, Tsuchihara K, Tsuboi M, Goto K, Ochiai A, Ishii G. Collagen type I induces EGFR-TKI resistance in EGFR-mutated cancer cells by mTOR activation through Akt-independent pathway. Cancer Sci. 2018;109(6):2063–73. https://doi.org/10.1111/cas.13624.
Lin TC. Gefitinib modulates stress fibers and tubular-like structure formation and attenuates angiogenesis in an in vivo chicken model of chorioallantoic membrane angiogenesis. Biochem Biophys Res Commun. 2020;526(3):568–73. https://doi.org/10.1016/j.bbrc.2020.03.102.
Acknowledgements
The constructive periodic discussions and support headed by the Managing Director, Dr. R. Vasanthakumar, Karpagam Educational Institutions are responsible for this work. The authors thank the CEO, Vice-Chancellor, and Registrar of KAHE for providing constructive support for the completion of this work.
Funding
This study is supported by the seed money grant from the host institution (No.KAHE/R-Acad/A1/Seed Money/035/2022). This work is also approved by the DST-INSPIRE fellowship to SK and SM (No: DST/INSPIRE Fellowship/[IF210086]).
Author information
Authors and Affiliations
Contributions
Conceiving of idea, methodology, experimental design, fund acquisition and manuscript revision—SM; execution of in vitro experiments and initial draft preparation—SK; execution of in vitro experiments—RS; execution of in vitro experiments—LP; initial manuscript drafting—BM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnamoorthy, S., Sabanayagam, R., Periyasamy, L. et al. Plumbagin as a preferential lead molecule to combat EGFR-driven matrix abundance and migration of cervical carcinoma cells. Med Oncol 41, 89 (2024). https://doi.org/10.1007/s12032-024-02332-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02332-6