Abstract
Plant-derived immunomodulators and antitumor factors have appealed lots of attention from natural product scientists for their efficiency and safety and their important contribution to well-designed targeted drug action and delivery mechanisms. Zerumbone (ZER), the chief component of Zingiber zerumbet rhizomes, has been examined for its wide-spectrum in the treatment of multi-targeted diseases. The rhizomes have been used as food flavoring agents in numerous cuisines and in flora medication. Numerous in vivo and in vitro experiments have prepared confirmation of ZER as a potent immunomodulator as well as a potential anti-tumor agent. This review is an interesting compilation of all the important results of the research carried out to date to investigate the immunomodulatory and anticancer properties of ZER. The ultimate goal of this comprehensive review is to supply updated information and a crucial evaluation on ZER, including its chemistry and immunomodulating and antitumour properties, which may be of principal importance to supply a novel pathway for subsequent investigation to discover new agents to treat cancers and immune-related sickness. In addition, updated information on the toxicology of ZER has been summarized to support its safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major global disease and the second leading cause of death [1]. According to the GLOBOCAN report, there are projected to be 2,001,140 new cases of cancer and 611,720 deaths from cancer in the United States in the year 2024 [2]. There are many risk factors that increase cancer mortality, including environmental factors such as unhealthy diet, exposure to air pollution, toxic drugs, physical inactivity, etc. [3]. Present selective cancer treatments have non-specific toxicity [4], very low efficacy [5], high costs [6], exert a lot of adverse effects [7], and also became resistance [8]; that altogether made cancers to have more mortality and poor prognosis. Vegetables, fruits, legumes, nuts and herbs have been shown to contain an important class of phytochemicals that exert therapeutic effects on a variety of human diseases and have been used in folk medicine since ancient times for their pharmacological effects and reduced side effects [9,10,11,12,13,14,15,16].
ZER (Fig. 1), a natural crystalline cyclic sesquiterpene, is the main biological element of Zingiber zerumbet Smith rhizome, which is shown in a both in vitro and in vivo studies to has significant and curable effects in chemotherapy approaches [17]. ZER has been shown to have therapeutic effects such as antipyretic, anti-hypersensitive, anti-inflammatory, antibacterial, antinociceptive, antioxidant, hepatoprotective, and also has immunomodulatory functions [18]. In addition, ZER can also act as an antitumor drug due to its specific properties to suppress angiogenesis and proliferation and induce apoptosis in a variety of cancer cell lines [19]. Numerous studies have shown that ZER has anti-proliferative effects in various human cancers, including cervical, breast, colon and liver, and that it selectively affects tumor cells compared to normal cells [19,20,21] (Fig. 2). In this article, we have reviewed some of these effects of ZER in various human cancers.
The potential mechanisms involved in cancer
Cancer, characterized by the autonomous expansion and spread of a somatic clone, is the second most common cause of death worldwide, and its prevalence is increasing [22,23,24]. Resistance to cell death, uncontrolled the proliferative signaling pathways, induction of angiogenesis, evasion of growth suppressors, enabling replicative immortality, and activation of invasion and metastasis are known hallmarks of cancer [25,26,27] Nucleotide changes, small additions and deletions, chromosomal rearrangements and copy number changes are somatic mutations that disrupt protein-coding or regulatory mechanisms of genes [28,29,30,31].
Natural products-ZER
Zingiber zerumbet Smith of the Zingiberaceae family, also known as lempoyang wild ginger, has many medicinal properties such as treating swelling, wounds, anorexia, parasitic diseases, and treating inflammation [32]. Inhibiting tumor organizer 12-O-tetradecanoylphorbol-13-acetate-leading to Epstein-Barr virus [33], suppressing dextran sodium sulfate-induced colitis [34], pro-inflammatory protein production, suppressing free radical generation, and cancer cell proliferation associated with apoptosis [35] are examples of hundreds of distinguishing features of this plant. ZER is a monocyclic compound with molecular formula C15H22O is used as a food phytochemicals with anti-cancer properties [36]. The rhizomes of Zingiber zerumbet are abundant in Southeast Asia and tropical countries such as India, Bangladesh, Malaysia, Nepal, and Sri Lanka [37].
Anti-cancer mechanisms of ZER
Today, despite advances in cancer treatment techniques, cancer is still one of the worst diseases, causing many deaths every year [38]. Available cancer therapies are mostly infectious and have caused a lot of terrible side effects and resistance [39]. Overall, there is a need to find a novel, alternative, effective and non-toxic treatment for cancer. Studies indicate that ZER inhibits proliferation, arrests the cell cycle and induces apoptosis in many types of cancer, including colon, liver, breast, lung and brain tumors, by modulating various proteins and signaling pathways [20, 40,41,42].
From a pathogenesis-wise perspective, the anti-cancer effects of ZER can be categorized accordingly:
-
1.
Genetic mutations: Zerumbone shows promise in regulating genetic mutations linked to cancer, particularly by downregulating oncogenic pathways. Its ability to inhibit the expression of critical genes such as RAS and MYC suggests a potential role in impeding the oncogenic potential and promoting DNA repair mechanisms.
-
2.
Epigenetic changes: Studies hint at Zerumbone's influence on epigenetic modifications, including DNA methylation and histone alterations. By modulating these patterns, Zerumbone could potentially affect the expression of genes involved in cancer progression by regulating their epigenetic landscape.
-
3.
Cell signaling pathways: Zerumbone’s impact on vital cell signaling pathways, such as PI3K/AKT/mTOR and MAPK/ERK, suggests its potential to disrupt aberrant cell growth and survival in cancer cells.
-
4.
Angiogenesis: Research explores Zerumbone’s anti-angiogenic effects, potentially inhibiting the formation of new blood vessels around tumors by interfering with pro-angiogenic factors like VEGF.
-
5.
Apoptosis: Zerumbone’s ability to induce apoptosis in cancer cells is noteworthy, preventing these cells from evading programmed cell death and contributing to limiting their survival.
-
6.
Immune system evasion: Although limited, studies propose that Zerumbone might modulate immune responses, potentially enhancing the immune system's ability to recognize and eliminate cancer cells, possibly through the regulation of immune checkpoint proteins.
-
7.
Inflammation: Recognized for its anti-inflammatory properties, Zerumbone may create an environment less conducive to cancer initiation and progression by attenuating chronic inflammation associated with cancer development.
-
8.
Metastasis: While requiring further investigation, some studies suggest that Zerumbone may impact cell motility and invasion, crucial processes in metastasis, potentially hindering the spread of cancer cells to distant sites.
-
9.
Metabolic reprogramming: evidence suggests that Zerumbone may influence cellular metabolism, potentially altering metabolic pathways like the Warburg effect and impacting the energy dynamics of cancer cells [31,32,33,34,35].
It is noteworthy that in various in vitro studies showed that ZER could also suppress the CXCR4 expression, NF-κB activity, and other proteins. In addition, this compound can also inhibit the AKT/STAT3/PI3K/mTOR/IL-6/JAK2 lines and the expression of related genetic factors such as ETV1, COX2, IL-6 and cyclin D1, thus suppressing the proliferation and angiogenesis activity of malignant cells by inducing cell cycle arrest and apoptosis. In addition, ZER has shown anti-cancer activity against tumor growth and metastasis in various mouse models and, rarely, in clinical trials [43]. So we looked at some in vitro and in vivo trials that showed the effectiveness of ZER as a treatment for different types of cancer. ZER leads to cell detoxification of oxidative, genotoxic, and carcinogenic chemicals by induction of GSH-related enzymes of stage II, including glutathione-transferase (GST) [37]. Inhibition of tumor cell growth, induction of apoptosis, differentiation and cytoprotective activity are the mechanisms by which ZER acts as an anti-proliferative agent [44]. Studies have shown that ZER prevents the proliferation of colon adenocarcinoma HepG2 cells in a dose-dependent manner and also inhibits the activation of the primary antigen of the Epstein-Barr virus [45]. ZER reduces the production of tumour necrosis factor-alpha (TNF-α) and interleukin-4 (IL-4) and suppresses LTC4 production from lung tissue, downregulates NF-KB and NF-KB gene expression, suppresses CXCR4 and HER2-overexpressing breast cancer cells [46]. Inhibition of leukaemia cells by stimulating Fas receptors and reduction of cyclin B1/CDK1 protein levels by inhibiting the G2/M cell cycle in HL-60 cells are other protective mechanisms of ZER [20, 47]. ZER reduces the expression of NF-kB and NF-kappa regulated gene, which increases in cases of carcinogenicity. It also prevents pancreatic and invasive breast cancer by reducing the expression of the chemokine receptor CXCR4 by inducing the reduction of CXCL12 [48, 49]. It is hypothesized that the carbonyl b-unsaturated group with the depletion of intracellular glutathione (GSH) causes the therapeutic effects of ZER [41, 50].
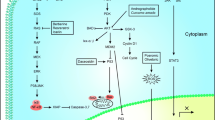
In vitro studies of the effects of ZER on lipid peroxidation in biological systems (phospholipid and cholesterol membrane oxidation) showed that ZER induce to the accumulation of cytosolic lipid droplets and protein dynamics/altered cell membrane organization, depolarizing the mitochondrial membranes and causing alteration of nuclear morphology and apoptosis [51]. ZER with obstructing the excretion of pro-inflammatory cytokines, stimulating NF-κB p65 in LPS-activated inflammation of THP-1 cell-derived macrophages, inhibiting mRNA and protein levels of TLR-2/4 prevents diabetes, cancer and atherosclerosis [52]. ZER stimulates Hsp90 ATPase activity and modifies cysteine residues that destabilise cytotoxicity and anti-cancer efficacy [53]. ZER anti-proliferative activity on the cell lines Hep-G2 (hepatocellular carcinoma, ATCC-HB-8065), LU (lung adenocarcinoma, ATCC-HTB-57), P338 (leukaemia, ATCC-CCl-46), MCF7 (breast cancer, ATCC-HTB-22) and SW 480 (colon adenocarcinoma, ATCC-CCL-228) is evident [54]. ZER has anti-inflammatory and anti-proliferative activities by inhibiting the activation of NF-κB and NF-κB-regulated gene expression caused by carcinogens (Fig. 3) [55].
ZER and glioma
The most common and deadly cancer of the adult central nervous system that is resistant to alkylating agents and other antineoplastic treatments is glioblastoma multiform (GBM) [56]. A number of signaling pathways have been implicated in glioma resistance to alkylating agents and/or the maintenance of brain tumor stem cells, including sonic hedgehog (SHH), Notch and Wnt-β-catenin [57]. ZER induces WOX1-overexpressed U373MG and U87MG cells, and transient overexpression of WOX1 (The WW domain-containing oxidoreductase gene) and blockade of SHH signaling can increase the radiosensitivity of GBM cells independent of p53 and WOX1 levels [58]. Activation of inhibitory κB (IκB) proteins. IκB kinase (IKK), followed by activation of the Akt-FKHR cascade and inactivation of caspase-3, contributes to the resistant to apoptotic process in GBM [59]. Apoptosis pathway of the GBM8401 cell includes inactivating IKKα that affecting to FOXO1 dephosphorylating, via Akt dephosphorylating or not, then inducing caspase-3 activation [60]. ZER treatment reduced cell viability and induced apoptosis in GBM cells by inactivating IKKα, resulting in suppressed FOXO1 and Akt phosphorylation and activation of caspase-3 protein and PARP [60].
ZER and breast cancer
Breast cancer is the second most common cause of cancer death in women and therefore requires special attention [61, 62]. Seventy percent of breast cancers are luminal carcinomas that have alpha estrogen receptor (ER) [63]. ZER binds to estrogen receptors (ERs) and mediates critical pathophysiological signaling pathways in breast cancer, known to be the most common malignancy in women worldwide [64]. The rhizome in ginger has a significant role in the care of a breast concert with inhibiting the migration of MDA‐MB‐231 cells [65]. The expression of integrin αvβ3 appears to play a key role in the development of bone marrow from breast cancer [66]. ZER, when co-administered with the TP5-iRGD peptide, has better antitumour activity by targeting the integrin αvβ3 [67].
ZER by inhibiting IKKβ kinase and thus preventing it from binding to NF-κB can lead to the ultimate induction of apoptosis [68]. ZER was noted that effects on the vitality of MCF-7 and MDA-MB-231 cells [69]. ZER also decrease Breast Cancer–leading to Bone Loss, inhibits Osteoclastogenesis with MDA-MB-231 breast cancer, and suppresses RANKL-leading to NF-KB Activation [70]. In a study, ZER was leading to decreased in Notch1 and Notch4 cleaved proteins, that produced in the inhibition of cellular migration and increased apoptosis. On the other hand, it caused the cleavage of Notch2 to rise the induction of presenilin-1 protein and Notch transcriptional activity [71]. Further, ZER is leading to suppressed IL-1βinduced cell invasion and migration in TNBC through the downregulation of NF-κB activity that inhibited MMP-3 and IL-8 expression [48, 72].
ZER caused a reduction in cell growth and proliferation by arresting the cell cycle in the G1 phase due to a reduction in CD1d expression and the lipid antigen presentation pathway [73]. CD44 shown to promote protumorigenic signaling and metastatic cascade [74]. ZER decreased expression of CD44 through EGFR ligands, TGF-α or EGF and also inhibited STAT3 phosphorylation that resulted reduction of tumor metastasis and progression [75].
ZER also reduced the tumor growth and caused Bax- and Bak-mediated apoptosis by inducing G2/M cell arrest [47]. The elevated levels of CXCR4 indicate that the patient has a high probability of lymph node metastasis [76]. ZER decreased CXCL12-Induced metastasis and invasion in breast cancer through downregulating the CXCR4 expression [77]. In addition, this natural compound reduce phosphorylation of TGF-β1-affected from Smad3 and Ki67 expression following that inhibit TGF-β1-induced MMP-2, FN, and MMP-9 expression, which lead to restrain the motility and tumorigenicity of triple-negative breast malignance cells [78].
In addition, Bcl-2-positive tumors with increased loss of apoptosis were associated with metastasis [79]. ZER reduced expression of Bcl-2 genes and increased Bax. ZER also N-acetyl cysteine (NAC) and elevated reactive oxygen species (ROS) which led to the NF-κB p65 activation. Therefore ZER has an apoptotic induction potential and increased cell cycle arrest at G2/M stage in GBM U-87 MG cells [80].
ZER and colorectal cancer
The incidence of colorectal cancer in the general population is about 5 to 6% and is the second most common cancer in the world [81]. The expression of miR-200c was increased in higher grade CRC and has been implicated in CRC tumor progression and aggressiveness via regulation of epithelial-to-mesenchymal (EMT) and mesenchymal-to-epithelial (MET) transition processes [82]. ZER has an important role on colorectal cancer (CRC), cancer stem cells (CSCs) and including EMT as one of the most rampant and lethal malignancies in the world by inhibiting the β-catenin pathway through miR-200c and inhibiting mesenchymal-epithelial transition and cancer stem cells characterizes [83].
This tropical ginger can increase tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), deletion of DR5 or DR4, decrease regulation of cFLIP and inhibit caspase-8 [84]. In addition, ZER has protective effects opposite bowel cancer in Enterotoxigenic Bacteroides fragilis (ETBF)-colonized AOM/DSS BALB/c mouse [85]. A study by Edagawa et al. demonstrated that during celecoxib and ZER treatment in human p53-deficient colorectal cancer cells, ATF3 promotes DR5 producting and apoptotic cell death [86]. ZER induces apoptosis of colon cancer by inhibiting the formation of colonic preneoplastic ACFs, and its anti-proliferative influences were noted effectiveness as an anti-cancer agent [87].
The majority of colorectal cancers express high levels of cyclin B1 [88], that ZER inhibited DNA synthesis and cyclin B1 expression as an antitumor and anti-cancer agent, especially on human colon cancer [89]. ZER decreases the proliferation of bowel cancer cells and induced apoptosis through translocation of phosphatidylserine, mitochondrial transmembrane dysfunction, and chromatin condensation [35]. ZER Modulated Fak/PI3k/NF-κB-uPA pathway and proved its anti-metastatic potential and Suppressed Human Colorectal Cancer Invasion on HCT-116 and SW48 cells [50].
In addition, ZER increases oxidative stress in a thiol-dependent ROS-independent pathway to enhance apoptosis and radiosensitivity of colorectal cancer cells while inhibiting the expression of radiation-induced DNA repair proteins DNA-PKcs and ataxia-telangiectasia mutated (ATM) through GSH depletion, leading to cell cycle arrest (G2/M) [17].
In one study, ZER was found to stimulate the expression of interleukin (IL)-1α, IL-1β, IL-6 and production of tumor necrosis factor (TNF)-α in human colon adenocarcinoma cell lines [90]. Further ZER treatment repressed NF-κB and heme oxygenase (HO)-1 that caused inhibition of the multiplicity and inflammation in colonic adenocarcinomas, induction of apoptosis and suppression of the proliferation [21]. ZER treatment suppressed TNF-alpha and downregulated HCT116 colon cancer cells proliferation [40]. ZER increased Bax, Caspase 3, Caspase 8, Caspase 9 and also caused enhancement of cell cycle stopping at G2/M stage by down regulated Bcl2 expression, mitochondrial membrane potential and the cellular antioxidant status [91].
ZER and cholangiocarcinoma
Cholangiocarcinoma (CCA) is the most common malignancy of the biliary tract and has increased significantly in recent decades [92]. EGFR signaling is involved in cholangiocarcinoma development and progression [93]. In a study on seventeen ZER derivatives has been shown the presence of amine, hydroxylamine, epoxyamine, and nitrile groups by interacting with the molecular target EGFR have the most effective anti-proliferative activity against KKU-100 cell lines with an IC50 level of 16.44 mM which can exhibit acutely anti-cancer activities opposed to CCA cells [94].
ZER and gastric cancer
Stomach cancer is the second most common cause of cancer deaths worldwide because it is usually detected in the late stages [95]. Cyclophilin A (CypA) was expressed at abnormally high levels in several types of cancer, including gastric cancer, and was implicated in cancer cell proliferation, cell migration/invasion, drug resistance and inhibition of apoptosis in several cancer cell types [96]. ZER block the action of cyclophilin A and promote mitochondrial pathway-interceded apoptosis, as a result, produce caspase-dependent apoptosis in gastric cancer cells [97]. Gene products controlled by NF-κB include the angiogenesis modulator vascular endothelial growth factor (VEGF), which supports cell survival and leads to the acquisition of chemoresistance [91]. ZER reduces NF-κB activities and the expression of VEGF, thereby inhibiting angiogenesis, leading to suppression of cell proliferation and tube formation in human umbilical vein endothelial cells [98].
ZER and leukemia
Leukaemia is characterized by starting in the bone marrow and resulting in high numbers of abnormal blood cells, which, like other cancers, arise from mutations in DNA [93]. In research on the murine leukaemia model using WEHI-3B cells by Rahman et al, ZER also induced the mitochondrial-dependent apoptotic pathway [99]. ZER stimulates the intrinsic apoptotic proteins (Caspase-3 and Caspase-9), releases Cytochrome c from the mitochondria, and following that cleavage of poly (adenosine diphosphate-ribose) polymerase (PARP) which led to arrest the Jurkat cells at G2/M stage with inactivation of cyclin B1 protein. As a result, ZER treatment showed the anti-proliferative effect on human lymphoblastic leukemia cell line [100, 101].
ZER suppresses K562 chronic myeloid leukaemia cell proliferation and colony formation due to DNA damage and upregulation of total histone H2AX, increased calcium, generation of ROS with activation of pro-caspase-3, -9 and PARP cleavage on Western blots, termed mitochondria-mediated apoptosis [102]. ZER treatment against CEM-ss leukemic cells enhanced the number of TUNEL-positive stains and the caspase-3 level of cells and also revealed membrane blabbing holes and cytoplasmic discharges which are characteristics of apoptosis [103].
In one study, two distinct pathways [mitochondrial and Fas (CD95)-mediated] were identified in ZER-treated NB4 cells. ZER inhibits the proliferation of leukaemic promyelocytic NB4 cells by inducing G2/M cell phase arrest followed by apoptosis via the onset of Fas (CD95)/Fas ligand (CD95L) expression associated with caspase-8 action. It also reduced B1/CDK1 protein cycling along with ATM/Chk1/Chk2 phosphorylation. In this study, both caspase-8 and -9 were cleaved into their active forms by treatment with ZER. ZER also induced the cleavage of Bax and Mcl-1 proteins, but not Bcl-2 or Bcl-XL [104, 105].
ZER and liver cancer
The incidence of liver cancer has increased and it is the third most common cause of cancer and leads to death [106]. Several studies have identified alterations and dysregulated expression of the phosphatidylinositol-3-kinase (PI3K)/serine-threonine protein kinase (Akt)/mammalian target of rapamycin (mTOR) pathway in hepatocellular carcinoma (HCC) [107]. ZER decreased proliferation and clonogenic survival of HCC cells and induced apoptosis via stopping cells at the G2/M stage due to the significant suppression of the STAT3 and PI3K/AKT/mTOR signaling pathways [108]. ZER enhanced Bax pro-apoptotic protein and inhibited Bcl-2 anti-apoptotic protein expression and leads to inducing apoptosis [44]. In addition, ZER induced mitochondria-regulated apoptosis and inhibited proliferation by upregulating Bax, decreased Bcl-2 protein expression, reduced oxidative stress, and as a result, lessening DEN/AAF-caused carcinogenesis in rat liver [109].
HCC is characterized by marked vascular abnormalities, arteriogenesis and capillarisation [110]. Another in vitro study about the anti-tumor effect of ZER on HCC demonstrates suppression angiogenesis in cells of HepG2 through suppression the expressions of VEGF, MMP-9, and VEGFR [86]. In a study noted that to prevent the proliferation and migration of HepG2 cell in a dose-dependent method, ZER decreased tube formation through HUVECs inhibits new blood vessel and tissue matrix formation and also reduces expression of molecular effectors of angiogenesis, MMP-9, VEGF, and VEGF receptor proteins [111]. Further ZER influence on nuclear localization of the transcription factor (Nrf2) that activated the Nrf2/ARE-dependent detoxification pathway and therefore showed the antioxidant role in the lipid peroxidation neutralization in hepatocytes [112]. ZER encapsulated by hydroxypropyl-β-cyclodextrin (HPβCD) recognized induced apoptosis and G2/M stage stopping in HepG2 cells beside the release of cytochrome c and damage of mitochondrial membrane potential and also increased Caspase 3/7, Caspase 8, and Caspase 9 with the depletion of BID divided by Caspase 8 [113]. ZER increased apoptosis and cell cycle arrest at G2/M stage in HepG2 cells via upregulated cytochrome c, p27, p38, Bcl‑2, caspase‑3 and‑9 expression through MAPK signaling pathway [114].
ZER and lung cancer
Lung cancer is the leading cause of cancer death worldwide [115]. Lipopolysaccharide (LPS) increased the expression of haem oxygenase (HO-1) and Nrf2 and lipid peroxidation, activation of antioxidant enzymes and activation of MMP-9 and myeloperoxidase (MPO), which was suppressed by ZER and led to a reduction in acute lung injury [116]. NF-κB and HO-1 signaling pathways reduce ROS production in lung cancer cells which followed by chemoresistance [117] and ZER decreased growth, inflammation, and expression of NF-κB and HO-1, which caused apoptosis, suppression of lung carcinogenesis, and inhibited the multiplicity [21].
In addition, ZER leads to loss of mitochondrial membrane potential resulting in cytochrome c production, activation of caspase-3 and -9, promotion of Bax and p53 expression and upregulation of ROS production. Thus, ZER induces increased susceptibility to cisplatin and mitochondrial apoptosis in non-small cell lung cancer (NSCLC) cells [118]. LIM kinase (LIMK) is a serine/threonine protein kinase that includes members LIMK1 and LIMK2, which protect cancer cells from death and promote cell proliferation and chemotherapeutic resistance. LIMK2 expression was also upregulated in radioresistant NSCLC cells [119] and in vitro showed that ZER suppressed LIM kinase 1 and 2 and AKT and FAK phosphorylation Non-Small Cell Lung Cancer A549 Cells and also reduced osteopontin through blocking ROCK1 expression [120].
ZER and oral cancer
Oral squamous cell carcinoma (OSCC) is one of the ten most common cancers worldwide, with high mortality and poor response to treatment [121]. Regarding the distant metastasis from the oral cancer and as over activation of PI3K/Akt signaling pathway in human oral cancers [122], A study of ZER treatment in oral squamous cell carcinoma by Zainal et al. showed that ZER suppressed OSCC proliferation, migration and invasion, and induction of G2/M cell phase exit and apoptosis by downregulating the expression of RhoA, CXCR4 proteins, and also decreased the PI3K-mTOR signaling pathway via inactivation of S6 and Akt proteins [123]. Regarding the apoptosis-resistant of oral cancer cells, ZER induced apoptosis via S and G2/M stages of cell cycle arrest due to its antiproliferative, antioxidant, anticancer, and anti-inflammatory effects on Human Laryngeal Carcinoma Cell Line Hep-2 [41].
ZER and cervical and ovarian cancer
Cervical cancer is known to be a major problem in most developing countries and the second most common cancer in women worldwide [124]. Ovarian cancers have the highest occurrence and mortality rate among gynecologic cancers that, unlike cervical cancer, there is no proper prevention program [125]. Thirteen percent of women with cervical cancer are diagnosed at an advanced stage of the disease that in contrast to localized type, there is no standard treatment for patients with metastatic cervical cancer and median survival is only 8 to 13 months [126]. ZER is an active agent that induces apoptosis, cytotoxicity and anti-migratory effects in cervical cancer cells by preventing cell migration of HeLa cells, reducing the production of MMP-2/9 and proangiogenic factor VEGF, and stimulating programmed cell death in HeLa cells through phosphatidylserine translocation, increased caspase 3 activity, DNA fragmentation, upregulation of the expression of pro-apoptotic protein Bax, cleaved caspase 3, cleaved PARP and downregulation of anti-apoptotic protein Bcl-2 [38].
In a study on ZER treatment in Cervical Intraepithelial Neoplasia (CIN) of Female BALB/c mouse, noted ZER reduced immunoexpressions of proliferating cell nuclear antigen and proved an anti-cancer effect on cervical cancer cells [127]. Regarding the overexpression of Bcl-2 and Bax in CIN [128], ZER modulated the expression of Bcl-2 gene and Bax protein and induced mitochondria-regulated apoptosis through the regression of CIN tissues [129]. IL-6 was significantly upregulated in ovarian cancer and subsequently promote a pro-inflammatory tumor microenvironment [130]. ZER suppressed the IL-6 levels secreted by both Caov-3 and HeLa cells and induced apoptosis by stopping cells at the G2/M phase [131]. ZER also upregulated the Caspase-3 cellular level in HeLa cells and originated distinctive morphological features of apoptosis concluded chromatin and nuclear condensation, multinucleation, cell shrinkage, membrane blebbing, holes, abnormalities of mitochondrial cristae, cytoplasmic extrusions and formation of apoptotic bodies [132].
ZER and pancreatic cancer
Pancreatic cancer has poor prognosis among solid tumors. And the response to chemotherapy is not so good [133]. CXCR 4/CXCL12 is associated with tumor invasion and metastasis in pancreatic cancer also induced chemoresistance [134] that ZER treatment decreased CXCR4 expression and inhibited CXCL12-induced invasion in pancreatic tumor cells, which leads to suppressed cancer metastasis [77]. Similar to CXCR4, IL8/CXCL8 could play an important role in tumor progression and angiogenesis [135], ZER reduced the mRNA expression and protein secretion of the main angiogenic factors VEGF and IL-8 in PaCa cells and blocked the PaCa-associated angiogenesis through the suppression of NF-κB and NF-κB-dependent proangiogenic genesis which leads to suppressed tube structure of human umbilical vein endothelial cells [136]. In a study using the pancreatic cancer cell lines AsPC-1, SW1990 and PANC-1, ZER treatment resulted in upregulation of p21 expression, p53 protein levels and ROS production. Therefore, ZER decreased cell viability and induced apoptosis via the p53 pathway [137].
ZER and prostate cancer
Prostate cancer in men has a significant prevalence and a good prognosis with early detection through prostate specific antigen (PSA) in the blood for and improved procedures with radiotherapy and surgical intervention [138]. Ataxia telangiectasia mutated (ATM) kinase is a 350 kDa nuclear protein kinase that is activated by DNA double-strand breaks to activate DNA repair, but is down-regulated in prostate cancer that induces resistance to radiotherapy [139]. In research on PC3 and DU145 prostatic cancer cells, emphasized that ZER increased the radiation effect on prostate cancer cells and reduced the radiation-caused expression of phosphorylated ATM. ZER also suppressed the expression of STAT3 and JAK2, which are implicated in DNA damage repair signaling [140].
A primary sensor and master regulator of ER stress is glucose-regulated protein 78 (GRP78), overexpression of which confers resistance to a variety of chemotherapy drugs [141]. In a study on anti-proliferative and apoptotic effects against DU-145 and PC-3 cell lines, ZER induced (ER) stress and mitochondrial damage by upregulation of GRP-78 and CHOP/GADD153 expression and the loss of mitochondrial membrane potential [142]. ZER also increased intracellular Ca2+ levels, which related to the structure of the active calpain I fragment and induced autophagy and apoptosis in human hormone-refractory prostate cancers (HRPCs) through tubulin binding and a Caspase-dependent way and dramatic LC3-II formation.
In addition, ZER suppressed microtubule assembly and increased MPM-2 expression, Mcl-1 protein expression, phosphorylation of Bcl-xL and Bcl-2, leading to the tubulin-binding effect. ZER also downregulated Cdc25C and increased the expression of C/EBP homologous protein (CHOP)/growth arrest and GRP-78 and DNA damage 153 (GADD153) [143]. Furthermore, ZER induced apoptosis via cell cycle arrest at the G0/G1 stage, and also ZER decreased the JAK2/STAT3/IL-6 signaling pathway and blocked the prostate cancer-associated genes including: IL-6, cyclin D1, COX2 (cytochrome c oxidase) and ETS variant 1 (ETV1) [144].
ZER and renal cell carcinoma
Renal cell carcinoma (RCC) accounts for approximately 3% of adult cancers and is characterized by resistance to conventional cancer treatments [145]. ZER treatment induced apoptosis in human renal cell carcinoma through inhibition of Bcl-2 and Gli-1, which caused chemoresistance of RCC, inhibition of cell viability and DNA fragmentation. On the other hand, stimulation of caspase-3 and -9 led to PARP cleavage [146]. STAT3 is aberrantly activated in several types of malignancy, including RCC, where it regulates the expression of genes involved in cell survival, proliferation and angiogenesis [147]. ZER stimulates JAK 1/2 and upstream kinases c-Src. It therefore inhibits the activation of STAT3 in RCC cells in a time- and dose-dependent manner. As a result, ZER suppresses proliferation and induces apoptosis in RCC. ZER induced the expression of the tyrosine phosphatase SHP-1, which is associated with its ability to block STAT3 activation [148].
ZER and skin cancer
One of the most common types of cancer, especially in fair-skinned populations, is skin cancer, which is divided into melanoma and non-melanoma skin cancers (NMSCs), affecting the skin in the form of basal cell carcinoma and squamous cell carcinoma (BCC and SCC, respectively) [149]. Mice genetically deficient in Nrf2 are highly susceptible to chemically induced skin tumorigenesis. They are also less responsive to the cytoprotective effects of some chemopreventive phytochemicals [150]. ZER with suppressed nuclear Nrf2 activation in HSF cells prevents from aging skin cells [151]. Reactive oxygen species such as NOX, iNOS, COX‐2 play a key role in skin tumorigenesis [150] and ZER decrease NOX, iNOS, COX‐2 with inhibiting mRNA expression. This mechanism is also seen in the colon [152]. Melanoma is known as a high malignancy tumor. ZER with suppressing the migration and proliferation of the melanoma cell line CHL-1, also decreasing mitochondrial activity that leads to the subsequent raising in ROS generation, decrease in MMP, and a reduction in mtDNA and ATP levels can be a valuable treatment option [42].
The xenobiotic-metabolizing enzymes (NQO1, GSTP1) and mRNA levels for manganese superoxide dismutase (MnSOD), glutathione S-transferase-P1, glutathione peroxidase-1 (GPx1), and NAD (P) H quinone oxidoreductase are examples on antioxidant that protect in cells the epidermis from tumorigenesis and could be upregulated over ZER treatment. ZER also reduced cyclooxygenase-2 (COX-2) expression, H2O2-induced oedema formation, ERK1 phosphorylation and leukocyte infiltration, suppressing the initiation and promotion stages of skin cancer [153]. ZER induced HO-1 expression via stimulation of Nrf2, which led to an antioxidant effect on skin carcinogenesis [43]. In a study on UVA-irradiated damages that caused skin cancer, noted ZER increased expression of c-glutamyl cysteine ligase (c-GCLC) and HO-1 genes and also upregulated antioxidant response element (ARE) and Nrf2 nuclear translocation that related to the PI3K/AKT, p38 MAPK, and PKC signaling [149]. In addition, ZER suppressed the expression of microphthalmia-associated transcription factor (MITF) and downregulated melanin aggregation in α-melanocyte stimulating hormone (α-MSH), leading to the induction of melanogenesis [154].
ZER and esophageal cancer
Adenocarcinoma and esophageal squamous cell carcinoma (SCC) of the esophagus is a serious malignancy with a poor prognosis and the eighth most common cancer in the world [155]. ZER can be useful in on the proliferation and apoptosis of the esophagus cancer EC-109 cells by downregulating the Bcl-2 protein expression and upregulating the P53 protein expression [156]. The results showed that Rac-1 was upregulated at the protein and mRNA levels in ESCC cancer and associated with lymph node metastasis were two independent factors for poor survival [157]. ZER decreased Rac1 protein by enhancing Rac1 ubiquitination through the proteasome-dependent inhibition pathway, which led to suppressed migration in human esophageal squamous cell carcinoma KYSE-150 and KYSE-30 cells [158] and may be a potential agent for targeting and therapy of ESCC.
Future directions
As reviewed above, many in vitro studies have shown the significant effect of ZER on various human cancers, but only a few in vivo studies on breast, lung, cervical, renal cell, colorectal and skin cancers have been conducted to demonstrate these effects. There are also no relevant clinical trials to test the safety and efficacy of ZER, so there is a paucity of in vivo evidence. In addition, some studies are needed to analyze the pharmacokinetic properties of this product, such as distribution, solubility, etc. As an example, previous research designed to improve the solvability of ZER and it was investigated to evaluate its relationship with hydroxypropyl-β-cyclodextrin (HPβCD) in containing compounds and demonstrated that the solvability of ZER dramatically improved with an increase in the release of HPβCD at 20 °C, thus indicating that this product could be consumed in this drug formation [159]. Therefore, in this field of medicine on cancer therapy with ZER, we urgently need to more studies in both in vivo and clinical trials and also pharmacokinetic features, to accredit its clinical use in various human cancers.
Data availability
The data supporting the conclusions of this article are all online.
References
Turrini E, Ferruzzi L, Fimognari C. Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxid Med Cell Longev. 2015;2015:1–19.
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics. CA. 2024;74:12–49.
Ho PJ, Lau HSH, Ho WK, Wong FY, Yang Q, Tan KW, et al. Incidence of breast cancer attributable to breast density, modifiable and non-modifiable breast cancer risk factors in Singapore. Sci Rep. 2020;10(1):1–11.
Moon OJ, Yoon CJ, Lee BR, Lee J. An optimally fabricated platform guides cancer-specific activation of chemotherapeutic drugs and toxicity-free cancer treatment. Adv Healthc Mater. 2022;11(15):2200765.
Reljic D, Herrmann HJ, Jakobs B, Dieterich W, Mougiakakos D, Neurath MF, et al. Feasibility, safety, and preliminary efficacy of very low-volume interval training in advanced cancer patients. Med Sci Sports Exerc. 2022;54(11):1817.
Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it. Mayo Clin Proc. 2012;87:935–43.
Nurgali K, Jagoe RT, Abalo R. Adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Lausanne: Frontiers Media; 2018. p. 245.
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers. 2014;6(3):1769–92.
Sailo BL, Banik K, Padmavathi G, Javadi M, Bordoloi D, Kunnumakkara AB. Tocotrienols: the promising analogues of vitamin E for cancer therapeutics. Pharmacol Res. 2018;130:259–72.
Hsieh YS, Yang SF, Sethi G. Natural bioactives in cancer treatment and prevention. BioMed Res Int. 2015;2015:182835.
Hasanpourghadi M, Looi CY, Pandurangan AK, Sethi G, Wong WF, Mustafa MR. Phytometabolites targeting the warburg effect in cancer cells: a mechanistic review. Current drug targets. 2017;18(9):1086–94.
Shanmugam MK, Warrier S, Kumar AP, Sethi G, Arfuso F. Potential role of natural compounds as anti-angiogenic agents in cancer. Current Vasc Pharmacol. 2017;15(6):503–19.
Tewari D, Nabavi SF, Nabavi SM, Sureda A, Farooqi AA, Atanasov AG, et al. Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention. Pharmacol Res. 2018;128:366–75.
Kunnumakkara AB, Bordoloi D, Harsha C, Banik K, Gupta SC, Aggarwal BB. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci. 2017;131(15):1781–99.
Kashyap D, Sharma A, Tuli HS, Sak K, Garg VK, Buttar HS, et al. Apigenin: a natural bioactive flavone-type molecule with promising therapeutic function. J Funct Foods. 2018;48:457–71.
Wang L, Do Dang Khoa Phan JZ, Ong P-S, Thuya WL, Soo R, Wong ALA, et al. Anticancer properties of nimbolide and pharmacokinetic considerations to accelerate its development. Oncotarget. 2016;7(28):44790.
Deorukhkar A, Ahuja N, Mercado AL, Diagaradjane P, Raju U, Patel N, et al. Zerumbone increases oxidative stress in a thiol-dependent ROS-independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med. 2015;4(2):278–92.
Singh YP, Girisa S, Banik K, Ghosh S, Swathi P, Deka M, et al. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J Funct Foods. 2019;53:248–58.
Kalantari K, Moniri M, Boroumand Moghaddam A, Abdul Rahim R, Bin Ariff A, Izadiyan Z, et al. A review of the biomedical applications of zerumbone and the techniques for its extraction from ginger rhizomes. Molecules. 2017;22(10):1645.
Haque MA, Jantan I, Arshad L, Bukhari SNA. Exploring the immunomodulatory and anticancer properties of zerumbone. Food Funct. 2017;8(10):3410–31.
Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124(2):264–71.
Pourgholi A, Dadashpour M, Mousapour A, Amandi AF, Zarghami N. Anticancer potential of silibinin loaded polymeric nanoparticles against breast cancer cells: insight into the apoptotic genes targets. Asian Pac J Cancer Prev APJCP. 2021;22(8):2587.
Alagheband Y, Jafari-gharabaghlou D, Imani M, Mousazadeh H, Dadashpour M, Firouzi-Amandi A, et al. Design and fabrication of a dual-drug loaded nano-platform for synergistic anticancer and cytotoxicity effects on the expression of leptin in lung cancer treatment. J Drug Deliv Sci Technol. 2022;73: 103389.
Hassani N, Jafari-Gharabaghlou D, Dadashpour M, Zarghami N. The effect of dual bioactive compounds artemisinin and metformin co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Appl Biochem Biotechnol. 2022;194(10):4930–45.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Jafari-Gharabaghlou D, Dadashpour M, Khanghah OJ, Salmani-Javan E, Zarghami N. Potentiation of folate-functionalized PLGA-PEG nanoparticles loaded with metformin for the treatment of breast cancer: possible clinical application. Mol Biol Rep. 2023;50(4):3023–33.
Firouzi-Amandi A, Tarahomi M, Rahmani-Youshanlouei H, Heris RM, Jafari-Gharabaghlou D, Zarghami N, et al. Development, characterization, and in vitro evaluation of cytotoxic activity of Rutin loaded PCL-PEG nanoparticles against Skov3 ovarian cancer cell. Asian Pac J Cancer Prev APJCP. 2022;23(6):1951.
Consortium ICG. International network of cancer genome projects. Nature. 2010;464(7291):993.
Dadashpour M, Ganjibakhsh M, Mousazadeh H, Nejati K. Increased pro-apoptotic and anti-proliferative activities of simvastatin encapsulated PCL-PEG nanoparticles on human breast cancer adenocarcinoma cells. J Clust Sci. 2023;34(1):211–22.
Sharifi-Azad M, Fathi M, Cho WC, Barzegari A, Dadashi H, Dadashpour M, et al. Recent advances in targeted drug delivery systems for resistant colorectal cancer. Cancer Cell Int. 2022;22(1):1–21.
Azar LK, Dadashpour M, Hashemi M, Zarghami N. Design and development of nanostructured co delivery of artemisinin and chrysin for targeting hTERT gene expression in breast cancer cell line: possible clinical application in cancer treatment. Asian Pac J Cancer Prev APJCP. 2022;23(3):919.
Abdul A, Al-Zubairi A, Tailan N, Wahab S, Zain Z, Ruslay S, et al. Anticancer activity of natural compound (Zerumbone) extracted from Zingiber zerumbet in human HeLa cervical cancer cells. Int J Pharmacol. 2008;4(3):160–8.
Murakami A, Takahashi M, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Biosci Biotechnol Biochem. 1999;63(10):1811–2.
Murakami A, Hayashi R, Takana T, Kwon KH, Ohigashi H, Safitri R. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combination. Biochem Pharmacol. 2003;66(7):1253–61.
Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, et al. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the α, β-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23(5):795–802.
Keong YS, Alitheen NB, Mustafa S, Aziz SA, Rahman MA, Ali AM. Immunomodulatory effects of zerumbone isolated from roots of Zingiber zerumbet. Pak J Pharm Sci. 2010;23(1):75–82.
Sidahmed HMA, Hashim NM, Abdulla MA, Ali HM, Mohan S, Abdelwahab SI, et al. Antisecretory, gastroprotective, antioxidant and anti-helicobcter pylori activity of zerumbone from Zingiber zerumbet (L.) Smith. PloS ONE. 2015;10(3):e0121060.
Saranya J, Dhanya B, Greeshma G, Radhakrishnan K, Priya S. Effects of a new synthetic zerumbone pendant derivative (ZPD) on apoptosis induction and anti-migratory effects in human cervical cancer cells. Chem-Biol Interactions. 2017;278:32–9.
Monisha J, Roy NK, Padmavathi G, Banik K, Bordoloi D, Khwairakpam AD, et al. NGAL is downregulated in oral squamous cell carcinoma and leads to increased survival, proliferation, migration and chemoresistance. Cancers. 2018;10(7):228.
Singh SP, Nongalleima K, Singh NI, Doley P, Singh CB, Singh TR, et al. Zerumbone reduces proliferation of HCT116 colon cancer cells by inhibition of TNF-alpha. Sci Rep. 2018;8(1):1–11.
Jegannathan SD, Arul S, Dayalan H. Zerumbone, a sesquiterpene, controls proliferation and induces cell cycle arrest in human laryngeal carcinoma cell line Hep-2. Nutr Cancer. 2016;68(5):865–72.
Yan H, Ren MY, Wang ZX, Feng SJ, Li S, Cheng Y, et al. Zerumbone inhibits melanoma cell proliferation and migration by altering mitochondrial functions. Oncol Lett. 2017;13(4):2397–402.
Shin J-W, Ohnishi K, Murakami A, Lee J-S, Kundu JK, Na H-K, et al. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev Res. 2011;4(6):860–70.
Sakinah SS, Handayani ST, Hawariah LA. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;7(1):4.
Alwi SSS, Nallappan M, Pihie AHL, Hawariah L. Zerumbone exerts antiproliferative activity via apoptosis on HepG2 cells. Malaysian J Biochem Mol Biol. 2007;15(1):19–23.
Singh CB, Nongalleima K, Brojendrosingh S, Ningombam S, Lokendrajit N, Singh L. Biological and chemical properties of Zingiber zerumbet Smith: a review. Phytochem Rev. 2012;11(1):113–25.
Sehrawat A, Arlotti JA, Murakami A, Singh SV. Zerumbone causes Bax-and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res Treatment. 2012;136(2):429–41.
Jeon M, Han J, Nam SJ, Lee JE, Kim S. Elevated IL-1β expression induces invasiveness of triple negative breast cancer cells and is suppressed by zerumbone. Chemico-Biol Interactions. 2016;258:126–33.
Mukherjee D, Zhao J. The role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res. 2013;3(1):46.
Hosseini N, Khoshnazar A, Saidijam M, Azizi Jalilian F, Najafi R, Mahdavinezhad A, et al. Zerumbone suppresses human colorectal cancer invasion and metastasis via modulation of FAk/PI3k/NFκB-uPA pathway. Nutr Cancer. 2019;71(1):159–71.
Rosa A, Caprioglio D, Isola R, Nieddu M, Appendino G, Falchi A. Dietary zerumbone from shampoo ginger: new insights into its antioxidant and anticancer activity. Food Funct. 2019;10(3):1629–42.
Kim M-J, Yun J-M. Molecular mechanism of the protective effect of zerumbone on lipopolysaccharide-induced inflammation of THP-1 cell-derived macrophages. J Med Food. 2019;22(1):62–73.
Nakamoto H, Amaya Y, Komatsu T, Suzuki T, Dohmae N, Nakamura Y, et al. Stimulation of the ATPase activity of Hsp90 by zerumbone modification of its cysteine residues destabilizes its clients and causes cytotoxicity. Biochem J. 2018;475(15):2559–76.
Van Truong V, Nam TD, Hung TN, Nga NT, Quan PM, Van Chinh L, et al. Synthesis and anti-proliferative activity of novel azazerumbone conjugates with chalcones. Bioorganic Med Chem Lett. 2015;25(22):5182–5.
Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-κ B and I κ B α kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24(46):6957–69.
Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Therapy-Nucleic Acids. 2013;2: e126.
Ulasov IV, Nandi S, Dey M, Sonabend AM, Lesniak MS. Inhibition of Sonic hedgehog and Notch pathways enhances sensitivity of CD133+ glioma stem cells to temozolomide therapy. Mol Med. 2011;17(1):103–12.
Chiang M-F, Chen H-H, Chi C-W, Sze C-I, Hsu M-L, Shieh H-R, et al. Modulation of Sonic hedgehog signaling and WW domain containing oxidoreductase WOX1 expression enhances radiosensitivity of human glioblastoma cells. Exp Biol Med. 2015;240(3):392–9.
Weng H-Y, Hsu M-J, Chen C-C, Chen B-C, Hong C-Y, Teng C-M, et al. Denbinobin induces human glioblastoma multiforme cell apoptosis through the IKKα–Akt–FKHR signaling cascade. Eur J Pharmacol. 2013;698(1–3):103–9.
Weng H-Y, Hsu M-J, Wang C-C, Chen B-C, Hong C-Y, Chen M-C, et al. Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells. J Biomed Sci. 2012;19(1):86.
DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA. 2014;64(1):52–62.
Loghmani H, Noruzinia M, Abdul Tehrani H, Taghizadeh M, Mohammad Hamid K. Association of estrogen receptors’ promoter methylation and clinicopathological characteristics in Iranian patients with breast cancer. Mol Biochem Diag J. 2014;1(1):21–33.
Lamb CA, Vanzulli S, Lanari CLM. Hormone receptors in breast cancer: more than estrogen receptors. 2019.
Eid EE, Azam F, Hassan M, Taban IM, Halim MA. Zerumbone binding to estrogen receptors: an in-silico investigation. J Recept Signal Transduct. 2018;38(4):342–51.
Al-Amin M, Eltayeb NM, Hossain CF, Khairuddean M, Rahiman SSF, Salhimi SM. Inhibitory activity of extract, fractions, and compounds from Zingiber montanum rhizomes on the migration of breast cancer cells. Planta Med. 2020;86(06):387–94.
Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin αvβ3 expression. Anticancer Res. 2005;25(1A):79–83
Eid E, S Alanazi A, Koosha S, A Alrasheedy A, Azam F, M Taban I, et al. Zerumbone Induces apoptosis in breast cancer cells by targeting αvβ3 integrin upon co-administration with TP5-iRGD peptide. Molecules. 2019;24(14):2554.
Fatima A, Abdul ABH, Abdullah R, Karjiban RA, Lee VS. Binding mode analysis of zerumbone to key signal proteins in the tumor necrosis factor pathway. Int J Mol Sci. 2015;16(2):2747–66.
Al-Amin M, Eltayeb NM, Khairuddean M, Salhimi SM. Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231. Nat Prod Res. 2019;35:1–5.
Sung B, Murakami A, Oyajobi BO, Aggarwal BB. Zerumbone abolishes RANKL-induced NF-κB activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res. 2009;69(4):1477–84.
Sehrawat A, Sakao K, Singh SV. Notch2 activation is protective against anticancer effects of zerumbone in human breast cancer cells. Breast Cancer Res Treatment. 2014;146(3):543–55.
Han J, Bae SY, Oh SJ, Lee J, Lee JH, Lee HC, et al. Zerumbone suppresses IL-1β-induced cell migration and invasion by inhibiting IL-8 and MMP-3 expression in human triple-negative breast cancer cells. Phytotherapy Res. 2014;28(11):1654–60.
Shyanti RK, Sehrawat A, Singh SV, Mishra J, Singh RP. Zerumbone modulates CD1d expression and lipid antigen presentation pathway in breast cancer cells. Toxicol In Vitro. 2017;44:74–84.
Louderbough JM, Schroeder JA. Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res. 2011;9(12):1573–86.
Kim S, Kil WH, Lee J, Oh S-J, Han J, Jeon M, et al. Zerumbone suppresses EGF-induced CD44 expression through the inhibition of STAT3 in breast cancer cells. Oncol Rep. 2014;32(6):2666–72.
Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The elevated level of CXCR4 is correlated with nodal metastasis of human breast cancer. Breast. 2005;14(5):360–7.
Sung B, Jhurani S, Ahn KS, Mastuo Y, Yi T, Guha S, et al. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68(21):8938–44.
Kim S, Lee J, Jeon M, Lee JE, Nam SJ. Zerumbone suppresses the motility and tumorigenecity of triple negative breast cancer cells via the inhibition of TGF-β1 signaling pathway. Oncotarget. 2016;7(2):1544.
Sierra A, Castellsagué X, Escobedo A, Lloveras B, García-Ramirez M, Moreno A, et al. Bcl-2 with loss of apoptosis allows accumulation of genetic alterations: a pathway to metastatic progression in human breast cancer. Int J Cancer. 2000;89(2):142–7.
Jalili-Nik M, Sadeghi MM, Mohtashami E, Mollazadeh H, Afshari AR, Sahebkar A. Zerumbone promotes cytotoxicity in human malignant glioblastoma cells through reactive oxygen species (ROS) generation. Oxidat Med Cell Longev. 2020;2020:1.
Lynch HT, De la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919–32.
Roh MS, Lee HW, Jung SB, Kim K, Lee EH, Park M-I, et al. Expression of miR-200c and its clinicopathological significance in patients with colorectal cancer. Pathol-Res Pract. 2018;214(3):350–5.
Dermani FK, Amini R, Saidijam M, Pourjafar M, Saki S, Najafi R. Zerumbone inhibits epithelial–mesenchymal transition and cancer stem cells properties by inhibiting the β-catenin pathway through miR-200c. J Cell Physiol. 2018;233(12):9538–47.
Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: evidence for an essential role of reactive oxygen species. Cancer Res. 2009;69(16):6581–9.
Hwang S, Jo M, Hong JE, Park CO, Lee CG, Rhee K-J. Protective effects of zerumbone on colonic tumorigenesis in enterotoxigenic Bacteroides fragilis (ETBF)-colonized AOM/DSS BALB/c mice. Int J Mol Sci. 2020;21(3):857.
Edagawa M, Kawauchi J, Hirata M, Goshima H, Inoue M, Okamoto T, et al. Role of activating transcription factor 3 (ATF3) in endoplasmic reticulum (ER) stress-induced sensitization of p53-deficient human colon cancer cells to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through up-regulation of death receptor 5 (DR5) by zerumbone and celecoxib. J Biol Chem. 2014;289(31):21544–61.
Kirana C, McIntosh GH, Record IR, Jones GP. Antitumor activity of extract of Zingiber aromaticum and its bioactive sesquiterpenoid zerumbone. Nutr Cancer. 2003;45(2):218–25.
Fang Y, Liang X, Jiang W, Li J, Xu J, Cai X. Cyclin b1 suppresses colorectal cancer invasion and metastasis by regulating e-cadherin. PLoS ONE. 2015;10(5): e0126875.
Rajan I, Rabindran R, Nithya N, Lakshmipriya T, Jayasree P, Kumar P. Assessment of cell cycle phase-specific effects of zerumbone on mitotically synchronous surface cultures of Physarum polycephalum. Protoplasma. 2014;251(4):931–41.
Murakami A, Miyamoto M, Ohigashi H. Zerumbone, an anti-inflammatory phytochemical, induces expression of proinflammatory cytokine genes in human colon adenocarcinoma cell lines. Biofactors. 2004;21(1–4):95–101.
Sithara T, Dhanya B, Arun K, Sini S, Dan M, Kokkuvayil Vasu R, et al. Zerumbone, a cyclic sesquiterpene from Zingiber zerumbet induces apoptosis, cell cycle arrest, and antimigratory effects in SW480 colorectal cancer cells. J Agric Food Chem. 2018;66(3):602–12.
Ghouri YA, Mian I, Blechacz B. Cancer review: cholangiocarcinoma. J Carcinogen. 2015;14:1.
Yoon J-H, Gwak G-Y, Lee H-S, Bronk SF, Werneburg NW, Gores GJ. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41(5):808–14.
Songsiang U, Pitchuanchom S, Boonyarat C, Hahnvajanawong C, Yenjai C. Cytotoxicity against cholangiocarcinoma cell lines of zerumbone derivatives. Eur J Med Chem. 2010;45(9):3794–802.
Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635.
Feng W, Xin Y, Xiao Y, Li W, Sun D. Cyclophilin A enhances cell proliferation and xenografted tumor growth of early gastric cancer. Digestive Dis Sci. 2015;60:2700–11.
Wang D, Li Y, Cui P, Zhao Q, Tan B-B, Zhang Z-D, et al. Zerumbone induces gastric cancer cells apoptosis: involving cyclophilin A. Biomed Pharmacotherapy. 2016;83:740–5.
Tsuboi K, Matsuo Y, Shamoto T, Shibata T, Koide S, Morimoto M, et al. Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol Rep. 2014;31(1):57–64.
Rahman HS, Rasedee A, How CW, Zeenathul NA, Chartrand MS, Yeap SK, et al. Antileukemic effect of zerumbone-loaded nanostructured lipid carrier in WEHI-3B cell-induced murine leukemia model. Int J Nanomed. 2015;10:1649.
Rahman HS, Rasedee A, Chartrand MS, Othman HH, Yeap SK, Namvar F. Zerumbone induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in Jurkat cell line. Nat Prod Commun. 2014;9(9):1934578X1400900904.
Rahman HS, Rasedee A, Abdul AB, Zeenathul NA, Othman HH, Yeap SK, et al. Zerumbone-loaded nanostructured lipid carrier induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in a human lymphoblastic leukemia cell line. Int J Nanomed. 2014;9:527.
Rajan I, Jayasree P, Kumar PM. Zerumbone induces mitochondria-mediated apoptosis via increased calcium, generation of reactive oxygen species and upregulation of soluble histone H2AX in K562 chronic myelogenous leukemia cells. Tumor Biol. 2015;36(11):8479–89.
Abdelwahab SI, Abdul AB, Mohan S, Taha MME, Syam S, Ibrahim MY, et al. Zerumbone induces apoptosis in T-acute lymphoblastic leukemia cells. Leukemia Res. 2011;35(2):268–71.
Xian M, Ito K, Nakazato T, Shimizu T, Chen CK, Yamato K, et al. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a Fas-and mitochondria-mediated pathway. Cancer Sci. 2007;98(1):118–26.
Huang G-C, Chien T-Y, Chen L-G, Wang C-C. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005;71(03):219–24.
Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a population-based case–control study. Hepatology (Baltimore, MD). 2011;54(2):463.
Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines. 2021;9(11):1639.
Wani NA, Zhang B, Teng K-Y, Barajas JM, Motiwala T, Hu P, et al. Reprograming of glucose metabolism by zerumbone suppresses hepatocarcinogenesis. Mol Cancer Res. 2018;16(2):256–68.
Taha MME, Abdul AB, Abdullah R, Ibrahim TAT, Abdelwahab SI, Mohan S. Potential chemoprevention of diethylnitrosamine-initiated and 2-acetylaminofluorene-promoted hepatocarcinogenesis by zerumbone from the rhizomes of the subtropical ginger (Zingiber zerumbet). Chemico-Biol Interact. 2010;186(3):295–305.
Sugimachi K, Tanaka S, Terashi T, Taguchi K-I, Rikimaru T, Sugimachi K. The mechanisms of angiogenesis in hepatocellular carcinoma: angiogenic switch during tumor progression. Surgery. 2002;131(1):S135–41.
Samad NA, Abdul AB, Rahman HS, Rasedee A, Tengku Ibrahim TA, Keon YS. Zerumbone suppresses angiogenesis in HepG2 cells through inhibition of matrix metalloproteinase-9, vascular endothelial growth factor, and vascular endothelial growth factor receptor expressions. Pharmacogn Mag. 2018;13(Suppl 4):S731-s6.
Nakamura Y, Yoshida C, Murakami A, Ohigashi H, Osawa T, Uchida K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004;572(1–3):245–50.
Muhammad Nadzri N, Abdul AB, Sukari MA, Abdelwahab SI, Eid EE, Mohan S, et al. Inclusion complex of zerumbone with hydroxypropyl-beta-cyclodextrin induces apoptosis in liver hepatocellular HepG2 Cells via Caspase 8/BID cleavage switch and modulating Bcl2/Bax ratio. Evid-Based Complem Altern Med. 2013;2013:810632.
Lv T, Zhang W, Han X. Zerumbone suppresses the potential of growth and metastasis in hepatoma HepG2 cells via the MAPK signaling pathway. Oncol Lett. 2018;15(5):7603–10.
Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–44.
Leung WS, Yang ML, Lee SS, Kuo CW, Ho YC, Huang-Liu R, et al. Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway. Int Immunopharmacol. 2017;46:194–200.
Wang L, Li Y, Yang S, Wang F, Hou Y, Cui W, et al. Gambogic acid synergistically potentiates cisplatin-induced apoptosis in non-small-cell lung cancer through suppressing NF-κB and MAPK/HO-1 signalling. Br J Cancer. 2014;110(2):341–52.
Hu Z, Zeng Q, Zhang B, Liu H, Wang W. Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. Biochimie. 2014;107:257–62.
Tian C, Peng Z, Chang L, Deng X, Jiang S, Han J, et al. Suppresses of LIM kinase 2 promotes radiosensitivity in radioresistant non-small cell lung cancer cells. Heliyon. 2023;9(11):e22090.
Kang CG, Lee H-J, Kim S-H, Lee E-O. Zerumbone suppresses osteopontin-induced cell invasion through inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung cancer A549 cells. J Nat Prod. 2016;79(1):156–60.
Remmerbach TW, Wottawah F, Dietrich J, Lincoln B, Wittekind C, Guck J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res. 2009;69(5):1728–32.
Aggarwal S, John S, Sapra L, Sharma SC, Das SN. Targeted disruption of PI3K/Akt/mTOR signaling pathway, via PI3K inhibitors, promotes growth inhibitory effects in oral cancer cells. Cancer Chemotherapy Pharmacol. 2019;83:451–61.
Zainal NS, Gan CP, Lau BF, Yee PS, Tiong KH, Abdul Rahman ZA, et al. Zerumbone targets the CXCR4-RhoA and PI3K-mTOR signaling axis to reduce motility and proliferation of oral cancer cells. Phytomedicine. 2018;39:33–41.
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27.
Hung C-F, Wu TC, Monie A, Roden R. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol Rev. 2008;222:43–69.
Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016. https://doi.org/10.3802/jgo.2016.27.e43.
Abdul AB, Abdelwahab SI, Bin Jalinas J, Al-Zubairi AS, Taha MM. Combination of zerumbone and cisplatin to treat cervical intraepithelial neoplasia in female BALB/c mice. Int J Gynecol Cancer. 2009;19(6):1004–10.
Wootipoom V, Lekhyananda N, Phungrassami T, Boonyaphiphat P, Thongsuksai P. Prognostic significance of Bax, Bcl-2, and p53 expressions in cervical squamous cell carcinoma treated by radiotherapy. Gynecol Oncol. 2004;94(3):636–42.
Abdelwahab SI, Abdul AB, Devi N, Taha MM, Al-zubairi AS, Mohan S, et al. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: involvement of mitochondria-regulated apoptosis. Exp Toxicol Pathol. 2010;62(5):461–9.
Liu X, Meng L, Chen L, Liang Y, Wang B, Shao Q, et al. IL-6 expression promoted by Poly (I: C) in cervical cancer cells regulates cytokine expression and recruitment of macrophages. J Cell Mol Med. 2020;24(3):2284–93.
Abdelwahab SI, Abdul AB, Zain ZN, Hadi AH. Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Int Immunopharmacol. 2012;12(4):594–602.
Abdel Wahab SI, Abdul AB, Alzubairi AS, Mohamed Elhassan M, Mohan S. In vitro ultramorphological assessment of apoptosis induced by zerumbone on (HeLa). J Biomed Biotechnol. 2009;2009: 769568.
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–22.
Zhang J, Liu C, Mo X, Shi H, Li S. Mechanisms by which CXCR4/CXCL12 cause metastatic behavior in pancreatic cancer. Oncol Lett. 2018;15(2):1771–6.
Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, et al. CXCL8/IL-8 and CXCL12/SDF-1α co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124(4):853–61.
Shamoto T, Matsuo Y, Shibata T, Tsuboi K, Nagasaki T, Takahashi H, et al. Zerumbone inhibits angiogenesis by blocking NF-kappaB activity in pancreatic cancer. Pancreas. 2014;43(3):396–404.
Zhang S, Liu Q, Liu Y, Qiao H, Liu Y. Zerumbone, a Southeast Asian ginger sesquiterpene, induced apoptosis of pancreatic carcinoma cells through p53 signaling pathway. Evid-Based Complement Alternat Med. 2012;2012:936030.
Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14(19):2410–34.
Truman J-P, Gueven N, Lavin M, Leibel S, Kolesnick R, Fuks Z, et al. Down-regulation of ATM protein sensitizes human prostate cancer cells to radiation-induced apoptosis. J Biol Chem. 2005;280(24):23262–72.
Chiang PK, Tsai WK, Chen M, Lin WR, Chow YC, Lee CC, et al. Zerumbone regulates DNA repair responding to ionizing radiation and enhances radiosensitivity of human prostatic cancer cells. Integrative Cancer Therapies. 2018;17(2):292–8.
Kosakowska-Cholody T, Lin J, Srideshikan S, Scheffer L, Tarasova N, Acharya J. HKH40A downregulates GRP78/BiP expression in cancer cells. Cell Death Dis. 2014;5(5):e1240.
Chan M-L, Liang J-W, Hsu L-C, Chang W-L, Lee S-S, Guh J-H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn-Schmiedeberg’s Arch Pharmacol. 2015;388:1223–36.
Chan ML, Liang JW, Hsu LC, Chang WL, Lee SS, Guh JH. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn-Schmiedeberg’s Arch Pharmacol. 2015;388(11):1223–36.
Jorvig JE, Chakraborty A. Zerumbone inhibits growth of hormone refractory prostate cancer cells by inhibiting JAK2/STAT3 pathway and increases paclitaxel sensitivity. Anti-cancer Drugs. 2015;26(2):160–6.
Messai Y, Noman MZ, Hasmim M, Janji B, Tittarelli A, Boutet M, et al. ITPR1 protects renal cancer cells against natural killer cells by inducing autophagy. Cancer Res. 2014;74(23):6820–32.
Sun Y, Sheng Q, Cheng Y, Xu Y, Han Y, Wang J, et al. Zerumbone induces apoptosis in human renal cell carcinoma via Gli-1/Bcl-2 pathway. Die Pharmazie. 2013;68(2):141–5.
Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K, et al. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer. 2010;102(11):1592–9.
Shanmugam MK, Rajendran P, Li F, Kim C, Sikka S, Siveen KS, et al. Abrogation of STAT3 signaling cascade by zerumbone inhibits proliferation and induces apoptosis in renal cell carcinoma xenograft mouse model. Mol Carcinogen. 2015;54(10):971–85.
Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49(9):978–86.
Chun K-S, Kundu J, Kundu JK, Surh Y-J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol Lett. 2014;229(1):73–84.
Hseu YC, Chang CT, Gowrisankar YV, Chen XZ, Lin HC, Yen HR. Zerumbone exhibits antiphotoaging and dermatoprotective properties in ultraviolet A-irradiated human skin fibroblast cells via the activation of Nrf2/ARE defensive pathway. Oxid Med Cell Longev. 2019;2019:4098674.
Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121(11):2357–63.
Murakami A, Tanaka T, Lee JY, Surh YJ, Kim HW, Kawabata K, et al. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int J Cancer. 2004;110(4):481–90.
Oh T-I, Jung H-J, Lee Y-M, Lee S, Kim G-H, Kan S-Y, et al. Zerumbone, a tropical ginger sesquiterpene of Zingiber officinale Roscoe, attenuates α-MSH-induced melanogenesis in B16F10 cells. Int J Mol Sci. 2018;19(10):3149.
Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19(34):5598–606.
Ma S, Lei Y, Zhang L, Wang J. Effects of zerumbone on proliferation and apoptosis of esophageal cancer cells and on P53 and Bcl-2 expression levels. Oncol Lett. 2018;16(4):4379–83.
Yang Q, Luo G-Y, Li Y, Shan H-B, Wang H-Y, Xu G-L. Expression of Rac-1 related to tumor depth, lymph node metastasis and patient prognosis in esophageal squamous cell carcinoma. Med Oncol. 2013;30:1–7.
Wang M, Niu J, Gao L, Gao Y, Gao S. Zerumbone inhibits migration in ESCC via promoting Rac1 ubiquitination. Biomed Pharmacotherapy. 2019;109:2447–55.
Eid EEM, Abdul AB, Suliman FEO, Sukari MA, Rasedee A, Fatah SS. Characterization of the inclusion complex of zerumbone with hydroxypropyl-β-cyclodextrin. Carbohydr Polym. 2011;83(4):1707–14.
Acknowledgements
The authors would like to express their gratitude to the Cancer Research Center, Semnan University of Medical Sciences, Semnan, Iran.
Funding
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1445)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis, A.S., S. P., D. H., and A.H.; the first draft of the manuscript, A.S., S. P., D. H.; Writing-original draft preparation, M.D.; Draw the figures, R.S. writing—review and editing, N.G. and R.S.; visualization, D.H; supervision, M.D.; project administration, M.D., and N. G.; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soroush, A., Pourhossein, S., Hosseingholizadeh, D. et al. Anti-cancer potential of zerumbone in cancer and glioma: current trends and future perspectives. Med Oncol 41, 125 (2024). https://doi.org/10.1007/s12032-024-02327-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02327-3