Abstract
The epithelial cell adhesion molecule (EpCAM) is a critical glycoprotein involved in cell cycle progression, proliferation, differentiation, migration, and immune evasion. Its role as a target for bispecific antibodies has shown promise in annihilating cancer cells. EpCAM's potential as a biomarker for tumor-initiating cells, characterized by self-renewal and tumorigenic capabilities, underscores its value in early cancer detection, immunotherapy, and targeted drug delivery. While EpCAM monotherapies have been met with limited success, bispecific antibodies targeting both EpCAM and other proteins have exhibited encouraging results in colorectal cancer (CRC) research. The integration of EpCAM-directed nanotechnology in drug delivery systems has emerged as a pivotal innovation in CRC treatment. Moreover, developing chimeric antigen receptor (CAR) T-cell and CAR natural killer (NK) cell therapies opens promising therapeutic avenues for EpCAM-positive CRC patients. Although preliminary, this review sets the stage for future advances. Additionally, this study advances our understanding of the role of non-coding RNAs in CRC, which may be pivotal in gene regulation and could provide insights into the molecular underpinning. The findings suggest that lncRNA, miRNA, and circRNA could serve as novel therapeutic targets or biomarkers, further enriching the landscape of CRC diagnostics and therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial cell adhesion molecule (EpCAM), previously known as CD326 or TACSTD1, is a single transmembrane glycoprotein expressed on the surfaces of some ordinary human epithelial cells and most malignant epithelial tumor cells [1]. Studies indicate that EpCAM facilitates cell interaction and communication. EpCAM is upregulated 100–1000 folds in various primary carcinoma tumor types compared to healthy tissues, rendering it a notable marker in cancer biology [2].
The overexpression of EpCAM is not merely a passive observation; it correlates with worse survival rates and poorer prognoses for cancer patients, including those diagnosed with colorectal cancer (CRC) [3,4,5]. This link is further reinforced by clinical trials [6]. Moreover, given its consistent expression in carcinoma cells, EpCAM has been actively researched as a biomarker for detecting circulating or metastatic carcinoma cells across different malignancies [7]. Considering that CRC ranks as the third most common cancer and leads to mortality, understanding the intricate relationship between CRC and EpCAM becomes crucial for CRC diagnostic advancements [8].
Furthermore, the role of EpCAM in the cellular processes and progression of CRC highlights the escalating interest in its therapeutic targeting [7]. The introduction of the EpCAM-specific antibody marked the inception of monoclonal antibody treatment in oncology in the 1990s [9]. Contemporary advancements in biotechnology and molecular biology have broadened the spectrum of therapeutic agents aimed at EpCAM. This repertoire extends beyond monoclonal antibodies to include bi- and even tri-specific antibodies, enabling simultaneous engagement with multiple targets for a more comprehensive therapeutic strategy [10, 11]. Additionally, EpCAM aptamers, single-stranded RNA or DNA molecules, are gaining traction as formidable drug delivery tools due to their high specificity and modifiability [12]. By conjugating these aptamers to specific drugs, they can direct treatment precisely to EpCAM-positive cells, potentially enhancing therapeutic outcomes while minimizing adverse effects [13]. As insights into the molecular intricacies of CRC deepen, the potential of EpCAM-focused therapeutic strategies in clinical scenarios presents an exciting frontier for research. Although there has been rapid progress in developing EpCAM-targeted antibodies and drug delivery mechanisms for CRC treatment in both in vivo and in vitro contexts, there is a conspicuous dearth of empirical evidence regarding their efficacy and safety in human cancer patients. This review aims to provide a detailed overview of the molecular structure of EpCAM, recent advancements in therapies targeting EpCAM-positive CRC cells, and the implications for patient prognosis.
As our understanding of cancer biology evolves, the intricate relationship between EpCAM and non-coding RNAs has come into the spotlight. Despite not coding for proteins, non-coding RNAs have garnered attention due to their pivotal roles in regulating gene expression and cellular functions. Notably, three primary classes of non-coding RNAs—microRNAs (miRNA), long non-coding RNAs (lncRNAs), and circular RNAs (circRNA)—have been implicated in the pathogenesis of various cancers, including CRC. Emerging research suggests potential interactions between these non-coding RNAs and the EpCAM pathway, which could influence CRC progression, diagnostic approaches, and treatment outcomes. In this review, we delve deeper into this relationship, striving to provide a holistic understanding of the combined role of EpCAM and non-coding RNAs in CRC.
Structure and expression of EpCAM
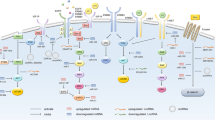
EpCAM, also known as epithelial cell adhesion molecule or CD326 is a type I transmembrane glycoprotein. The gene encoding EpCAM, GA733-2, is located on chromosome 2p21 and is comprised of 9 exons. It falls under the TACSTD1 gene family umbrella, which is known for its vital role in cell proliferation, tumorigenicity, and growth [14]. Unlike classical adhesion molecules in the immunoglobulin superfamily, EpCAM exhibits unique structural characteristics [15]. While it shares functional similarities with adhesion molecules, it does not belong to the four primary families of cell adhesion molecules [16, 17]. Structurally, EpCAM consists of an extracellular domain (EpEX), a single transmembrane domain, and a short intracellular domain (EpICD). Delving deeper into its molecular structure, EpCAM's extracellular domain (EpEX) is responsible for mediating calcium-independent homophilic cell–cell adhesions, it can be cleaved and shed by the tumor necrosis factor α converting enzyme, while the intracellular domain (EpICD), upon being released by the presenilin 2-containing γ-secretase complex, translocates to the cell nucleus. In the nucleus, EpICD forms a complex with other proteins, including Lef-1, FHL2, and β-catenin. This nuclear complex is instrumental in regulating the expression of genes associated with the epithelial-mesenchymal transition (EMT), a process critical in cancer metastasis and progression [18] (Fig. 1A).
In normal human tissues, EpCAM is expressed in most human epithelial cells. It is expressed on the basolateral membrane of epithelial cells and serves as a molecule that enhances cellular adhesion to the extracellular matrix and other substrates [17]. Typically, EpCAM protein expression is minimal in human epithelial tissues during the embryonic phase. However, postnatally, it remains consistently expressed in most epithelial cells. Among various human tissues, the colon showcases the most pronounced EpCAM expression, highlighting its significance in CRC [17]. Contrarily, specific cells like epidermal keratinocytes, hepatocytes, thymic cortical epithelial cells, and squamous stratified epithelial cells exhibit no EpCAM expression.
Meanwhile, EpCAM is widely expressed in human cancer cells, especially adenocarcinoma and squamous cell carcinoma, both are common types of CRC. High levels of EpCAM can activate oncogenes such as C-myc and cyclin A and E, accelerate the cell cycle, and boost cell proliferation. Tumor cells with EpCAM overexpression have high proliferation and strong invasion, and EpCAM down-regulation through RNA interference was conformed to decrease these factors [19]. EpCAM also plays a role in Th2 differentiation and immune escape by blocking major histocompatibility complex (MHC) class II antigen presentation and creating defective CD4+T cells, helping the tumor escape CD4+T cell-dependent immune response and promoting tumorigenesis [20]. Moreover, overexpression of EpCAM is shown to increase levels of the transcription factor slug, and activation of the PTEN/Akt/mTOR signaling pathway, both of which are associated with EMT, leading to increased cell motility and migration in colorectal tumor cells [21].
In summary, the functions and impacts of EpCAM are diverse and context-dependent, highlighting its dualistic nature in maintaining normal epithelial cell behavior and contributing to the malignancy of CRC cells.
EpCAM and diagnosis of colorectal carcinoma
As of now, surgery remains the primary therapeutic approach for most CRC, often supplemented by chemotherapy and radiotherapy [22]. To ensure the best therapeutic outcomes, early diagnosis is imperative. Consequently, the identification of biomarkers, such as EpCAM, plays a vital role in improving prognosis [23]. In transplantation experiments conducted on NOD-SCID mice, only the cells expressing EpCAM were capable of effectively initiating the development of invasive tumors, which persisted across serial transplantations [24]. This persistence underscores that EpCAM-positive cells exhibit characteristics akin to cancer stem cells (CSCs). A comprehensive review of over 150 CRC tumor specimens revealed a significant trend: cells exhibiting heightened EpCAM expression (EpCAM +) shared characteristics with hepatic stem cells and demonstrated a markedly enhanced invasive capacity compared to EpCAM- cells [24]. These results suggest that EpCAM serves as a biomarker for identifying cancer stem cells within a tumor population, which is significant in the early diagnosis of malignant cancers such as colorectal cancer [25]. Furthermore, in CRC diagnostics, elevated EpCAM levels suggest the possible prevalence of these aggressive tumor stem cells, thus indicating a heightened cancer risk. Research by Wen-Sy Tsai et al. demonstrated that when combined with established diagnostic tools like CEA, EpCAM + circulating tumor cells (CTCs) notably reduced false positives, thereby augmenting disease detection rates [26]. Research by Xin Shou et al. put forward a new Six-gene Assay, based on six genes (EpCAM and CEA, CK19, MUC1, EGFR, and C-Met), proving that it was more sensitive and accurate than CEA Gene Assay in diagnosing CRC [27]. These integrated approaches promise to refine the accuracy of CRC diagnostics.
In addition, alterations in the EpCAM gene structure in CRC have garnered significant academic interest. Prior studies have explored its association with clinicopathologic and molecular factors, including the expression of DNA mismatch repair proteins and the methylation status of the MLH1 promoter. Among 168 microsatellite instability-high (MSI-H) colorectal carcinomas, complete loss of EpCAM was exclusively observed in MSH2-deficient MSI-high CRCs [28]. In contrast, partial loss primarily manifested in MLH1-deficient tumors. Another investigation evaluated the methylation of the EpCAM promoter, microsatellite instability (MSI), and the CpG island methylator phenotype across extensive cohorts of human CRCs [29]. The results indicated that a complete germline loss of EpCAM distinctly marks Lynch syndrome-associated CRC, while a partial loss may suggest the presence of invasive tumor components in CRC.
Moreover, EpCAM-mRNA has been proposed as a potential detective marker for assessing CRC metastasis. Zhang and colleagues employed quantitative RT-PCR to examine EpCAM-mRNA across 60 exudate samples sourced from the thorax and peritoneum. Their findings confirmed that EpCAM-mRNA can efficiently differentiate benign from malignant exudates, boasting a sensitivity of 75.0% and an accuracy rate of 81.6% [30]. This diagnostic performance surpassed traditional cytological examination. Given the correlation between mRNA and protein expression, it is worth noting that peritoneal metastasis is a frequent and stubborn complication in advanced CRC stages. Thus, EpCAM's role in predicting disease progression and outcomes is invaluable.
Notably, EpCAM has also emerged as a pivotal DNA aptamer in advancing CRC diagnostics, particularly in the sensitive detection of CRC exosomes. By capitalizing on the interactions between graphene oxide and DNA aptamers, researchers have fine-tuned a novel technique that prioritizes EpCAM for CRC exosome detection. When CRC exosomes are present, they bind to aptamers, especially the EpCAM aptamer, leading to the recovery of fluorescence from fluorophore-labeled aptamers that had been previously suppressed by graphene oxide. To amplify the diagnostic signal, DNase I is employed to digest single-stranded DNA aptamers on the exosome surface. This step facilitates more interactions between the exosomes and fluorescent aptamer probes, particularly the EpCAM probe. Clinical validation of this approach using blood serum samples successfully differentiated CRC patients from healthy individuals, underscoring the significant diagnostic potential of EpCAM in CRC [31].
In conclusion, while current EpCAM-centric research in CRC diagnosis remains in its infancy, all existing studies herald its prospective utility. There's a pressing need for further research to corroborate these diagnostic mechanisms and further delve into the potential incorporation of EpCAM in CRC diagnostic practices.
EpCAM and colorectal cancer treatment
Monoclonal antibodies targeting the EpCAM have been investigated for their potential to combat CRC. This therapeutic strategy employs two principal mechanisms to eradicate cancer cells: activation of the complement system and antibody-dependent cell-mediated cytotoxicity (ADCC). Edrecolomab, a murine monoclonal IgG2 antibody against the EpCAM antigen, recognizes the tumor-associated antigen 17-1A and has been used to treat breast cancer and CRC. It has shown benefits in recurrence-free and overall survival, especially when used as a part of adjuvant chemotherapy [9, 32]. A more recent development is the human anti-EpCAM monoclonal IgG1 antibody, Adecatumumab (MT201), which has shown increased efficacy in ADCC compared to Edrecolomab. Preliminary studies, such as those by Schmidt et al., have suggested that MT201 may not induce significant tumor regression as a single agent but can slow tumor progression, advocating for further investigation into its use for tumors with high EpCAM expression or minimal residual disease [33]. Despite these advancements, the role of EpCAM monoclonal antibodies in the treatment of CRC remains underexplored. Most of the research has been confined to breast cancer thus far [34, 35]. The variability in response and the complexity of tumor biology necessitates a combination of therapies tailored to individual patient profiles, and EpCAM monoclonal antibodies' standalone use in CRC treatment appears insufficient. Clinical trials targeting EpCAM have yielded unsatisfactory results, chiefly due to the antibodies' suboptimal specificity and low affinity, highlighting that this area is still nascent and requires further refinement [36]. Thus, researchers should focus on integrating these therapies into a multifaceted treatment approach, possibly in conjunction with other targeted therapies or immune checkpoint inhibitors, to enhance their therapeutic potential.
Bispecific antibodies, capable of concurrently targeting T cells and cancer cells, are emerging as a promising immunotherapeutic modality. An EpCAM/CD3 bispecific single-chain antibody, MT110, has shown efficacy in activating CD8+T cells, leading to the secretion of pro-inflammatory cytokines and subsequent lysis of tumor cells. Preclinical studies have demonstrated MT110's effectiveness against metastatic ovarian cancer in murine models. Encouraging results from Phase I clinical trials indicate that MT110 is generally well-tolerated and biologically active in patients with advanced EpCAM-expressing cancers, including gastric, colorectal, and lung carcinomas [37, 38]. Catumaxomab, another EpCAM/CD3-targeting trifunctional bispecific antibody, demonstrates promising safety profiles and potential efficacy in the treatment of CRC, as evidenced by early-phase clinical trials [39]. This therapeutic agent engages CD3+T cells via its antigen-binding sites, simultaneously targeting EpCAM receptors on the surface of tumor cells. The innovative design of catumaxomab includes a fragment crystallizable (Fc) domain, which further recruits and activates natural killer (NK) cells. This engagement results in the phagocytosis of tumor cells and triggers cell death through the release of pro-apoptotic cytokines, including IL-2, IL-12, and TNF-α [40,41,42]. What’s more, researchers have recently discovered a novel tri-specific T-cell-recruiting antibody for the treatment of CRC consisting of single-domain antibodies directed against CD3, epidermal growth factor receptor (EGFR), and EpCAM, which can specifically kill cancer cells expressing EGFR and/or EpCAM, while improving in vitro potency and significantly prolonging survival time in vivo [11]. These findings may help make Bispecific antibodies better and improve their anti-tumor efficacy in the future.
Moreover, EpCAM-targeted aptamers can facilitate the delivery of specific anti-tumor agents, thereby effectively eradicating CRC cells. A prevailing challenge in contemporary cancer chemotherapy is the safe and efficient localization of therapeutic agents at the site of disease [43]. EpCAM aptamers serve as an ideal mechanism for targeting CRC cells, as they exhibit a specific affinity for EpCAM. Compared to antibodies, these aptamers offer numerous advantages, including their smaller size, reduced potential for immunogenicity, enhanced stability, straightforward synthesis, and versatile modifiability. Research has demonstrated that when curcumin-loaded lipopolymer-lecithin hybrid nanoparticles are conjugated with EpCAM-targeted RNA aptamers, they preferentially bind to and are internalized by CRC cells, leading to significantly improved cytotoxicity compared to untargeted nanoparticles. Such functionalization of nanoparticles with EpCAM aptamers enhances the specificity and efficiency of drug delivery to CRC cells, signifying a potent strategy for cancer treatment. Furtherly, the integration of nanotechnology in drug delivery systems presents novel opportunities to augment the therapeutic efficacy of treatments like EpCAM aptamers, while also reducing adverse effects. For instance, the encapsulation of Tanshinone II-A within EpCAM-targeted nanoparticles (EpCAM-TSIIA-NPs) not only increases the drug's solubility and cytotoxicity but also provides superior targeting capabilities [12]. These nanoparticles specifically inhibit tumor growth and metastasis by stabilizing the β-catenin destruction complex, thus mitigating the nuclear actions of β-catenin that are implicated in tumor progression. The potential of this technology extends to various pharmacological agents, including chemotherapeutics like 5-fluorouracil, the cytotoxic agent DM1, and even common drugs such as aspirin, illustrating a versatile platform for cancer therapy. EpCAM aptamer-based strategies epitomize a promising frontier in the targeted treatment of CRC, showcasing the synergy between molecular biology and nanotechnology to improve patient outcomes.
Finally, the EpCAM-based treatment landscape for CRC is evolving with the advent of novel therapeutic approaches. Chimeric antigen receptor (CAR) T-cell therapy, a treatment paradigm originally effective in hematological malignancies, is now being explored for its potential in combating solid tumors, including CRC. This transition addresses the challenge posed by the immunosuppressive tumor microenvironment characteristic of solid tumors. CAR T cells engineered to target EpCAM have demonstrated selective cytotoxicity against cancer cell lines with high EpCAM expression [44]. In parallel, the role of EpCAM in activating oncogenic signaling pathways like AKT and MAPK has been targeted by the development of neutralizing antibodies. EpAb2-6, an EpCAM-neutralizing antibody, has shown potential in inhibiting these signaling pathways, promoting apoptosis, and enhancing the cytotoxic activity of CD8+T cells. The combination of EpAb2-6 with Atezolizumab, an anti-PD-L1 antibody, has led to near-complete tumor eradication in preclinical models, thereby revealing a synergistic potential that warrants further exploration. Such combination strategies, which capitalize on EpCAM expression in CRC tumors, could represent a significant advance in cancer immunotherapy [45, 46]. Collectively, these findings underscore the promise of EpCAM-associated CAR T-cell therapy and associated immunotherapeutic strategies in the treatment of CRC, emphasizing the importance of personalized and targeted approaches in the future of cancer therapy.
In summary, while the war against CRC is far from won, the horizon is brightening with these emerging therapies. The breakthrough of monoclonal antibodies, bispecific antibodies, EpCAM-based drug delivery, and so on (Table 1), heralds a new era in the management of colorectal cancer, bringing hope for more effective and personalized treatment options.
EpCAM and colorectal cancer prognosis
The intricate role of EpCAM in oncogenesis underscores a multifaceted paradigm in cancer biology and prognostication. On one hand, EpCAM's loss on the cell membrane correlates with increased tumor cell dissociation and locoregional metastasis in colorectal carcinoma [47]. This association is reinforced by beta-catenin's nuclear translocation in the absence of membranous EpCAM, signifying EMT and potential for invasive cancer phenotypes [47]. On the other hand, the presence of EpCAM, particularly truncated EpCAM variants, portends a dismal clinical prognosis. This variant's expression correlates strongly with advanced stages of colorectal cancer, higher histological grades, and vascular invasion, leading to diminished survival rates [48]. Related studies further demonstrated that EpCAM can promote colon cancer progression and metastasis by modulating epidermal growth factor receptor signaling [46]. Consequently, the EpCAM and its variant emerge as prognostic biomarkers, indicative of aggressive cancer behavior and warranting further investigation into its oncogenic mechanisms. In cancer metastasis, EpCAM's role becomes even more intriguing. The discovery of EpCAM-negative CTCs that carry a brain metastasis-selected marker signature undermines the conventional reliance on EpCAM as a universal CTC marker [49]. These cells exhibit potent invasiveness and the capacity to form metastatic brain lesions, suggesting a pivotal role for EpCAM-negative CTCs in the metastatic cascade, particularly in the brain [49].
Turning to the practical applications of EpCAM in predicting colorectal cancer outcomes, the dichotomous nature of EpCAM expression demands recognition. Predictive models incorporating EpCAM status must consider its variable expression patterns, suggesting a nuanced role for EpCAM and advocating for a customized approach in its clinical use as a biomarker.
The prognostic landscape for CRC has evolved with the inclusion of circulating biomarkers, notably EpCAM, in liquid biopsies. Studies illustrate EpCAM's significant role in enriching CTCs and CTMs for early CRC recurrence prediction, highlighting its advantage over traditional serum markers like CEA and CA19-9 [50]. The detection of EpCAM-positive CTCs/CTMs using a microfluidic chip-based imaging platform correlates negatively with patient survival, suggesting a higher recurrence rate in advanced-stage CRC [50].
Furthermore, advances in single-molecule array technology have enabled the development of highly sensitive immunoassays for detecting EpCAM-expressing extracellular vesicles (EVs). These assays differentiate cancerous from non-cancerous plasma samples, providing a non-invasive diagnostic tool [51]. This assay demonstrates superior diagnostic accuracy for CRC patients by comparing CD9-CD63 and EpCAM-CD63 signals across patient cohorts [51]. Flow cytometry has also been instrumental in enumerating and subtyping EVs, revealing that metastatic CRC patients have higher blood levels of EpCAM-positive EVs, correlating with reduced overall survival and lower response rates to systemic therapy [52]. This finding underscores the prognostic value of EpCAM-positive EVs as a blood-based biomarker for risk stratification and treatment optimization in CRC.
At the tissue level, the clinical relevance of EpCAM extends to its role in Neutrophil extracellular traps (NETs) within CRC tissues. High-resolution microscopy has revealed the presence of citrullinated histone H3, a marker for citrullinated NETs, closely associated with extracellular DNA, a NETs characteristic. These NETs, significantly associated with higher tumor grades and lymph node metastasis, implicate a role in tumorigenesis and metastatic progression, facilitated by an EpCAM-mediated EMT-like process in CRC cells [53].
The cBio Cancer Genomics Portal (http://cbioportal.org) is an invaluable open-access resource for exploring cancer genomics data. Analysis of 13 datasets, including those from DFCI, Genentech, TCGA, and MSK, reveals EpCAM alterations in CRC. These datasets indicate that mutations play a crucial role in EpCAM alterations (Fig. 2A), and although copy number alterations are infrequent, the presence of shadow deletions notably increases the mutation count (Fig. 2B). This evidence further substantiates the link between EpCAM alterations and CRC. Our analysis, based on these 13 datasets, indicates that cases with EpCAM alterations exhibit improved 3-year overall survival (Fig. 2C), suggesting EpCAM's potential as a prognostic biomarker for CRC patients.
A changes of EpCAM in colorectal cancer using 13 bowel-related datasets from cbioportal. B correlation plot for EpCAM, samples with EpCAM shadow deletion have a significantly higher mutation count. C survival analysis. Patients of colorectal cancer with an EpCAM alteration have better 3-year overall survival than those without an EpCAM alteration
In conclusion, while the complete analysis of the mechanisms linking EpCAM to cancer prognosis remains to be elucidated, the diverse applications of EpCAM, from liquid biopsy markers to tissue-based NETs, provide a comprehensive prognostic framework for CRC. There is a need for better methods to detect the structure and expression of EpCAM for improved prognostication of CRC clinical outcomes.
EpCAM and non-coding RNAs
The emerging complexity of the interaction between EpCAM and non-coding RNAs in CRC is beginning to be elucidated, offering new insights into the molecular underpinnings of CRC pathogenesis and highlighting potential therapeutic targets (Fig. 1B). Recent research has shed light on the regulatory role of ncRNAs, particularly miRNAs, lncRNAs, and circRNAs, in modulating EpCAM expression and functionality.
Terminal differentiation-induced ncRNA (TINCR), a lncRNA implicated in cell differentiation, has been demonstrated to interact directly with EpCAM. Significantly reduced in CRC and metastatic CRC cell lines, TINCR's downregulation contributes to tumor progression activities. What’s more, RNA pull-down assays show that the loss of TINCR leads to augmented hydrolysis of EpCAM, facilitating the release of EpICD and subsequent activation of the Wnt/β-catenin signaling pathway, thereby promoting tumorigenesis [54]. Conversely, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), another lncRNA, has been identified as regulating pro-migratory markers, including EpCAM, which intimates a tumor-suppressive function in CRC. The influence of MALAT1 on EpCAM suggests its role as a negative regulator of tumor progression [55].
MiRNAs have been recognized for their preferential expression in EpCAM + /CD44 + CRC cells, known to be enriched in CSCs. MiR-221, associated with CRC proliferation and metastasis, is observed to increase the organoid-forming capacity—a characteristic of CSCs—in CRC cells. Additionally, miR-221 targets the QKI-5 isoform of the Quaking gene, affecting EpCAM expression levels and thereby influencing CRC cell viability and tumor growth [56]. Furthermore, a study conducted by the University of Copenhagen has demonstrated that miRNA profiling of EpCAM + EVs extracted from plasma and serum can facilitate early detection and intervention in CRC. This research has identified eight miRNAs derived from tumors that may serve as non-invasive biomarkers for CRC detection, including miR-16-5p, miR-23a-3p, miR-23b-3p, miR-27a-3p, miR-27b-3p, miR-30b-5p, miR-30c-5p, and miR-222-3p. This work underscores a blood-based, non-invasive approach for sensitive cancer detection with clinical application potential [57].
In addition to miRNAs and lncRNAs, advancements have underscored the significance of circRNAs associated with EpCAM. Investigations into public circRNA databases (http://www.circbase.org/) have pinpointed EpCAM-associated circRNAs among the myriad of circRNAs cataloged in human samples, presenting new opportunities for understanding the functional consequences of EpCAM in CRC. Among the 65,731 cataloged circRNAs, 5 EpCAM-associated circRNAs have been detected, meriting further investigation into EpCAM's role.
The complex interplay between EpCAM and these ncRNAs forms a sophisticated regulatory network that modulates CRC progression. TINCR and MALAT1 seem to exert EpCAM-mediated suppression of CRC proliferation and metastasis, while miR-221 appears to augment these processes. Unraveling these molecular interactions opens avenues for developing targeted therapeutics and identifying novel biomarkers for CRC, underscoring the imperative for continued research into the EpCAM-ncRNA axis in CRC.
Conclusion and future perspectives
This review has highlighted the complex biological functions of EpCAM within the tumorigenic context, particularly in CRC. EpCAM's involvement in cellular processes fundamental to tumor progression such as cell cycle regulation, proliferation, differentiation, and immune evasion positions it as a critical biomarker and therapeutic target.
The therapeutic landscape has been invigorated by the advent of EpCAM-specific antibodies, including monoclonal and innovative bispecific agents. These agents exploit the antigenic specificity of EpCAM to mediate targeted cytotoxicity against CRC cells, thus heralding a new frontier in immuno-oncology. Despite these advances, the success of such targeted therapies has been tempered by the complexity of EpCAM's regulation and its multifaceted role in cancer pathophysiology, which remains incompletely understood.
Future investigations are mandated to dissect the molecular intricacies governing EpCAM expression and its downstream signaling cascades. This will not only refine our current therapeutic approaches but also facilitate the design of novel interventions that can be integrated into multimodal treatment regimens. Moreover, the emerging role of non-coding RNAs in post-transcriptional regulation presents a promising area of research, offering potential mechanisms to modulate EpCAM activity and thus CRC progression.
As we expand our molecular purview, there is a compelling need for translational studies that bridge the gap between benchtop discoveries and bedside applications. Anticipated advances in the characterization of EpCAM interactions and the impact of non-coding RNAs may lead to groundbreaking precision medicine strategies, shifting the paradigm of CRC management towards more personalized and effective treatment modalities.
The confluence of these future scientific endeavors will undeniably enrich the armamentarium against CRC, optimizing patient outcomes through an augmented understanding of tumor biology and the consequent innovation of targeted therapies. The potential of EpCAM-centric research in oncology thus will represent a cornerstone in the quest for eradicating colorectal cancer, reinforcing the imperative for sustained investigative rigor and interdisciplinary collaboration in the field.
Data Availability
All relevant supporting data are cited properly within the manuscript.
Abbreviations
- EpCAM:
-
Epithelial cell adhesion molecules
- CRC:
-
Colorectal cancer
- miRNA:
-
MicroRNAs
- LncRNAs:
-
Long non-coding RNAs
- circRNAs:
-
Circular RNAs
- EpEX:
-
EpCAM extracellular domain
- EpICD:
-
EpCAM intracellular domain
- EMT:
-
Epithelial-mesenchymal transition
- MHC:
-
Major histocompatibility complex
- FDA:
-
Food and Drug Administration
- CSCs:
-
Cancer stem cells
- MSI:
-
Microsatellite instability
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- CAR:
-
Chimeric antigen receptor
- CTCs:
-
Circulating tumor cells
- EVs:
-
Extracellular vesicles
- NETs:
-
Neutrophil extracellular traps
- TINCR:
-
Terminal differentiation-induced ncRNA
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
References
Calabrese G, Crescenzi C, Morizio E, et al. Assignment of TACSTD1 (alias TROP1, M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2 (alias TROP2, M1S1) to human chromosome 1p32 by in situ hybridization. Cytogenet Cell Genet. 2001;92:164–5.
Liu Z, Zhang C, Cui B, et al. Targeted EpCAM-binding for the development of potent and effective anticancer proteins. Biomed Pharmacother. 2023;161: 114443.
Spizzo G, Went P, Dirnhofer S, et al. High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat. 2004;86:207–13.
Spizzo G, Went P, Dirnhofer S, et al. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol. 2006;103:483–8.
Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9.
Goossens-Beumer IJ, Zeestraten EC, Benard A, et al. Clinical prognostic value of combined analysis of Aldh1, Survivin, and EpCAM expression in colorectal cancer. Br J Cancer. 2014;110:2935–44.
Patriarca C, Macchi RM, Marschner AK, et al. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68–75.
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Riethmuller G, Schneider-Gadicke E, Schlimok G, et al. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes’ C colorectal carcinoma. German Cancer Aid 17–1A Study Group. Lancet. 1994;343:1177–83.
Golubovskaya V, Sienkiewicz J, Sun J, et al. mRNA-lipid nanoparticle (LNP) delivery of humanized EpCAM-CD3 bispecific antibody significantly blocks colorectal cancer tumor growth. Cancers (Basel). 2023;15:28650.
Tapia-Galisteo A, Sanchez Rodriguez I, Aguilar-Sopena O, et al. Trispecific T-cell engagers for dual tumor-targeting of colorectal cancer. Oncoimmunology. 2022;11:2034355.
Zhang Y, Li C, Jia R, et al. PEG-poly(amino acid)s/EpCAM aptamer multifunctional nanoparticles arrest the growth and metastasis of colorectal cancer. Biomater Sci. 2021;9:3705–17.
Li Y, Duo Y, Zhai P, et al. Dual targeting delivery of miR-328 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Nanomedicine (Lond). 2018;13:1753–72.
Litvinov SV, Velders MP, Bakker HA, et al. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–46.
Armstrong A, Eck SL. EpCAM: a new therapeutic target for an old cancer antigen. Cancer Biol Ther. 2003;2:320–6.
Fagotto F. EpCAM as modulator of tissue plasticity. Cells. 2020;9:2128.
Yahyazadeh Mashhadi SM, Kazemimanesh M, Arashkia A, et al. Shedding light on the EpCAM: An overview. J Cell Physiol. 2019;234:12569–80.
Kuan II, Liang KH, Wang YP, et al. EpEX/EpCAM and Oct4 or Klf4 alone are sufficient to generate induced pluripotent stem cells through STAT3 and HIF2alpha. Sci Rep. 2017;7:41852.
Munz M, Kieu C, Mack B, et al. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23:5748–58.
Gutzmer R, Li W, Sutterwala S, et al. A tumor-associated glycoprotein that blocks MHC class II-dependent antigen presentation by dendritic cells. J Immunol. 2004;173:1023–32.
Wang MH, Sun R, Zhou XM, et al. Epithelial cell adhesion molecule overexpression regulates epithelial-mesenchymal transition, stemness and metastasis of nasopharyngeal carcinoma cells via the PTEN/AKT/mTOR pathway. Cell Death Dis. 2018;9:2.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502.
Peiris-Pages M, Martinez-Outschoorn UE, Pestell RG, et al. Cancer stem cell metabolism. Breast Cancer Res. 2016;18:55.
Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010;52:280–1.
Clarke MF. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380:2237–45.
Tsai WS, Hung WS, Wang TM, et al. Circulating tumor cell enumeration for improved screening and disease detection of patients with colorectal cancer. Biomed J. 2021;44:S190–200.
Shou X, Li Y, Hu W, et al. Six-gene assay as a new biomarker in the blood of patients with colorectal cancer: establishment and clinical validation. Mol Oncol. 2019;13:781–91.
Kim JH, Bae JM, Kim KJ, et al. Differential features of microsatellite-unstable colorectal carcinomas depending on EPCAM expression status. Korean J Pathol. 2014;48:276–82.
Kim JH, Bae JM, Song YS, et al. Clinicopathologic, molecular, and prognostic implications of the loss of EPCAM expression in colorectal carcinoma. Oncotarget. 2016;7:13372–87.
Zhang KH, Cao F, Fu QB, et al. Detection of mRNAs of GA733 genes by RT-PCR in exfoliated cells of pleural and peritoneal effusions and its clinical values. Intern Med. 2007;46:1489–94.
Wang H, Chen H, Huang Z, et al. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta. 2018;184:219–26.
Riethmuller G, Holz E, Schlimok G, et al. Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788–94.
Schmidt M, Scheulen ME, Dittrich C, et al. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann Oncol. 2010;21:275–82.
Prang N, Preithner S, Brischwein K, et al. Cellular and complement-dependent cytotoxicity of Ep-CAM-specific monoclonal antibody MT201 against breast cancer cell lines. Br J Cancer. 2005;92:342–9.
Richter CE, Cocco E, Bellone S, et al. High-grade, chemotherapy-resistant ovarian carcinomas overexpress epithelial cell adhesion molecule (EpCAM) and are highly sensitive to immunotherapy with MT201, a fully human monoclonal anti-EpCAM antibody. Am J Obstet Gynecol. 2010;203(582):e1-7.
Eyvazi S, Farajnia S, Dastmalchi S, et al. Antibody based EpCAM targeted therapy of cancer, review and update. Curr Cancer Drug Targets. 2018;18:857–68.
Kebenko M, Goebeler ME, Wolf M, et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE(R)) antibody construct, in patients with refractory solid tumors. Oncoimmunology. 2018;7: e1450710.
Khosla AA, Jatwani K, Singh R, et al. Bispecific antibodies in lung cancer: a state-of-the-art review. Pharmaceuticals (Basel). 2023;16:1.
Sluiter N, de Cuba E, Kwakman R, et al. Adhesion molecules in peritoneal dissemination: function, prognostic relevance and therapeutic options. Clin Exp Metastasis. 2016;33:401–16.
Seimetz D. Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab). J Cancer. 2011;2:309–16.
Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–21.
Heiss MM, Strohlein MA, Jager M, et al. Immunotherapy of malignant ascites with trifunctional antibodies. Int J Cancer. 2005;117:435–43.
Gullotti E, Yeo Y. Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol Pharm. 2009;6:1041–51.
Li W, Zhou Y, Wu Z, et al. Targeting Wnt signaling in the tumor immune microenvironment to enhancing EpCAM CAR T-cell therapy. Front Pharmacol. 2021;12: 724306.
Chen HN, Liang KH, Lai JK, et al. EpCAM signaling promotes tumor progression and protein stability of PD-L1 through the EGFR pathway. Cancer Res. 2020;80:5035–50.
Lee CC, Yu CJ, Panda SS, et al. Epithelial cell adhesion molecule (EpCAM) regulates HGFR signaling to promote colon cancer progression and metastasis. J Transl Med. 2023;21:530.
Gosens MJ, van Kempen LC, van de Velde CJ, et al. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20:221–32.
Seeber A, Untergasser G, Spizzo G, et al. Predominant expression of truncated EpCAM is associated with a more aggressive phenotype and predicts poor overall survival in colorectal cancer. Int J Cancer. 2016;139:657–63.
Zhang L, Ridgway LD, Wetzel MD, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5:180ra48.
Hao YJ, Chang LW, Yang CY, et al. The rare circulating tumor microemboli as a biomarker contributes to predicting early colorectal cancer recurrences after medical treatment. Transl Res. 2023;263:1–9.
Mego M, De Giorgi U, Dawood S, et al. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer. 2011;129:417–23.
Brocco D, Simeone P, Buca D, et al. Blood circulating CD133+ extracellular vesicles predict clinical outcomes in patients with metastatic colorectal cancer. Cancers (Basel). 2022;14:1.
Stehr AM, Wang G, Demmler R, et al. Neutrophil extracellular traps drive epithelial-mesenchymal transition of human colon cancer. J Pathol. 2022;256:455–67.
Zhang ZY, Lu YX, Zhang ZY, et al. Loss of TINCR expression promotes proliferation, metastasis through activating EpCAM cleavage in colorectal cancer. Oncotarget. 2016;7:22639–49.
Amodio N, Raimondi L, Juli G, et al. MALAT1: a druggable long non-coding RNA for targeted anti-cancer approaches. J Hematol Oncol. 2018;11:63.
Mukohyama J, Isobe T, Hu Q, et al. miR-221 targets QKI to enhance the tumorigenic capacity of human colorectal cancer stem cells. Cancer Res. 2019;79:5151–8.
Ostenfeld MS, Jensen SG, Jeppesen DK, et al. miRNA profiling of circulating EpCAM(+) extracellular vesicles: promising biomarkers of colorectal cancer. J Extracell Vesicles. 2016;5:31488.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors provided critical revisions of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose. The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, X., Wang, S., Liang, Q. et al. Unraveling the multifaceted role of EpCAM in colorectal cancer: an integrated review of its function and interplay with non-coding RNAs. Med Oncol 41, 35 (2024). https://doi.org/10.1007/s12032-023-02273-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02273-6