Abstract
Prostate cancer is an epithelial malignant tumor occurring in the prostate and is the most common malignant tumor in the male genitourinary system. In recent years, the incidence of prostate cancer in China has shown a trend of sudden increase. The search for new and effective drugs to treat prostate cancer is therefore extremely important.The canonical Wnt/β-catenin signaling pathway has been shown to be involved in the regulation of tumor proliferation, migration and differentiation. Activation of the canonical Wnt/β-Catenin signaling pathway in the prostate has oncogenic effects. Drugs targeting the canonical Wnt/β-catenin signaling pathway have great potential in the treatment of prostate cancer. In this study, we found that Gastrodin could significantly inhibit the proliferation of prostate cancer cell line PC3 and DU145. Oral administration Gastrodin could significantly inhibit the tumor growth of PC3 cells subcutaneously injected. Gastrodin has an inhibitory effect on canonical Wnt/β-Catenin signaling pathway in Prostate cancer, and this inhibitory effect can be abolished by Wnt/β-Catenin agonist LiCl. These findings raise the possibility that Gastrodin can be used in the treatment of Prostate cancer by targeting canonical Wnt/β-Catenin signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most frequent cancer and the fifth-leading cause of cancer-related death in men [1]. Prostate cancer is one of the most common cancers in the United States and Japan and is the leading cause of cancer-related deaths [2, 3]. In China, prostate cancer has also been increasing dramatically at a rate of 10% per year in recent years, especially as China is entering an aging society, and a peak incidence is likely to occur in the next decade. Most patients are already in the middle or late stages of prostate cancer when diagnosed [4,5,6]. Although the survival time of patients is greatly prolonged with continuous advances in diagnosis and treatment, the 5-year overall survival rate of some highly malignant patients remains low [7, 8].
Many advanced prostate cancers initially respond to androgen ablative therapy, but later develop an aggressive androgen non-dependent phenotype that is resistant to conventional therapy and metastasizes to lymph nodes and bone [9,10,11]. The multidrug resistance exhibited by prostate cancer has been the main cause of tumor recurrence, metastasis, and even treatment failure, which is the most common and difficult problem to overcome in clinical prostate cancer treatment [12,13,14]. The efficacy of current chemotherapeutic drugs against prostate cancer is not very satisfactory and searches for active ingredients from natural substances are an important development strategy for the development of anti-prostate cancer drugs. Therefore, the search for effective herbal or bioactive ingredients for the treatment of Prostate cancer has become a new trend [15]. As the use of herbal medicine becomes more widespread, increasing interests have switched to the natural components from herbs.
Gastrodin (4-hydroxybenzyl alcohol-4-o-β-D-glucoside), its molecular weight is 286.28 (Fig. 1A). Gastrodin is a traditional ingredient in Chinese medicine, which has been shown to have the functions of anti-inflammation, anti-oxidation, anti-apoptosis, anti-central nervous aging [16,17,18,19,20,21,22]. In tumors, gastrodin has the potential to alleviate tumorigenesis through a GTP-Ras-dependent pathway [23]. It can represses transplanted H22 hepatic ascitic tumor cell growth by stimulating anticancer immune response [24]. But whether gastrodin regulates the development of Prostate Cancer is unclear. Basing on the anti-tumor activity of gastrodin, we chose to study its function in Prostate Cancer.
Gastrodin inhibits the activity of Prostate cancer cells. A Chemical structures of Gastrodin. B CCK8 assay results of PC3 cell treated with Gastrodin (0, 1, 2, 4, 6 mM) for 24 h, 48 h, and 72 h. C CCK8 assay results of DU145 cell treated with Gastrodin (0, 1, 2, 4, 6 mM) for 24 h, 48 h, and 72 h. **, p < 0.01; *, p < 0.05. n.s., no significant difference
In this study, we will investigate the effect of Gastrodin on prostate cancer by observing the effect of Gastrodin on the proliferation of PC3 cells and DU145 cells in vivo and in vitro. Finally, we found that Gastrodin could inhibit the proliferation of PC3 and DU145 cells and the expression of proliferation-related molecules in vitro. Furthermore, Gastrodin could inhibit the growth of PC3 cell xenografts. Mechanically, gastrodin inhibits canonical Wnt/β-catenin signaling pathway so that inhibits prostate cancer cell proliferation. In conclusion, gastrodin exhibited anti-tumor effect on prostate cancer cells, suggesting its potential as a new therapeutic medicine for prostate cancer treatment.
Material and methods
Animal model
Sixteen six-week-old male Balb/c nude mice, weighted 20–23.5 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijiing, China) and treated in accordance with the Guide for the Care and Use of Laboratory Animals. Mice used in this study were sourced from the SPF animal room and maintained in a temperature-controlled room (24 °C ± 2 °C) with a 12-h light/dark cycle and 40–70% humidity, and had ad libitum access to food and water. PC3 cells were collected by trypsin digestion and resuspended in PBS. Briefly, the animals were anesthetized by inhaling 2.0% isoflurane and oxygen/nitrous oxide mixed gas. After anesthesia, the mice were grasped and fixed with the left hand, the syringe was pierced parallel to the axilla of the mice, and 200 µl of injection solution was injected (5 × 106 cells per mice, 8 mice per group). Slowly rotate and pull out the syringe, put the mice back into the cage for further feeding, observe the status of the mice and the appearance of tumors in the axilla every 2 or 3 days. When the tumor volume reached 100mm3, Gastrodin (50 mg/kg) was given to the mice by gavage everyday. According to the tumor size, the mice were euthanized by inhaling excessive isoflurane, tumors were collected, weighed and photographed. At the time of sacrifice, the tumor volume was no more than 2000mm3, the tumor weight was no more than 10% of the animal’s original body weight, and the weight loss of mice was no more than 15% of the original body weight. During the experiment, the mice did not suffer from virus infection.
All animal experiments were reviewed and approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University (APPROVAL#WDRM20211204C).
Immunohistochemistry staining
The tissues was fixed in neutral formalin and embedded in paraffin. For immunohistochemistry staining, paraffin samples were cut into 5-μm sections and repaired the antigen by using citrate buffer. Then primary antibody (anti-Ki67, GB13030-2,1:100 dilution, Servicebio, Wuhan, China) was added and incubated overnight at 4 °C After washing, the secondary antibody (Rabbit Two-step Detection Kit (Rabbit Enhanced Polymer Detection System; PV-9001, ZSGB-BIO, Beijing, China) was added at 37 °C for 30 min, and DAB (ZLI-9018, ZSGB-BIO, Beijing, China) was used as the chromogen. and hematoxylin (G1004, Servicebio, Wuhan, China) was used to stain the nucleus. After the staining was completed, the staining was observed under a microscope (ECLIPSE 80i, Nikon) and photographed.
Cell culture and treatment
The prostate cancer cell lines (PC3, DU145) were purchased from the BeNa Culture Collection. The PC3 cells cells were cultured with RPMI 1640 (Gibco, C11875500BT) complete medium containing 10% serum (Kangyuan, super grade) and 1% dual antibody (Biosharp, BL505A) in 5% CO2 incubator at 37 °C, and DU145 cells were cultured in Dulbecco modified Eagle medium (DMEM) (Bio-Channel, BC-M-005) complete medium containing 10% serum and 1% dual antibody in 5% CO2 incubator at 37 °C.
Cell viability assays
Cells were inoculated into 96-well plates (Thermo,167,008) (3000 cells/well). 24 h after inoculation, different concentrations of Gastrodin (0, 1, 2, 4, 6 mm, dissolved in DMSO) were added. After that, the culture was continued in the incubator at 37 °C for 24, 48, 72 or 96 h. After reaching the predetermined time, the cell viability was detected using the CCK8 kit (Bimake, B34304) according to the instructions.
Colony formation assay
2000 cells/well were seeded into six-well plates for colony formation assays. 24 h after inoculation, different concentrations of Gastrodin (0, 1, 2, 4 mM, dissolved in DMSO) were added after the cells were completely adherent to the wall. After 9-day’s culture (changing the medium every 2 days and Gastrodin was maintained), cells were fixed using 4% paraformaldehyde (Biolight, BLRE150) at room temperature for 15 min and stained with 0.1% crystal violet (hushi, SCRC71012314) at 37 °C for 20 min. Then visible colonies were photographed and counted.
Quantitative real-time PCR (qRT-PCR) assay
Total RNA was extracted from cells and tumor tissues using TRIzol Reagent (Invitrogen, 15,596–026), and then reverse transcribed to cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, 04896866001), according to the manufacturer’s instructions. The SYBR Green PCR Master Mix (Roche, 04887352001) was used for the qRT-PCR, with β-ACTIN as the internal reference. RT-PCR results were analyzed using relatively quantitative methods. The ΔCt value of each group was obtained by comparing the Ct value of the target gene with the Ct value of internal reference, and then the experimental group and the control group were calculated to obtain ΔΔCt. The 2-ΔΔCt method was used to compare the expression of the same gene between groups. The primers are listed in Table 1.
Western blot analysis
The total proteins of cells and tumor tissues were collected using RIPA lysate buffer (Termo Scientifc, Rockford, IL), and the protein concentration was quantified using BCA Protein Quantification Kit according to the instruction. Protein samples (20 ug) were uniformly loaded onto SDS-PAGE, and then electro transferred to PVDF membrane. Subsequently, the PVDF membrane was blocked with 5% non-fat milk for 1 h and incubated overnight at 4 °C with primary antibody against β-catetin, C-Myc, Cyclind1, and β-ACTIN (loading control for normalization). The membranes were washed three times with TBST buffer, and then bound to secondary antibodies at 37 °C for 1 h. The signal was visualized by enhanced chemiluminescence (ECL). The primary antibodies are listed in Table 2.
Statistical analysis
All data are presented as the mean ± SD and analyzed with SPSS software (version. 21.0). When the data conform to the normal distribution, the differences between the two groups were compared with a two-side student’s t-test. The differences among multiple groups (containing three groups) were analyzed with One-way ANOVA that the Bonferroni analysis for the data of homogeneous variance and Tamhane’s T2 analysis for heteroscedasticity data. When the data showed abnormal distribution, the non-parametric test (Kruskal–Wallis test) was used for analysis. P < 0.05 was considered significant difference.
Results
Gastrodin inhibits the activity of prostate cancer cells
First, we analyzed PC3 and DU145 cells activity under different concentration conditions and drug stimulation at different times. Cell viability was detedted by CCK8 assay, and the results showed that 1 mM Gastrodin could significantly inhibit the activity of PC3 cells and DU145 cells at 48 h and 72 h. The cells treated with Gastrodin showed reduced viability in a dose-dependent manner. When the cells were treated with 6 mM Gastrodin, the cell viability was almost half that of the control group (Fig. 1B-C). These findings indicated that Gastrodin can inhibit the activity of prostate cancer cells.
Gastrodin inhibits the proliferation of prostate cancer cells invitro
We then performed the colony formation assays to investigate the influence of Gastrodin on the proliferation of prostate cancer cells. Consistent with the results of cell viability studies, 1 mM gastrodin significantly inhibited the clone formation of PC3 and DU145 cells, and the effect of gastrodin was dose-dependent (Fig. 2A-B). Furthermore, we detected the expression of proliferation-related molecules by qRT-PCR and Western blot, the results showed that mRNA expression levels (Fig. 2C-D) and protein levels (Fig. 2E-H) of MYC proto-oncogene, bHLH transcription factor (MYC) and cyclin D1(CCND1) were significantly inhibited by Gastrodin in PC3 and DU145 cells. In summary, Gastrodin can significantly inhibit Prostate cancer cell proliferation in vitro.
Gastrodin inhibits Prostate cancer cells proliferation in vitro A, B Clone formation and quantitative results of PC3 and DU145 cells treated with Gastrodin (0, 1, 2, 4 mM). C, D The mRNA levels of MYC and CCND1 in PC3 cells (C) and DU145 cells (D) treated with Gastrodin (0, 1, 2, 4 mM).Actb was used as a reference control. E–H Representative western blot images and quantitative results of MYC and CyclinD1 in PC3 cells (E, F) and DU145 cells (G, H) treated with Gastrodin (0, 1, 2, 4 mM). GPADH was used as a loading control.**, p < 0.01; *, p < 0.05. n.s., no significant difference
Gastrodin inhibits the growth of PC3 cell xenografts
In order to further study the effect of Gastrodin on prostate cancer tumor growth, PC3 cells were used to enstablished Prostate cancer xenograft models. We found that the tumor growth rate of Gastrodin group was significantly slower than that of control group and the size of tumor volume was significantly smaller in the Gastrodin administered group compared with the control group (Fig. 3A-C). Tumor mass was also significantly reduced in the Gastrodin administration group compared to the control group(Fig. 3D). The results of KI67 immunohistochemical staining of tumor tissue showed the KI67-positive cells in Gastrodin administered group was lesser than control group (Fig. 3E). Subsequently, qRT-PCR and Western blot analysis showed that the mRNA and protein levels of MYC and CCND1 were significantly down-regulated in tumor tissues of Gastrodin group (Fig. 3F-H).Totally, our findings proved that Gastrodin can significantly inhibit the tumor growth of Prostate cancer.
Gastrodin inhibits the growth of PC3 cell xenografts A Representative images of PC3 cell xenografts mice treated with or without Gastrodin (50 mg/kg). B Representative images of tumors from PC3 cell xenografts mice treated with or without Gastrodin (50 mg/kg). C Tumor volumes analyzed results after inoculation. D The tumor weight of PC3-injected mice. E Representative images and quantitative results the results of Ki67 immunohistochemical staining in tumor tissue. Scale bar,100 μm. F The mRNA levels of MYC and CCND1 in tumor tissue treated with or without Gastrodin (50 mg/kg). Actb was used as a reference control. G, H Representative western blot images and quantitative results of MYC and CyclinD1 in tumor tissue treated with or without Gastrodin (50 mg/kg). GPADH was used as a loading control.**, p < 0.01; *, p < 0.05. n.s., no significant difference
Gastrodin inhibits the activation of canonical Wnt/β-catenin signaling pathway
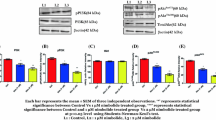
Previous studies have demonstrated that the activation of Wnt/β-catenin signalling is a frequent driver event in human cancer [25]. Gastrodin has been shown to inhibit canonical Wnt/β-catenin signaling activation in microglia [26]. Therefore, we hypothesized that Gastrodin may also inhibit the development of prostate cancer by inhibiting the activation of canonical Wnt/β-catenin signaling pathway. To test this hypothesis, we collected PC3 cells treated with gastrodin and tumor tissue from PC3 cells xenograft models to detect the expression of β-catenin, a key protein of canonical Wnt/β-catenin signaling pathway. Results showed that the expressions of β-catetin was reduced to different degrees after different concentrations of Gastrodin treatment (Fig. 4A-D). In addition, we added LiCl, an activator of Wnt/β-catenin signaling, to gastrodin treated PC3 cells, we found that the inhibitory effect of gastrodin on canonical Wnt/β-catenin signalling pathway was completely abolished (Fig. 4E-H). Furthermore, the result of the CCK8 experiment showed that LiCl abolished the inhibitory proliferation effect of Gastrodin on PC3 cells (Fig. 4I). These results reflected that Gastrodin can inhibit the activation of canonical Wnt/β-catenin signaling pathway in Prostate cancer.
Gastrodin inhibits the activation of canonical Wnt/β-catenin signaling pathway A, B Representative western blots and quantitative results of β-catenin in PC3 cells treated with Gastrodin (0, 1, 2, 4 mM).β-ACTIN was used as a loading control. C, D Representative western blot and quantitative results of β-catenin in tumors of PC3 cells xenografts treated with Gastrodin (0, 50 mg/kg). β-ACTIN was used as a loading control. E–H Representative western blot and quantitative results of β-catenin, C-Myc and CyclinD1 in PC3 cells treated with Gastrodin (0, 4 mM) or LiCL (0, 25 μM). β-ACTIN was used as a loading control. I CCK8 assay results of PC3 cell treated with Gastrodin (0, 4 mM) or LiCL (0, 25 μM) for 24 h, 48 h, 72 h and 96 h.For B and D,*vs Control group, For F-I *vs Control group, n.s. vs Control-LiCL group,**, p < 0.01; *, p < 0.05
Discussion
Prostate cancer is the most prevalent malignant tumor in men in Europe and the United States, and its incidence is increasing in China. Epidemiological surveys have shown a strong correlation between dietary factors and the development of prostate cancer. It is suggested that the ingredients may have a role in the prevention, treatment and prevention of tumor progression in prostate cancer. Therefore, in recent years, herbal medicine has been increasingly used in the treatment of prostate cancer in both Eastern and Western countries [27]. Gastrodin, a main chemical constituent of gastrodia, has been reported to inhibit tumor growth,such as gastrodin was capable of repressing transplanted H22 ascitic hepatic tumor cell growth in vivo with low toxicity [24]. Here,we found that in vitro, gastrodin (1 mM) could significantly inhibited the proliferation of PC3 and DU145 cells and in vivo, gastrodin (50 mg/kg) also markedly restrained the growth of xenograft by gavage.
The C-MYC oncogene has been implicated in the development of many human cancers.Under normal circumstances, expression level of c-myc is low, and once activated, it could cause a high proliferation and malignant transformation [28]. CCND1 gene encodes protein CyclinD1, which regulates cell cycle and is also a prognostic and predictive factor for different cancers [29]. Here, the result of qRT-PCR and western blot showed that gastrodin significantly inhibited the transcription and protein levels of myc and ccnd1 in prostate cancer cell (PC3 and DU45 cells) and also PC3 cell xenograft tumor tissues.
The Wnt/β-catenin signaling pathway, also called the canonical Wnt signaling pathway, is a conserved signaling axis involved in the regulation physiological processes of tumor such as proliferation, differentiation, apoptosis and metastasis, and the Wnt/β-catenin signaling pathway has aslo become an important target for clinical targeted therapy of tumors [30]. β-catenin, a key mediator of the Wnt/β-catenin pathway, which regulating the stabilization of the destruction complex and then adjusting β-catenin levels, could lead to early events in carcinogenesis when in abnormal regulation [31].It has been reported that the active ingredients extracted from traditional Chinese medicine can inhibit tumorigenesis by targeting the Wnt/β-catenin pathway. Such as Scutellarin may ameliorate colitis-associated colorectal cancer by weakening Wnt/β-catenin signaling cascade [32]. In our study, we found that gastrodin can inhibit the protein levels of β-catenin and proliferation associated molecules C-Myc and Cyclin D1. Furthermore, when LiCl, the agonist of Wnt/β-catenin pathway was administrated, the regulatory effect of gastrodin was abolished. These results suggested that gastrodin may exert its function of inhibiting the proliferation of Prostate Cancer by inhibiting the canonical Wnt/β-catenin signaling pathway. However, the activation of the canonical Wnt/β-catenin signaling pathway is a complex process involving multiple molecules. The phosphorylation and proteasome degradation of β-catenin is only a few of the Wnt/β-catenin signaling pathways. It remains unclear that how gastrodin regulates the protein levels of β-catenin and thus the activation of canonical Wnt/β-catenin signaling pathway.
In summary, the study showed that gastrodin had significant proliferation inhibitory activity on PC3 cells and DU145 cells in vitro, and inhibited tumor growth rate, reduced tumor size and improved anti-tumor efficacy in tumor-bearing mice. Gastrodin can inhibits the activation of canonical Wnt/β-catenin signaling pathway. The above experimental results laid the mechanical foundation for the study of the effect of gastrodin on tumor, and also provided the experimental basis for the use of gastrodin for therapy in oncology.
Data availability
The data and material that support the findings of this study are available in this paper.
References
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Barry MJ, Simmons LH. Prevention of prostate cancer morbidity and mortality: primary prevention and early detection. Med Clin North Am. 2017;101:787–806. https://doi.org/10.1016/j.mcna.2017.03.009.
Divella R, Daniele A, Savino E, Paradiso A. Anticancer effects of nutraceuticals in the mediterranean diet: an epigenetic diet model. Cancer Genomics Proteomics. 2020;17:335–50. https://doi.org/10.21873/cgp.20193.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. https://doi.org/10.3322/caac.21338.
Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21041447.
Nuhn P, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99. https://doi.org/10.1016/j.eururo.2018.03.028.
Kensler KH, Rebbeck TR. Cancer progress and priorities: prostate cancer. Cancer Epidemiol Biomarkers Prev. 2020;29:267–77. https://doi.org/10.1158/1055-9965.EPI-19-0412.
Valdespino V, Tsagozis P, Pisa P. Current perspectives in the treatment of advanced prostate cancer. Med Oncol. 2007;24:273–86. https://doi.org/10.1007/s12032-007-0017-9.
Morgans AK, et al. Quality of life during treatment with chemohormonal therapy: analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol. 2018;36:1088–95. https://doi.org/10.1200/JCO.2017.75.3335.
Armstrong AJ, et al. Development and validation of a prognostic model for overall survival in chemotherapy-naïve men with metastatic castration-resistant prostate cancer. Ann Oncol. 2018;29:2200–7. https://doi.org/10.1093/annonc/mdy406.
Kregel S, et al. Differential modulation of the androgen receptor for prostate cancer therapy depends on the DNA response element. Nucleic Acids Res. 2020;48:4741–55. https://doi.org/10.1093/nar/gkaa178.
Liu J, et al. Cyclodextrin-functionalized gold nanorods loaded with meclofenamic acid for improving N(6)-methyladenosine-mediated second near-infrared photothermal immunotherapy. ACS Appl Mater Interfaces. 2022;14:40612–23. https://doi.org/10.1021/acsami.2c09978.
Letang A, et al. Bone uptake in prostate cancer patients: diagnostic performances of PSMA-RADS v1.0, clinical, biological, and 68 Ga-PSMA-11 PET features to predict metastasis after biochemical recurrence. Clin Nucl Med. 2022;47:e529–39. https://doi.org/10.1097/RLU.0000000000004259.
Nelson PS, Montgomery B. Unconventional therapy for prostate cancer: good, bad or questionable? Nat Rev Cancer. 2003;3:845–58. https://doi.org/10.1038/nrc1210.
Di YN, Yu B, Kong WJ. Gastrodin ameliorates oleic acid-induced fat accumulation through activation of AMPK pathway in HL-7702 cells. Chin Pharmacol Bull. 2015;31:39–44. https://doi.org/10.3969/j.issn.1001-1978.2015.01.010.
He J, et al. Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson’s disease. Food Funct. 2021;12:7816–24. https://doi.org/10.1039/d1fo00847a.
Yang H, et al. Gastrodin modified polyurethane conduit promotes nerve repair via optimizing Schwann cells function. Bioact Mater. 2022;8:355–67. https://doi.org/10.1016/j.bioactmat.2021.06.020.
Chen J, et al. Gastrodin reduces IL-1beta-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomed Pharmacother. 2018;97:642–51. https://doi.org/10.1016/j.biopha.2017.10.067.
Ye T, et al. Gastrodin alleviates cognitive dysfunction and depressive-like behaviors by inhibiting ER stress and NLRP3 inflammasome activation in db/db mice. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19123977.
An SJ, et al. Gastrodin decreases immunoreactivities of gamma-aminobutyric acid shunt enzymes in the hippocampus of seizure-sensitive gerbils. J Neurosci Res. 2003;71:534–43. https://doi.org/10.1002/jnr.10502.
Zhang F, et al. Early intervention of gastrodin improved motor learning in diabetic rats through ameliorating vascular dysfunction. Neurochem Res. 2020;45:1769–80. https://doi.org/10.1007/s11064-020-03039-6.
Heo JC, et al. Anti-tumor activity of Gastrodia elata Blume is closely associated with a GTP-Ras-dependent pathway. Oncol Rep. 2007;18:849–53.
Shu G, Yang T, Wang C, Su H, Xiang M. Gastrodin stimulates anticancer immune response and represses transplanted H22 hepatic ascitic tumor cell growth: Involvement of NF-kappaB signaling activation in CD4+ T cells. Toxicol Appl Pharmacol. 2013;269:270–9. https://doi.org/10.1016/j.taap.2013.02.019.
Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21:5–21. https://doi.org/10.1038/s41568-020-00307-z.
Yao YY, et al. Gastrodin attenuates proliferation and inflammatory responses in activated microglia through Wnt/β-catenin signaling pathway. Brain Res. 2019;1717:190–203. https://doi.org/10.1016/j.brainres.2019.04.025.
de la Taille A, et al. Herbal therapy PC-SPES: in vitro effects and evaluation of its efficacy in 69 patients with prostate cancer. J Urol. 2000;164:1229–34. https://doi.org/10.1097/00005392-200010000-00021.
Kominami R, Niwa O. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci. 2006;97:575–81. https://doi.org/10.1111/j.1349-7006.2006.00218.x.
Seiler R, Thalmann GN, Rotzer D, Perren A, Fleischmann A. CCND1/CyclinD1 status in metastasizing bladder cancer: a prognosticator and predictor of chemotherapeutic response. Mod Pathol. 2014;27(1):87–95. https://doi.org/10.1038/modpathol.2013.125.(2013).
Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. https://doi.org/10.1186/s13045-020-00990-3.(2020).
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu C, Wang C, Ye L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):307. https://doi.org/10.1038/s41392-021-00701-5.(2021).
Zeng S, Chen L, Sun Q, Zhao H, Yang H, Ren S, Liu M, Meng X, Xu H. Scutellarin ameliorates colitis-associated colorectal cancer by suppressing Wnt/β-catenin signaling cascade. Eur J Pharmacol. 2021;906:174253. https://doi.org/10.1016/j.ejphar.2021.174253.
Acknowledgements
Not applicable.
Funding
This work was supported by Research Project of Hubei Provincial Health Commission 2021–2022 (WJ2021M086) and Esophageal and Gastric Malignancies Clinical Research Center Project of Hubei Clinical Medical Research Center.
Author information
Authors and Affiliations
Contributions
WM supervised the whole work. ML supervised the design, experiments, data analysis and manuscript preparation. YL and AW carried out the main work. YC, TM and BD participated in the experiments and data analysis. ZS participated in the manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were reviewed and approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University (APPROVAL#WDRM 20211204C).
Patient consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, YM., Wu, AD., Chen, Y. et al. Gastrodin inhibits prostate cancer proliferation by targeting canonical Wnt/β-catenin signaling pathway. Med Oncol 41, 32 (2024). https://doi.org/10.1007/s12032-023-02254-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02254-9