Abstract
Cancer stem cells (CSCs) are associated with metastasis and recurrence in prostate cancer as well as other cancers. We aimed to enhance the sensitivity of cabazitaxel in prostate cancer cell therapy by targeting CSCs with a Wnt inhibitor and salinomycin pretreatment. PC3, DU-145, and LNCaP human prostate cancer cells were exposed to Wnt/β-catenin pathway inhibitor CCT036477 (iWnt) with salinomycin for 48 h, followed by cabazitaxel treatment for 48 h. Cell viability, mRNA, and protein expression changes were evaluated by MTT, RT-qPCR, and Western blot assays, respectively. Apoptosis was determined by image-based cytometry, and cell migration was assessed by wound healing assay. Three-dimensional culture was established to assess the malignant phenotype and stemness potential of transformed or cancer cells. CD44 + CSCs were isolated using magnetic-activated cell sorting system. Pretreatment of PC3, DU-145, and LNCaP cells with salinomycin iWnt significantly sensitized the cells to cabazitaxel therapy. Spheroid culture confirmed that the treatment modality was more effective than a single administration of chemotherapy. The pretreatment of PC3 cells increased the rate of apoptosis compared to single administration of cabazitaxel, which downregulated Bcl-2 and upregulated caspase 3, caspase 8 expressions. The pretreatment suppressed cell migration, downregulated the expression of Sox2 and Nanog, and significantly reduced CD44 + CSC numbers. Notably, the treatment modality reduced pAKT, p-P38 MAPK, and pERK1/2. The data suggest that pretreatment of prostate cancer cells with salinomycin and Wnt inhibitor may increase the efficacy of cabazitaxel therapy by inhibiting cell proliferation and migration, and eliminating cancer stem cells.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most common cancer in men, after lung cancer, and ranks sixth in cancer-related deaths [1]. Despite advances in treatment, drug resistance, recurrence, and metastasis remain the major cause of cancer-related death [2]. Androgen deprivation therapy (ADT) is used in combination with a gonadotropin-releasing hormone agonist or antagonist for prostate cancer that has advanced to the metastatic stage [3]. The majority of patients respond to ADT by suppressing effective testosterone, but the disease may progress to metastatic castration-resistant prostate cancer (mCRPC). The introduction of new agents in the treatment, such as abiraterone acetate, enzalutamide, apalutamide, darolutamide, cabazitaxel, radium-223, and sipuleucel-T, has significantly advanced the treatment of mCRPC [4]. However, the optimal treatment for advanced prostate cancer has yet to be determined. Docetaxel is the first systemic therapy to improve survival in men with mCRPC, but increased chemoresistance reduces success. Cabazitaxel is the standard second-line chemotherapy for patients with mCRPC [5]. In the treatment of cabazitaxel, side effects such as neutropenia, leukopenia, and anemia develop, leading to inadequate median progression-free survival [5]. Metastasis, recurrence, resistance to chemotherapy, and radiotherapy are the main reasons for the failure of cancer treatments. Cancer stem cells (CSCs) may be among the main reason for the failure of the therapy encountered. Therefore, the development of CSC-targeted strategies may increase treatment efficacy.
Cancer stem cells are a subpopulation of tumor cells and are rare in the tumor mass. The diverse properties of these specialized cells enable them to survive standard treatments, leading to metastatic disease and tumor recurrence [6, 7]. The self-renewal and differentiation property of CSCs contributes to the initiation and maintenance of tumor growth. Several reports suggest that CSCs share numerous molecular features with mesenchymal cells, thus CSCs are considered leading players in metastasis [8]. In addition, these cells have higher intrinsic resistance to chemotherapy and radiotherapy than bulk cancer cells and may cause intra-tumor heterogeneity, complicating clinical treatment [9]. As tumor initiators, CSCs can be considered promising targets for better therapeutic outcome. However, various difficulties, such as the lack of suitable experimental models in defining the CSC subpopulation, targeting cancer and CSCs without harming normal cells, make targeting these cells difficult.
Salinomycin (C42H70O11), a member of the polyether ionophore antibiotics [10], has been shown to sensitize clinically used chemodrugs such as doxorubicin, trastuzumab, gemcitabine, and tamoxifen [11]. The molecular mechanisms are yet to be fully understood, but there is ample evidence that salinomycin, identified through high-throughput screening, exerts a selective effect on the elimination of CSCs [12]. Its biological effects include increasing intracellular reactive oxygen molecules, decreasing ATP levels, causing autophagy-related apoptosis, inducing endoplasmic reticulum stress, sequestration of iron in lysosomes, triggering DNA damage, and inhibiting DNA repair [13, 14]. Inhibition of the Wnt signaling cascade, AKT, and mitogen-activated protein kinase (MAPK) pathways in cancer cells by salinomycin administration plays a role in the indicated effects [15, 16]. Salinomycin and several of its nanoparticle-based therapy formulations have been tried to abolish prostate CSCs, but their potential for use with Wnt inhibitors and prior to cabazitaxel administration has not been tested.
Wnt/β-catenin signaling is of significant importance in cellular processes such as embryonic development, stem cell self-renewal, differentiation, and carcinogenesis [17, 18]. Aberrant Wnt/β-catenin signaling plays a critical role in bulk tumor expansion, metastasis, and treatment response by facilitating CSC regeneration, proliferation, and differentiation in many cancers including breast, colorectal, lung, ovarian, pancreatic, prostate, and melanoma [18, 19]. The Wnt/β-catenin pathway is frequently activated in late-stage prostate cancer through a variety of mechanisms, including cross-talk with other signaling pathways, altered expression of Wnt ligands, growth factors, cytokines produced by the damaged tumor microenvironment. Wnt signal-targeted therapeutics such as monoclonal antibodies, small-molecule hedgehog inhibitors, and β-catenin inhibitors are in clinical trials or preclinical studies for the treatment of patients with Wnt-induced cancer. A Wnt signaling inhibitor (2′Z, 3′E)-6-bromoindirubin-3′-oxime or CCT036477 (inhibitor XI) blocks transcription at β-catenin without altering its levels [20, 21]. CCT036477 is a cell-permeable indole derivative that inhibits proliferation of various cancer cells, development of embryos, and expression of Wnt target genes.
This study evaluated the efficacy of pretreatment with salinomycin and Wnt signal inhibitor prior to cabazitaxel therapy on prostate cancer cells and their CSCs populations.
Materials and methods
Cell culture
Human prostate PC3, DU-145, and LNCaP cancer cells were purchased from the ATCC (Manassas, VA, USA). The cells were maintained in complete DMEM/Ham’s F-12 medium (Winsent, Quebec, Canada) supplemented with 10% fetal bovine serum (Life Technologies, CA, USA) and penicillin/streptomycin. Wnt inhibitor (XI, CCT036477, Sigma-Aldrich, Darmstadt, Germany), salinomycin, and cabazitaxel (Cayman Chemical, Michigan, ABD) were reconstituted in dimethyl sulfoxide (DMSO) and stored at −20 °C until use. Bovine retinal pigment epithelial cells (RPE) were obtained by the ophthalmologist from eyes taken immediately after slaughter of cattle at the abattoir and grown in complete DMEM medium for three weeks [22]. After examining for contamination, these cells were used to determine possible cytotoxic effects of pharmacological agents on primary cells. PC3, DU-145, and bovine RPE cell doubling times are close to each other (approximately 30 h), while for LNCaP cells the time is 60 h [23, 24].
Time– and dose–response assessment
The cytotoxic effects of the Wnt inhibitor (iWnt), salinomycin, and cabazitaxel on cell survival were determined by MTT test. First, PC3, DU-145, LNCaP, and bovine RPE cells were seeded at a density of 104 cells/well in 96-well culture dishes (Nest Scientific, China) for 16 h. Then, the cells were treated with cabazitaxel at concentrations of 0.39 nM, 0.78, 1.56, 3.12, 6.25, 12.5, 25, 50, 100 nM; iWnt at concentrations of 0.39 µM, 0.78, 1.56, 3.12, 6.25, 12.5, 25, 50, 100 µM iWnt; or salinomycin at concentrations of 0.078 µM, 0.156, 0.312, 0.625, 1.25, 2.5, 5, 10, 20 µM. The experiments were repeated at least three times using eight wells for each dose. The effects of various concentrations of iWnt (Fig. 1A), salinomycin (Fig. 1B), and cabazitaxel (Fig. 1C) administrations for 24–72 h on cell survival were determined by MTT assay in a microplate reader at 570 nm (Multiskan GO, Thermo Scientific, Vantaa, Finland). Since 48 h is preferred for combination applications, the IC50 values of the agents were calculated using the MTT test results in this incubation period.
Salinomycin, Wnt inhibitor, and cabazitaxel inhibit prostate cancer cell proliferation in a dose- and concentration-dependent manner. PC3 cells were exposed to the various concentrations of the pharmacological agents for 24–72 h. At the end of the treatments, MTT test was performed and cell viability rates were determined compared to the control groups. *P < 0.01 compared to untreated control (0), #P < 0.05 compared to untreated control (0)
Determination of apoptosis by annexin V assay
To evaluate the apoptotic efficacy of the treatment modalities, the cells (1 × 105 cells/well) were seeded in six-well plate for overnight followed by exposure to a combination of 0.5 µM salinomycin and 6.25 µM iWnt for 48 h. Subsequently, the medium of the cells was then replaced with 2.5 nM cabazitaxel in fresh medium and incubated for an additional 48 h. At the end of the treatment period, the cells were harvested with trypsin/EDTA, centrifuged, and washed twice with PBS. The resulting cell pellet was stained with the Annexin V/PI kit (Life Technologies, Carlsbad, CA) according to the kit instructions. Dead, viable, and apoptotic cell proportions were counted in an image-based cytometer (Life Technologies). Untreated cells were treated with DMSO, a solvent of pharmacological agents with a final concentration not exceeding 0.1%.
RNA extraction and RT-qPCR
PC3 cells were first exposed to a combination of 0.5 µM salinomycin and 6.25 µM iWnt for 48 h, then treated with single 2.5 nM cabazitaxel as described in the previous section. Total RNA was extracted from PC3 cells using commercial kit (Thermo Fisher Scientific, MA, USA), then reverse transcribed using the high-capacity cDNA kit (Applied Biosystems, Victoria, Australia). Quantitative mRNA expression analyses of the selected genes were achieved on a real-time PCR system using (Applied Biosystems StepOne, Foster City, CA) using SYBR Green Master Mix (Thermo Fisher Scientific, MA). Reaction conditions were 1 cycle of initial denaturation at 50° °C for 2 min and 95 °C for 10 min; 40 cycles 95 °C for 15 s and 60 °C for 1 min. The quantification of fold enrichments of targets relative to 2−ΔΔCt was calculated using the expression GAPDH used as an internal reference. Primer pairs for RT-PCR are given in Table 1 (PRZ Biotech, Ankara, Turkey).
Protein expression by Western blot
After PC3 cells were treated as described in the previous section, protein homogenates were extracted using RIPA lysis buffer (containing 50 mM Tris–Cl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP-40, 100 μg/ml PMSF) (Thermo Fisher Scientific). Fifty μg protein samples were separated by 4–12% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Life Technologies). Then, the membranes were blocked and exposed to primary antibodies against anti-caspase 3, Bax, E-Cadherin, Vimentin, Wnt, phosphoP38, AKT, pAKT, pERK, Bcl-2, and β-actin (Novus Biologicals, Littleton, CO, USA) at 4 °C overnight. After washing the membranes twice in tris-buffer saline, they were incubated at room temperature with anti-rabbit/anti-mouse IgG using the chemiluminescence Western blot substrate kit (Thermo Fisher Scientific). The intensities of the bands were determined on the imaging system (Bio-Rad ChemiDoc MP System, Carlsbad, CA, USA). β-actin was used as to optimize the amount of loaded protein for the signal intensities.
Three-dimensional (3D) spheroid cultures
Mono- and multi-spheroid cultures were applied to evaluate the efficacy of the treatment in sphere sizes. To prevent cells from adhering to the bottom surface of the plates in a mono-sphere suspension, 96-well culture dishes were covered with 1.5% agarose. After washing and drying the plates, a single-spheroid suspension was obtained by leaving 2 × 103 cells in each well. Cells were simultaneously exposed to 2.5 nM cabazitaxel, 0.5 µM salinomycin, and 6.25 µM iWnt agents. 50 µl of fresh medium containing the treatment agents was added to the respective well every three days for 10 days. Seven spheroids were used for each treatment. On days 0 and 10, images were taken under the microscope (Zeiss, Germany), and the diameters of the spheres were measured using the imaging software. On day 10, spheroids from the same group were harvested with a pipette tip, cells were dissociated with trypsin, suspended in trypan blue solution, and viable cells were counted by with a hemocytometer.
In multi-spheroid cultures method, the bottoms of 75 cm2 plates were coated with agarose as indicated in the single spherical method, 1 × 106 PC3 cells were pipetted into each well, and processed as above. Colonies were photographed on days 0, 7, and 10 of incubation.
Identification of the number of stem cells in the population
CD44 + cell subpopulations were sorted by column selection using CD44-PE monoclonal antibody conjugated to magnetic microbeads (Miltenyi Biotec, Gladbach, Germany) as described previously [25]. Briefly, PC3 cells remaining in flasks after treatment with agents were trypsinized and washed with PBS. Next, cells were incubated with 100 µl of MACS and 10 µl of anti-PE CD44 microbeads (Miltenyi Biotec) for 20 min at + 4 °C. Subsequently, cells were pelleted and suspended in 1 ml of MACS buffer and then passed through the column. CD44 + cancer stem cells in the columns removed from the magnetic field were counted after eluting.
Wound healing assay
The effect of the treatment strategies on cell migration was evaluated by wound healing assay. For this, 9 × 105 PC3 cells were seeded in 6-well plates; wound was created in the midline of the well with a pipette tip after one day of incubation for adhesion. Floating cells were removed from the media, and then the cells were exposed to 2.5 nM cabazitaxel, 0.5 µM salinomycin + 6.25 µM iWnt, or the combination of the three agents in serum-free medium, and left to incubate for 24 h. At the end of the period, the remaining wound intervals were determined by phase-contrast microscope (10 × objective).
Statistical analysis
Data were evaluated with SPSS software (19.0; SPSS, Chicago, IL), with differences between treatment groups determined by analysis of variance (ANOVA) followed by Duncan’s multiple range test. Results were expressed as mean ± standard deviation (SD) and each experiment was repeated at least three times. P < 0.05 was considered statistically significant. The pharmacological agent values that killed half of the cells (IC50) were determined in GraphPad Prism 7.0 software (San Diego, CA, USA).
Results
Effect on cell survival
To determine the cytotoxic effects of the agents on prostate cancer cell survival, cells were exposed to different concentrations of iWnt (CCT036477), salinomycin, and cabazitaxel for 24, 48, and 72 h. Wnt inhibitor significantly reduced the PC3 cell viability in a dose- and time-dependent manner (Fig. 1A). While salinomycin treatment for 24 h did not cause significant cytotoxicity except at the maximum dose used, 48 and 72 h of applications produced strong cytotoxicity (Fig. 1B). Cabazitaxel used at the nanomolar levels significantly suppressed the cell viability at all administrated time periods (Fig. 1C). The IC50 values were calculated after 48 h of salinomycin, iWnt, and cabazitaxel administrations to PC3 cells were 0.5, 6.25, and 2.5 nM, respectively (Fig. 1A–C).
Combination treatment
Cells were exposed to drugs individually or as alternatives to determine the appropriate combination therapy. For this, PC3 (Fig. 2A), LNCaP (Fig. 2B), or DU-145 (Fig. 2C) cells were treated with 2.5 nM cabazitaxel, 0.5 µM salinomycin, or 6.25 µM iWnt for 48 h, then the pharmacological agents were removed and incubated for an additional 48 h. There was no significant difference in cell therapy between the individual use of each agent or the combined use of salinomycin with iWnt, salinomycin with cabazitaxel, or iWnt with cabazitaxel as determined by MTT test. However, pretreatment of PC3, DU-145, or LNCaP cells with 0.5 µM salinomycin and 6.25 µM iWnt for 48 h followed by exposure to only 2.5 nM cabazitaxel in fresh medium for an additional 48 h significantly increased treatment efficacy. Compared with cabazitaxel individual therapy, pretreatment of PC3 cells with salinomycin and iWnt followed by cabazitaxel further reduced cell survival by 25% (Fig. 2A). Combination therapy reduced cell survival by 75% compared to the untreated group. A similar effect was also observed in LNCaP (Fig. 2B) and DU-145 cells (Fig. 2C) in the pretreatment.
Pretreatment inhibits prostate cancer cell survival. PC3 (A), LNCaP (B), or DU-145 (C) cells were exposed to 0.5 µM salinomycin (Sal), 6.25 µM Wnt inhibitor (iWnt), or 2.5 nM cabazitaxel (Cab) for 48 h, the medium was replaced with fresh agent-free medium and incubated for another 48 h. The cells (Sal + iWnt group) were exposed to 0.5 µM salinomycin and 6.25 µM Wnt inhibitor for 48 h and then incubated with agent-free medium for 48 h as before. Sal + Cab or iWnt + Cab group cells were first treated for 48 h with 0.5 µM salinomycin or 6.25 µM Wnt inhibitor for 48 h, then incubated for another 48 h with only 2.5 nM cabazitaxel in fresh medium. Finally, Sal + iWnt + Cab group cells were treated with 0.5 µM salinomycin and 6.25 µM Wnt inhibitor for 48 h and then incubated for 48 h with only 2.5 nM cabazitaxel in fresh medium. Cell survivals were determined using the MTT test after the indicated applications and plotted as a percentage compared to the control group. Similar treatment was applied to retinal pigment epithelial (RPE) cells obtained as primary cells from slaughtered cattle to represent normal healthy cells (D). *P < 0.01 compared to untreated and Sal + iWnt + Cab, **P < 0.05 compared to untreated control (0)
Bovine eye RPE cells were used to determine the possible cytotoxic effect of established treatment strategies on primary normal cells. Neither single nor combined treatments produced a significant cytotoxic effect on RPE cell survival compared to the control group (P > 0.05) (Fig. 2D).
Induction of apoptosis
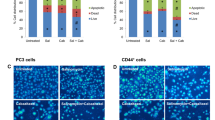
The results of cytometric analysis supported the MTT survival results. Co-administration of iWnt with salinomycin to PC3 cells induced apoptosis at a rate similar to cabazitaxel single therapy (Fig. 3A). However, the 40% apoptosis rate in these two different treatments increased to 67% after pretreatment of iWnt with salinomycin. There was no difference between the groups in the percentage of non-apoptotic cell death (Fig. 3). Molecular events underlying apoptosis induced by salinomycin and iWnt administration before cabazitaxel treatment were evaluated by RT-PCR and western blot. Pretreatment of cells significantly upregulated the expression of caspase 3 (Fig. 3B), caspase 8 (Fig. 3C), cytochrome c (Fig. 3D), and apaf-1 (Fig. 3E) compared to untreated or cabazitaxel single exposure, while decreased the protein expression of Bcl-2 (Fig. 3G). Surprisingly, the treatment modalities did not alter Bax protein expression (Fig. 3F). Calculated from protein expression data after B-actin corrections, the Bax/Bcl-2 ratios were 1.17, 1.84, and 3.27 in cabazitaxel, salinomycin + iWnt, and pretreated cells, respectively.
Cabazitaxel administration after salinomycin and Wnt pretreatment leads to the highest apoptosis. PC3 cells were treated with 0.5 µM salinomycin (Sal) and 6.25 µM Wnt inhibitor (iWnt) for 48 h and then incubated for 48 h with 2.5 nM cabazitaxel (Cab) in fresh medium (A). Apoptosis assessment was performed on an image-based cytometer using Annexin V/PI dye. Total RNA samples were extracted from cells where similar applications were made, cDNA was synthesized and caspase 3 (B), caspase 8 (C), cytochrome C (D), and Apaf-1 (E) transcription analyses were performed by real-time PCR. Bax (F) and Bcl-2 (G) protein expression levels were determined by Western blot. Protein amounts were optimized with β-actin. *P < 0.01 compared to untreated, **P < 0.05 compared to the others
Suppression of stemness properties
Due to the similarity of in vivo models, the efficacy of treatment strategies on PC3 cell survival was determined by single-spheroid testing [26]. Cabazitaxel application after salinomycin + iWnt pretreatment of cells declined spheroid diameter compared to single and combined treatment (Fig. 4A), while reducing the survival rate to the maximum level (Fig. 4B). According to the viability test performed on the cells found in spheroid structures, cabazitaxel administration after pretreatment enhances cell death by 75% compared to the cabazitaxel alone and salinomycin + iWnt groups, this rate increases to 90% compared to the untreated cells (Fig. 4B).
The treatment modality effectively reduces the spheroid diameter and the number of viable cells in the structure. A mono-sphere suspension of PC3 cells was established in 96-well culture dishes as described in Sect. “Three-dimensional (3D) spheroid cultures.” Cells were simultaneously exposed to 2.5 nM cabazitaxel (Cab), 0.5 µM salinomycin (Sal), and 6.25 µM Wnt inhibitor (iWnt). 50 µl of fresh medium containing the treatment agents was added to the respective well every three days for 10 days. Images were taken under the microscope on days 0 and 10 (A). At day 10, spheroids from the same group were harvested with a pipette tip, cells were separated with trypsin, suspended in trypan blue solution, and viable cells were counted with a hemocytometer (B). *P < 0.01 compared to untreated, **P < 0.01 compared to the others
Multiple spheroid cultures showed that cabazitaxel administration after salinomycin + iWnt pretreatment was the most effective strategy to reduce the size of the colonies (Fig. 5A). Although cabazitaxel or salinomycin + iWnt applications were effective on colony shrinkage, the most effective result was obtained with cabazitaxel treatment after pretreatment. The properties of the treatments to reduce the number of CSCs in spheroid structures were determined by magnetic column separation using antibodies specific to CD44 stem cell surface receptor. Administration of cabazitaxel alone, salinomycin + iWnt, and application of cabazitaxel following salinomycin + iWnt treatment decreased CD44 + CSC numbers in spheroid structures by 17.8, 32.1, and 71.4%, respectively, compared to untreated cells (Fig. 5B). RT-PCR analysis showed significant suppression of stem cell markers Sox2 (Fig. 5C) and Nanog (Fig. 5D) mRNA expression of stem cell-targeted pretreatment. Salinomycin + iWnt reduce Sox2 expression by 70%, cabazitaxel after pretreatment of two agents reduces by 80%, while cabazitaxel alone is ineffective (Fig. 5C). All the three treatments downregulated the expression of Nanog, while salinomycin + iWnt suppressed it by 80% and cabazitaxel after pretreatment by 90% (Fig. 5D). Single cabazitaxel administration downregulated it by 50%.
Pretreatment enhances the multi-spheroid inhibition potency of cabazitaxel. Multi-spheroid PC3 culture was established in 75 cm2 plates as described in Sect. “Three-dimensional (3D) spheroid cultures.” Cells were simultaneously exposed to 2.5 nM cabazitaxel (Cab), 0.5 µM salinomycin (Sal), and 6.25 µM Wnt inhibitor (iWnt) for ten days. Fresh medium containing the therapeutic agents was added every three days at the same concentrations. Images were taken under the microscope (A). Collected colonies were trypsin digested. CD44 + cell subpopulations were sorted by column selection using CD44-PE monoclonal antibody conjugated to magnetic microbeads as previously described. Columns were removed from the magnetic field, and CD44 + cells were counted after elution (B). Total RNA samples were extracted from colonies collected after treatments, Sox2 (C) and Nanog (D) mRNA transcription analyses were quantitatively determined by real-time PCR. *P < 0.01 compared to untreated, **P < 0.01 compared to the others
Inhibition of cell migration
Abnormal migration of cancer cells promotes metastatic progression. Therefore, inhibition of cell migration can prevent the formation of secondary foci by spreading the disease to distant tissues. Cabazitaxel single treatment suppressed PC3 cell migration at a low level, while salinomycin + iWnt administration markedly suppressed it (Fig. 6A, B). The highest cell migration inhibition was obtained with cabazitaxel treatment after salinomycin + iWnt administration, with the migration pathway similar to the zero hour (Fig. 6A, B). The last treatment modality made a significant difference in the prevention of cell migration compared to all groups. We checked whether the treatment modalities altered the protein expression of E-cadherin and vimentin, which are involved in the epithelial–mesenchymal transition. Cabazitaxel treatment increased E-Cadherin protein expression twofold compared to the untreated group; this increase was threefold in salinomycin + iWnt or pretreated cabazitaxel cells (Fig. 6C). On the other hand, cabazitaxel single treatment did not change vimentin protein expression, while salinomycin + iWnt significantly suppressed it. Salinomycin + iWnt administration before cabazitaxel produced maximum inhibition of vimentin expression compared to all other groups (Fig. 6D).
The pretreatment strategy maximally inhibits cell migration. PC3 cells were densely seeded in six-well plates, a day after incubation wounds were created in the midline of the wells with a pipette tip. According to the wells, 2.5 nM cabazitaxel (Cab), 0.5 µM salinomycin (Sal) + 6.25 µM Wnt inhibitor (iWnt), or the combination of three agents were added in serum-free medium and then incubated for 24 h. At the end of the period, the remaining wound spaces were imaged under a phase-contrast microscope (10 × objective) (A). Relative wound gap by treatments (B). E-cadherin (C) and vimentin (D) protein expression levels were determined by western blot. Protein amounts were optimized with β-actin. *P < 0.01 compared to untreated, **P < 0.01 compared to the others, #P < 0.05 compared to Cab
Modulation of cell signaling pathways
Major intracellular signaling pathways involved in cell growth, proliferation, and cell migration of the treatment modality were evaluated. Single administration of cabazitaxel downregulated the expression of phospho-p38 MAPK by 30% compared to untreated cells, while the downregulation was increased to 60% in salinomycin + iWnt pretreated group (Fig. 7A). The reduction in salinomycin + iWnt treatment without cabazitaxel was only 10%. While pERK expression decreased by 20% in cabazitaxel single application or salinomycin + iWnt treatment, pretreatment increased cabazitaxel efficacy to 40% (Fig. 7B). Treatment modalities did not affect AKT protein expression (Fig. 7C), pretreatment sensitized cells to cabazitaxel therapy and strongly downregulated phospho-AKT expression (Fig. 7D). The decrease in expression was lower in salinomycin + iWnt and insignificant in single cabazitaxel therapy (Fig. 7D). Cabazitaxel treatment decreased Wnt expression by 20% compared to untreated cells, while salinomycin + iWnt increased the rate to 30% (Fig. 7E). Wnt expression was reduced to 50% in cabazitaxel pretreated with salinomycin + iWnt (Fig. 7E).
Treatment modality regulates major intracellular signaling pathways. PC3 cells were treated with 0.5 µM salinomycin (Sal) and 6.25 µM Wnt inhibitor (iWnt) for 48 h, the medium was replaced with fresh medium containing 2.5 nM cabazitaxel (Cab) and incubated for an additional 48 h. Protein expressions of phosphorylated P38 (A), pERK (B), AKT (C), pAKT, and Wnt (D) were determined by Western blot. β-actin was used as a loading control. *P < 0.01 compared to untreated, **P < 0.05 compared to the others
Discussion
Currently, the treatment of castration-resistant prostate cancer is still limited. Studies have shown that natural compounds have a synergistic effect with chemotherapeutic drugs, revealing negligible side effects in patients as well as their chemo-preventive potential. Salinomycin is a natural antibiotic that selectively inhibits crucial intracellular signaling pathways such as AKT, Wnt, Hedgehog, and Notch, suppresses the ATP-binding cassette transporter in CSCs, and thereby inhibits cell growth, proliferation, and metastasis [27, 28] in various cancer types such as breast cancer [29], renal cell carcinoma [30], and prostate cancer [16] in vitro or xenograft mouse models. There is substantial evidence to support the theory that CSCs play a role in tumor initiation, intra-tumor heterogeneity, high metastatic potential, and the development of treatment resistance [31]. The Wnt/β-catenin signaling pathway involved in stem cell regeneration cross-talks with the Notch and Sonic Hedgehog pathways, which have implications for therapeutic interventions in cancers. Cross-talk between these pathways supports the maintenance of CSCs and regulates cancer cell motility [32]. We thus hypothesized that targeting CSCs with different approaches besides parent cell therapy increases chemotherapy efficacy. To test this, androgen-sensitive LNCaP or insensitive PC3 and DU-145 prostate cancer cells were pretreated with salinomycin and Wnt/β-catenin inhibitor prior to cabazitaxel treatment. The results showed that the treatment modality could significantly reduce the number of CSCs found within the cancer cell population, inhibit spheroid formation and cell migration compared to the conventional application.
Various preclinical studies show that salinomycin alone or in combination with 5-fluorouracil, gemcitabine, cabazitaxel, and radiotherapy [16, 33, 34] exhibits increased anti-tumoral activity compared to extensive chemotherapy. In the present study, salinomycin, Wnt inhibitor, or cabazitaxel inhibited cell proliferation when administered alone. Different combinations of these agents produced a similar effect. However, pretreatment of cells with a combination of salinomycin and Wnt inhibitor prior to cabazitaxel treatment strongly suppressed cell proliferation, spheroid formation, and cell migration compared to single cabazitaxel treatment. Inhibition of cell proliferation has been shown to be largely apoptosis-mediated. Cabazitaxel treatment combined with pretreatment caused a significant increase in caspase 3, 8 expression and a rise in Bax/Bcl-2 ratio. The anti-tumor activity of salinomycin mainly consists of abolishing the nuclear translocation of NF-κB proteins [29], inducing the caspase 3-related cell death pathway [35], and interrupting HIF-1α/VEGF [36], resulting in induction of apoptosis and autophagy. A low Bax/Bcl-2 ratio was characteristic for resistant cells and a high Bax/Bcl-2 ratio was characteristic for sensitive cells [37]. Inhibition of cancer cell and CSCs proliferation is mediated by regulation of AKT, ERK, and p38 MAPK phosphorylation [16, 38]. Induction of intracellular oxidative stress and decreased antioxidant capacity [27] and mitochondrial membrane depolarization [39] may have played a role in stimulating apoptosis in the prostate cancer treatment modality. Here, the increased Bax/Bcl-2 ratio and downregulation of critical signaling pathways provide strong evidence that combined pretreatment prior to cabazitaxel treatment promotes apoptosis.
Wnt/β-catenin activation is required for prostate CSC self-renewal and the ability to maintain expression of the androgen receptor [40]. It has been shown that this signaling contributes prostate cancer heterogeneity [41], docetaxel resistance [42], and metastasis [43]. Although salinomycin inhibits the Wnt/β-catenin signaling pathway, the addition of a Wnt inhibitor to the pretreatment further enhanced the efficacy of cabazitaxel therapy. Thus, strong blocking of this pathway inhibits the proliferation of CSCs as well as the death of the parent cancer cell. We have recently shown that simultaneous administration of prostate cancer cells with salinomycin and cabazitaxel has a synergistic but limited inhibition of cell proliferation [16]. The current modality killed 75% of the parent cells, compared to 52% in the previous one. In addition, with the new modality, CD44 + CSCs survival decreased to 28%, whereas concomitant salinomycin + cabazitaxel treatment only reduced it to 59% [16]. Thus, the use of Wnt/β-catenin inhibitor and salinomycin as a pretreatment provides therapeutic advantage by increasing the sensitivity of cells to cabazitaxel. Tumor spheroids provide an in vitro model that more closely mimics the physiological environment of tumors in vivo than monolayer cultures. Under appropriate culture conditions, individual CSCs can transform into 3-dimensional structures called spheroids or tumorspheres, which have high characteristics of growth, migration, clonogenic survival, and anchorage-independent aggression [44]. The pretreatment modality significantly reduced the number of CSCs present within the PC3 population. It has been shown that salinomycin downregulates the expression of Sox2 and Oct3/4 in human ovarian CSCs, while its combination with paclitaxel reduces the spheroid-forming abilities of the chemotherapeutic agent [45]. Here, treatment modality reduced the stemness marker expression and spheroid-forming ability of CSCs. Salinomycin has been shown to reduce the characteristics of CSCs by suppressing Aldehyde Dehydrogenase 1 and CD44 expressions and Wnt/β-catenin signaling [16, 28]. Biological activities of CSCs such as self-renewal are regulated by critical pluripotent transcription factors such as Oct4, Sox2, Nanog, KLF4, and Myc [46]. Thus, downregulation of Sox2 and Nanog expression of the prostate cancer cells may have an adverse effect on the biology, self-renewal, and proliferation of CSCs.
Wnt/β-catenin signaling promotes cancer progression as well as metastasis through the induction of epithelial–mesenchymal transition (EMT) [47, 48]. EMT enables cells to detach from the tissue and acquire increased motility by losing their basoapical polarity. Cells that display decreased levels of E-cadherin and increased expression of vimentin are associated with drug resistance and promote EMT [49, 50]. Conversely, increased E-cadherin expression has been shown to enhance sensitivity to chemotherapy, with mesenchymal-like tumor cells exhibiting chemoresistance [51]. Our study demonstrated that the combination of Wnt/β-catenin inhibitor and salinomycin prior to cabazitaxel treatment significantly inhibits prostate cancer cell migration by downregulating vimentin expression and upregulating E-cadherin expression. It was previously shown that the administration of salinomycin impedes the migration of prostate cancer cells through the suppression of NF-ĸB expression and the reduction of aldehyde dehydrogenase activity [27]. Targeting CD44 cells with salinomycin can decreases EMT and metastasis in vivo by decreasing CD44 expression as well as downregulating vimentin and upregulating E-Cadherin in prostate cancer [52]. Salinomycin has been shown to have a similar effect in different types of cancer, for example, upregulated E-cadherin and downregulated vimentin expression in salinomycin therapy was associated with the suppression of metastasis and invasion of bladder cancer cells [53].
In conclusion, it was shown in this study that pretreatment of prostate cancer cells with salinomycin and Wnt inhibitor suppressed proliferation and migration. The significant reduction in the number of CSCs in the population points to the effectiveness of the treatment modality. Treatment efficacy may be partially attributed to the AKT, ERK, and p38 MAPK pathways. The data suggest that administration of salinomycin and blocking the Wnt/β-catenin signaling pathway before chemotherapy may be a new and promising approach for effective treatment and perhaps prevention of recurrence of prostate cancer.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697–711.
Yu EM, Aragon-Ching JB. Advances with androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother. 2022;23(9):1015–33.
George DJ, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18(4):284–94.
Chen JR, et al. Comparison of systemic treatments for metastatic castration-resistant prostate cancer after docetaxel failure: a systematic review and network meta-analysis. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2021.789319.
Alfarouk KO, et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71–71.
Ritch C, Cookson M. Recent trends in the management of advanced prostate cancer. F1000Res. 2018;7:1513.
Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781.
Takebe N, et al. Targeting notch, hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12(8):445–64.
Anadón A, Martínez-Larrañaga MR. Veterinary drugs residues: coccidiostats. In: Motarjemi Y, editor. Encyclopedia of food safety. Waltham: Academic Press; 2014. p. 63–75.
Kim JH, et al. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br J Pharmacol. 2011;162(3):773–84.
Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59.
Yu J, et al. Salinomycin triggers prostate cancer cell apoptosis by inducing oxidative and endoplasmic reticulum stress via suppressing Nrf2 signaling. Exp Ther Med. 2021;22(3):946.
Versini A, et al. Salinomycin derivatives kill breast cancer stem cells by lysosomal iron targeting. Chemistry. 2020;26(33):7416–24.
Qi D, et al. Salinomycin as a potent anticancer stem cell agent: state of the art and future directions. Med Res Rev. 2022;42(3):1037–63.
Erdogan S, et al. The synergistic anticancer effect of salinomycin combined with cabazitaxel in CD44+prostate cancer cells by downregulating wnt, NF-kappa B and AKT signaling. Mol Biol Rep. 2022;49(6):4873–84.
Ng LF, et al. WNT signaling in disease. Cells. 2019;8(8):826.
Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165.
Borah A, et al. Targeting self-renewal pathways in cancer stem cells: clinical implications for cancer therapy. Oncogenesis. 2015;4:e177.
Ewan K, et al. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res. 2010;70(14):5963–73.
Fu SF, et al. N-methyl-N-nitrosourea induces zebrafish anomalous angiogenesis through Wnt/β-Catenin pathway. Ecotoxicol Environ Saf. 2022;239:113674.
Fronk AH, Vargis E. Methods for culturing retinal pigment epithelial cells: a review of current protocols and future recommendations. J Tissue Eng. 2016;7:2041731416650838.
Oka MS, Landers RA, Bridges CD. A serum-free defined medium for retinal pigment epithelial cells. Exp Cell Res. 1984;154(2):537–47.
Su CY, et al. Analyzing the expression of biomarkers in prostate cancer cell lines. In Vivo. 2021;35(3):1545–8.
Erdogan S, Turkekul K. Neferine inhibits proliferation and migration of human prostate cancer stem cells through p38 MAPK/JNK activation. J Food Biochem. 2020. https://doi.org/10.1111/jfbc.13253.
Han SJ, Kwon S, Kim KS. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021;21(1):152.
Ketola K, et al. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br J Cancer. 2012;106(1):99–106.
Liu Q, et al. Salinomycin suppresses tumorigenicity of liver cancer stem cells and Wnt/Beta-catenin signaling. Curr Stem Cell Res Ther. 2021;16(5):630–7.
Tyagi M, Patro BS. Salinomycin reduces growth, proliferation and metastasis of cisplatin resistant breast cancer cells via NF-kB deregulation. Toxicol In Vitro. 2019;60:125–33.
Liu L, et al. Salinomycin suppresses cancer cell stemness and attenuates TGF-beta-induced epithelial-mesenchymal transition of renal cell carcinoma cells. Chem Biol Interact. 2018;296:145–53.
Barbato L, et al. Cancer stem cells and targeting strategies. Cells. 2019;8(8):926.
Patni AP, et al. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma-clinical implications. Cell Oncol. 2021;44(3):473–94.
Klose J, et al. Salinomycin: anti-tumor activity in a pre-clinical colorectal cancer model. PLoS ONE. 2019;14(2):e0211916.
Gehrke T, et al. Combination of salinomycin and radiation effectively eliminates head and neck squamous cell carcinoma cells in vitro. Oncol Rep. 2018;39(4):1991–8.
Arafat K, et al. Inhibitory effects of salinomycin on cell survival, colony growth, migration, and invasion of human non-small cell lung cancer A549 and LNM35: involvement of NAG-1. PLoS ONE. 2013;8(6):e66931.
Dewangan J, et al. Salinomycin inhibits breast cancer progression via targeting HIF-1alpha/VEGF mediated tumor angiogenesis in vitro and in vivo. Biochem Pharmacol. 2019;164:326–35.
Raisova M, et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117(2):333–40.
Kim KY, et al. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci. 2017;18(5):1088.
Kim KY, et al. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun. 2011;413(1):80–6.
Lee SH, et al. Androgen signaling is a confounding factor for beta-catenin-mediated prostate tumorigenesis. Oncogene. 2016;35(6):702–14.
Wang F, et al. Wnt/beta-catenin signaling contributes to prostate cancer heterogeneity through reciprocal suppression of H3K27 trimethylation. Biochem Biophys Res Commun. 2020;527(1):242–9.
Bian P, et al. Activated Wnt/f3-catenin signaling contributes to E3 ubiquitin ligase EDD-conferred docetaxel resistance in prostate cancer. Life Sci. 2020;254:116816.
Fang F, et al. CD147 promotes epithelial-mesenchymal transition of prostate cancer cells via the Wnt/beta-catenin pathway. Exp Ther Med. 2020;20(4):3154–60.
Liao JQ, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE. 2014;9(1):e84941.
Lee HG, et al. Salinomycin reduces stemness and induces apoptosis on human ovarian cancer stem cell. J Gynecol Oncol. 2017. https://doi.org/10.3802/jgo.2017.28.e14.
Yang L, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8.
Zhang Y, et al. Bortezomib potentiates antitumor activity of mitoxantrone through dampening Wnt/beta-catenin signal pathway in prostate cancer cells. BMC Cancer. 2021. https://doi.org/10.1186/s12885-021-08841-1.
Yu X, et al. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30(16):1868–79.
Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24(6):1838–51.
Wang W, et al. Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling. Chin J Cancer. 2017;36(1):35.
Witta SE, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66(2):944–50.
Shang Z, et al. A switch from CD44(+) cell to EMT cell drives the metastasis of prostate cancer. Oncotarget. 2015;6(2):1202–16.
Qu H, et al. Effect of salinomycin on metastasis and invasion of bladder cancer cell line T24. Asian Pac J Trop Med. 2015;8(7):578–82.
Acknowledgements
We would like to thank Drs. Ruveyde Garip and Sultan Kaya (Trakya University, School of Medicine, Department of Ophthalmology) for their support in the preparation of bovine retinal pigment epithelial cells.
Funding
This study was funded by the Trakya University Research Project Foundation (Project no: 2020-01), Edirne, Turkey.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experiments were performed and analyzed by Riza Serttas. The first draft of the manuscript was written by Suat Erdogan and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial, personal, or professional conflict of interest.
Ethical approval
Not applicable. This article does not contain any studies with human participants or live animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Serttas, R., Erdogan, S. Pretreatment of prostate cancer cells with salinomycin and Wnt inhibitor increases the efficacy of cabazitaxel by inducing apoptosis and decreasing cancer stem cells. Med Oncol 40, 194 (2023). https://doi.org/10.1007/s12032-023-02062-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02062-1