Abstract
Glioblastoma (GBM) is the most frequent brain cancer and more lethal than other cancers. Characteristics of this cancer are its high drug resistance, high recurrence rate and invasiveness. Invasiveness in GBM is related to overexpression of matrix metalloproteinases (MMPs) which are mediated by wnt/β-catenin and induced by the activation of signaling pathways extracellularly activated by the cytokine neuroleukin (NLK) in cancer stem cells (CSC). Therefore, in this work we evaluated the effect of the tetrose saccharide, erythrose (Ery), a NLK inhibitor of invasiveness and drug sensitization in glioblastoma stem cells (GSC). GSC were obtained from parental U373 cell line by a CSC phenotype enrichment protocol based on microenvironmental stress conditions such as hypoxia, hipoglycemia, drug exposition and serum starvation. Enriched fraction of GSC overexpressed the typical markers of brain CSC: low CD133+ and high CD44; in addition, epithelial to mesenchyme transition (EMT) markers and MMPs were increased several times in GSC vs. U373 correlating with higher invasiveness, elongated and tubular mitochondrion and temozolomide (TMZ) resistance. IC50 of Ery was found at nM concentration and at 24 h induced a severe diminution of EMT markers, MMPs and invasiveness in GSC. Furthermore, the phosphorylation pattern of NLK after Ery exposition also was affected. In addition, when Ery was administered to GSC at subIC50, it was capable of reverting TMZ resistance at concentrations innocuous to non-tumor cancer cells. Moreover, Ery added daily induced the death of all GSC. Those findings indicated that the phytodrug Ery could be used as adjuvant therapy in GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain and central nervous system cancers represent an important life-threatening disease for children and adults worldwide. Among them, glioblastoma (GBM) is the most common primary and devastating malignant brain tumor due to its extremely high recurrence risk [1]. GBM is defined for its poor prognoses; despite the use of surgery and adjuvant radiation therapy, most patients succumb in the range of 14.6–20.5 months after diagnosis, and only 15% of patients can survive until 2 years, a limited life expectancy concerning other types of cancer [2,3,4]. In addition, GBM is resistant to multimodal therapy with surgery followed by radiation and temozolomide, a DNA alkylating agent used for its capacity to cross the blood–brain barrier [5]. However, even though temozolomide (TMZ) has been the most effective drug for GBM treatment, a best 5-year survival rate of only 9.8% is achieved, and most patients successfully treated with combined therapy eventually had tumor recurrence and death [6]. Thus, searching for more specific and directed therapies for treating this tumor is a main topic of interest.
Among the principal features of GBM are an invasive behavior and the presence of cancer stem cells (CSC), which favors a high recurrence due to its tendency to rapidly disseminate within normal brain parenchyma making complete surgical resection almost impossible [7], which limits importantly the response to further treatment [8]. High expression of chemokine receptors in CSC and their activation induce a signaling cascade that promotes the expression of calcium-containing proteases (MMPs, zinc-dependent, calcium-containing endopeptidases), which have been regarded as the principal contributor to GBM CSC invasiveness [9, 10]. MMPs are known to be the main proteolytic enzymes that play a fundamental role in the degradation and remodeling of the components of the extracellular matrix (ECM), connective tissue and basement membrane components, which allows cancer cells to invade adjacent tissues. In addition, MMPs are overexpressed in GBM cell lines and biopsies of patients compared with low-grade astrocytoma or normal brain samples [11], favoring a worse overall survival probability. Therefore, it is suggested that anti-MMP therapy can be beneficial for GBM patients.
Thus, the inhibition of MMPs has been proposed as a possible therapeutic target to stop or at least delay tumor invasion and, ultimately, prolong patient survival [11]. However, in the last years, MMPs inhibition attempts in experimental therapy for GBM have been unsuccessful. Therefore, its inhibition with synthetic (such as marimastat, solimastat, metastat and others) and natural inhibitors has had, so far, little clinical benefit due to the development of severe side effects during treatment in preclinical phases [12].
Phytodrugs have been used for cancer treatment, particularly for inhibiting early stages of invasiveness. This is the case of Ery, which is a tetrose intermediary of the pentose phosphate pathway that is obtained principally from rhubarb (Polygonaceae) roots [13]. Ery is a competitive inhibitor of the cytokine neuroleukin (NLK) or autocrine motility factor (AMF) [14]. NLK also performs enzymatic functions in the cytosol as the glycolytic enzyme hexose phosphate isomerase (HPI) or is secreted, depending on the cellular context, as a cytokine to induce invasiveness by binding extracellularly to its gp78 receptor (AMFR) [14, 15]. In GBM a high NLK expression was associated with a worse patient prognosis, and knockdown of NLK diminished GBM cell migration [16].
Previously we reported in a model of breast CSC a diminished content of epithelial to mesenchyme transition (EMT) proteins including MMP-1, migration and invasion, after adding Ery at nanomolar concentrations [17]. Therefore, in the present study we evaluated the role of Ery in the inhibition of invasive function in GSC.
Materials and methods
Cell culture
GBM human U373 cells isolated from brain epithelium; human breast metastatic cell line MDA-MB-231 (as invasiveness control); normal human umbilical vein endothelial normal cells HUVEC and normal mouse fibroblast NIH3T3 cells (as control of non-cancer cell), were grown in Dulbecco-MEM medium (DMEM) with 25 mM of glucose (high glucose) and supplemented with 10% fetal bovine serum (GIBCO; Rockville, USA) plus 10,000 U penicillin/streptomycin (Sigma; Steinheim, Germany) at an initial density of 1 × 106 cells/mL. Cells were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37 °C for 2–3 days until a confluence of 80% was reached. U373 cells used in the present work proceeded from ATCC (American Type Culture Collection; Rockville, MD, USA).
Drug and natural compounds treatment
U373 and GSC (1 × 105 cells/0.5 mL) were cultured in 24-well plates overnight. Afterward, Ery was added at 0.1, 1, 10, 100 µM; 1 and 10 mM for further 24 h incubation. Then, cellular viability was determined by trypan blue stain assay. The concentration required to inhibit 50% of cellular viability (IC50) was determined after 24 h drug exposure using the Origin 8 software. The same methodology was used for the obtention of IC50 of TMZ in GSC and U373 cells for 72 h.
Glioblastoma stem cells (GSC) enriched fraction
GSC were selected and isolated (enriched fraction) from U373 parental line following the microenvironmental stress protocol previously reported [17]. Briefly, U373 cells (1 × 106 cells/mL) were exposed to hypoglycemia (2.5 mM glucose) plus Temozolomide 500 nM under normoxia (20% O2) for 12 h. Then the medium was changed for DMEM 25 mM plus Etoposide 500 mM and cells were exposed to hypoxia (0.1% O2) in an Oxygen Control Chamber (Coy Laboratory, Grass Lake, MI) for 12 h. Finally, cells were cultured in serum-free DMEM containing 25 mM glucose and placed in a humidified atmosphere of 5% CO2/95% air at 37 °C for 24 h until their use. Surviving cells from this protocol were named GSC.
Glioblastoma stem cells sphere formation
GBM spheres of cancer stem cells were generated by using the floating sphere-forming assay [18]. Briefly, GSC and U373 cells (1 × 105 cells) were grown in Erlenmeyer flasks with free serum-DMEM and placed immediately under slow (20–50 rpm) orbital shaking for 10 days at 37 °C in 95% air/5% CO2. Fresh DMEM was added every 2–3 days to remove cellular debris and planktonic cells not-forming spheroids. GBM sphere size was measured at different culture times with a graduated reticule in an inverted phase contrast microscope (1/10 mm; Zeiss, NY, USA).
Western blot
U373 and GSC cells (5 × 106 cells/mL) were dissolved in RIPA (PBS 1 × pH 7.2, 1% IGEPAL NP40, 0.1% SDS and 0.05% sodium deoxycholate) lysis buffer plus 1 mM PMSF (phenyl methanesulfonyl fluoride) and 1 tablet of complete protease inhibitors cocktail (Roche, Mannheimm, Germany), as previously it was described [19]. Protein samples (40 µg) were resuspended in loading buffer plus 5% β-mercaptoethanol and loaded onto 10 or 12.5% polyacrilamide gel under denaturalizing conditions. Electrophoretic transfer to PVDF membranes (BioRad; Hercules, CA, USA) was followed by overnight immunoblotting with 1:1000 dilution of CD133, CD44, Oct 3/4, vimentin, NOTCH, β-catenin, MMP-1, MMP-9, NLK, receptor gp78, β and α-tubulin antibodies (Santa Cruz; Santa Cruz, CA, USA) at 4 °C. The hybridization bands were revealed with the corresponding secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology, CA, USA). The signal was detected by chemiluminescence using the ECL-Plus detection system (Amersham Bioscience; Little Chalfont, Buckinghamshire, UK). Densitometry analysis was performed using the Scion Image Software (Scion; Bethesda MD, USA) and normalized against its respective load control. The percentage of each band represents the mean ± SD of at least three independent experiments.
Immunoprecipitation assays
To reveal NLK/P-ser and NLK/-thr, proteins were immunoprecipitated by incubating total protein of U373 (control or Ery-treated) with the NLK or IgG1 as control (1 µg) for 1 h plus protein A-sepharose (Sigma-Aldrich), and further detected with anti- NLK antibody using its correspondent secondary antibodies and following manufacturer instructions. Densitometry analysis was performed using the Scion Image Software (Scion, Bethesda MD, USA).
Detection of NLK protein in the extracellular milieu
Extracellular neuroleukin (NLK, or hexose phosphate isomerase HPI) protein detection was assayed in free-cell DMEM from 24 h U373. Cell-free medium was incubated with 10% trichloroacetic acid (TCA) at 4 °C overnight [19]. Afterwards, the mixture was centrifuged once at 10,000 rpm for 30 min. The sediment was resuspended in loading buffer and loaded onto 12.5% SDS-PAGE gels. Electrophoretic transfer to PVDF membranes (BioRad; Hercules, CA, USA) was followed by overnight immunoblotting with 1:500 dilution of NLK antibodies (Santa Cruz, CA USA) at 4 °C. The hybridization bands were revealed with the mouse secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology). The signal was detected by chemiluminescence as described above and albumin was used as load control.
Invasiveness and wound healing assay
U373 and GSC (5 × 104 cells/mL) in serum-free DMEM were seeded on the upper compartment of Boyden chambers (Trevigen Inc., Helgerman, USA). The lower compartment was filled with DMEM without serum. After 24 h at 37 °C, invasive cells in the lower compartment were loaded with 60 nM calcein AM (acetomethylester) for 60 min. Calcein fluorescence was detected at 485 nm excitation and 520 nm emission in a microplate reader (NunclonTM, Roskilde, Denmark) [19]. For control assays, MDA-MB-231 metastatic canonical breast cancer cells were used. For migration, a wound healing assay was used. Briefly, cell monolayers cultured in Petri dishes were wounded using a blue plastic sterile tip, washed with PBS buffer and incubated with fresh DMEM without serum. After 24 h, the medium was removed and fresh DMEM without serum was added. Cellular migration distance from the border to the center of the Petri dish was documented in micrographs [19].
Transmission electron microscopy
To evaluate the mitochondria morphology from U373 and GSC, standard transmission electron microscopy (TEM) was used. Briefly, the samples were fixed in a mixture of 4% paraformaldehyde/2.5% glutaraldehyde in PBS for 3 h, washed in PBS, post-fixed in 1% osmium tetroxide for 2 h, washed with PBS, dehydrated for incubating in a graded series of ethanol, incubated in propylene oxide, and then embedded in propylene oxide/epoxy resin (1:1) for 18 h and finally incorporated in Eppendorf microtubes and polymerized at 60 °C for 48 h [20].
Statistical analysis
Data are expressed as mean ± standard deviation of the indicated number of independent experiments. The experimental and control groups were statistically compared using ANOVA with P values < 0.01 or < 0.05 as significance criteria.
Results
GSC metastatic phenotype
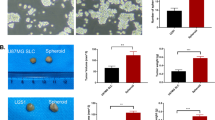
GSC obtained and named in this way after microenvironmental stress protocol (hypoxia, hypoglycemia, drugs exposition and serum starvation) [17] showed a high viability of ≈ 85%, inclusive, its morphology remained unchanged in comparison with the control (parental line) (Fig. 1a). As previously it was reported [17], breast CSC obtained with the same protocol only showed 30% of viability with a fibroblastoid morphology very different from its parental line. Current findings in GSC probably indicated high resistance and proportion of the subpopulation of CSC inside the cell line. Therefore, to evaluate above, GSC markers and EMT proteins were measured in U373 and GSC. CD133+ and CD44 low, well established GSC and mesenchymal markers; increased 9.3 times and diminished 67% vs parental cell line, respectively (Fig. 1b). In addition, the pluripotency marker Oct3/4 and the metastasis and colonization factor NOTCH increased 3–7.7 times vs. parental cell line. Finally, a crucial transcription factor in EMT phenotype, β-catenin was increased 3 times with the concomitant overexpression (from 1.1 to 8.9 times) of their target matrix metalloproteinases 1 and 9; and vimentin, key proteins involved in migration and invasiveness; the protein MMP-1 did not change in both lines, while MMP-9 increased 10 times in GSC vs. U373 (Fig. 1b). Another key feature of CSC phenotype is the capacity of sphere-forming in absence of growth factor of serum. GSC formed spheres from 330 ± 25 μm of diameter at day 10 without fetal bovine serum (serum-free DMEM) (Fig. 1c). In contrast, U373 parental cell line did not form spheres from more than 50 μm of diameter in the same conditions. Furthermore, GSC were more potent in its capacity of invasiveness (from 2.4 to 4.6 times), in Boyden chamber, in comparison with other breast cancer cell line highly metastatic, MDA-MB-231 and its counterpart MDA-MB-231 CSC (Fig. 1d). These results of invasiveness were similar to the migration evaluated by wound healing assay (Fig. 1d). Electron microscopy showed the presence of typical elongated and tubular mitochondria in GSC with granular structures inside (Fig. 1e). This type of mitochondrial morphology was similar to that reported in MDA-MB-231 cells, a high metastatic cell line [21]. On the other hand, the morphology of U373 mitochondria was preferentially cylindrical (Fig. 1e).
a Morphology of GSC and its parental line (U373). b Stemness biomarkers and EMT of GSC U373. c Sphere formation of GCS and U373. d Invasiveness and migration potential. e Representative transmission electron micrographs; the arrows indicate the mitochondrion in U373 and GSC. Bar = 200 μm in a, c and d and 1 μm, 500 nm or 200 nm in e. The data shown represent mean ± S.D. of at least 4 independent experiments. *P ≤ 0.05 and **P ≤ 0.01 vs. U373 (for b and c) or MDA-MB-231 in d
Neuroleukin in GSC
The neuroleukin (NLK or HPI/AMF, hexose phosphate isomerase/autocrine motility factor) has been reported to be secreted in cancer via an autocrine route and probably specific phosphorylation [14]. To evaluate the phosphorylation status in NLK, we immunoprecipitated total and extracellular NLK and P-Ser and P-Thr. We found that in intracellular total protein both control and GSC had the same content of NLK although phosphorylation in P-Thr was more evident (14 times) that in P-Ser in GSC compared with the control (Fig. 2a). The pattern of the content of NLK in extracellular milieu (supernatants) was completely different. NLK was oversecreted almost 40 times in GSC versus control and both P-Ser and P-Thr were also increased 3.4–5 times; On the other hand, U373 control showed a similar pattern of phosphorylation between P- Ser and P-Thr (Fig. 2a). In addition, we found that the NLK receptor, gp78, was increased in 5 times in GSC vs. U373 control (Fig. 2b). To evaluate if NLK recognizes its receptor in GSC we immunoprecipitated NLK and revealed with gp78. We found that NLK co-immnoprecipitated preferentially in GSC versus parental cell line (Fig. 2c). Because Ery is a competitive inhibitor of NLK (HPI/AMF) [17], we obtained the IC50 of Ery in control U373 (3.5 ± 0.3 μM) and GSC (345 ± 7 nM) at 24 h. After adding during 24 h the IC50 of Ery in control and GSC, we observed that NLK stopped precipitating with gp78 in GSC, preferentially, indicating a loss of interaction between NLK and gp78 (Fig. 2c). Those findings indicated that NLK oversecreted is susceptible of interaction with its receptor gp78 and Ery blocks this interaction.
Ery alters phosphorylation and invasive profile in GSC
Because Ery inhibited the interaction between NLK and gp78 in GSC, we evaluated its phosphorylation status. GSC were subjected to IC50 of Ery during 24 h and protein content was measured. Firstly, we observed that Ery induced a severe diminution of NLK intracellularly and extracellularly (both in more than 90%) (Fig. 3a). These observations correlated with a loss of phosphorylation of P-Ser and P-Thr in intracellular and extracellular NLK from 59 to 96% in comparison with GSC without Ery (Fig. 3a). In addition, subIC50 concentrations of Ery in GSC did not induce a decrease in NLK, but did induce a diminution in P-Ser and P-Thr (Fig. 3a). Since the NLK was inhibited to be secreted in extracellular milieu of GSC, we evaluated the protein content of GSC markers, pluripotency and EMT. All proteins were diminished from 10 to 96.6% after Ery adding during 24 h, except CD44, which increased 2 times vs. GSC without Ery (Fig. 3b). Concomitant with EMT proteins diminution, invasiveness in Boyden chamber was inhibited by 73.5%, same as migration (Fig. 3c).
a Intracellular and extracellular pattern of phosphorylation of NLK after Ery treatment of GSC. b Stemness Biomarkers of GSC and EMT and c invasiveness and migration after Ery treatment (Bar = 200 μm). The data shown represent mean ± S.D. of at least 4 independent experiments. *P ≤ 0.05 and **P ≤ 0.01 vs. GSC without Ery (for a and b) or U373 in c
Ery reverts TMZ resistance in GSC
Another major problem in GBM treatment includes the drug resistance. Because Ery induced a severe diminution of invasiveness, and it is reported that it can induce cell death [22], we evaluated if we could sensitize GSC to the effect of the canonical treatment with TMZ. For the evaluation of drug sensitization in GSC, IC50 of TMZ was obtained in U373 (350 ± 20 μM at 72 h) and was similar to the previously reported at 72 h [23]. However, GSC were more resistant to the same drug, the IC50 was of 632 ± 25 μM and therefore, GSC were not susceptible to IC50 found in U373 (Fig. 4a). On the other hand, IC50 of Ery (345 nM) from GSC was able to revert TMZ resistance at 350 μM in GSC, inclusive the same effect was observed even with only 50 μM (sub IC50) of TMZ (Fig. 4a). Same doses (TMZ 50 μM and Ery 345 nM) were applied to non-tumor cells (HUVEC and NIH3T3) without an effect on cell viability (Fig. 4b). Although these observations could seem successful, when the remaining GSC cells survivors from this treatment were re-cultured in complete DMEM without drugs, they remained viable at least until 144 h (Fig. 4a) without loss of viability. Hence, a new scheme of treatment was followed in which the first combination (TMZ 50 μM and Ery 345 nM) was applied for 24 h and then only IC50 of Ery from GSC was added every day in fresh medium. At day 5 all remaining cells succumb completely (Fig. 4c and d). The same treatment scheme was ineffective in human HUVEC cells (Fig. 4c). In contrast, in GSC without Ery, cells remaining viable (Fig. 4d). Those observations indicated that Ery could be used as adjuvant treatment in recurrent GSC with TMZ (Fig. 5) without apparent effects on the viability of non-tumor cells.
a Combination therapy of TMZ with Ery on GSC. b Combination therapy of TMZ with Ery on non-cancer cells. c Ery as adjuvant therapy in the treatment of recurrent GSC. d GSC with or without treatment scheme. Bar = 200 μm. The data shown represent mean ± S.D. of at least 4 independent experiments. **P ≤ 0.01 vs. U373 (a) or treatment in GSC at t = 0 (c)
Targets of action of Ery and TMZ in GSC. Expelled NKL from GSC interacts with its receptor (gp78) and activates signaling pathways involved in stemness; therefore, the stem population remains in the tumor. The combinatory therapy of TMZ with Ery has 2 different targets, the DNA and the NLK/AMFR interaction, respectively. As a result, the GSC population dies and could prevent recurrence in the patient
Discussion
Glioblastoma stem cells (GSC)
It is believed that GBM, the most common primary brain tumor, is initiated and maintained by the presence of a subpopulation of self-renewing stem-like cells known as glioma stem cells (GSC). It is characterized by increased resistance to radiotherapy and chemotherapy, which may lead to a failure in the treatment; high invasiveness and tumor recurrence rates, in turn, promote the malignant progression and short survival period [24] which makes to GBM a high-grade and difficult to handle tumor. Current strategies to treat GMB introduced since 2005 with safe resection, radiotherapy and/or TMZ only has shown a scarce increase of 2.5 months of patient survival [25] and they are therapies not directed to the main features of GBM i. e. recurrency and invasiveness.
To evaluate the possible effect on invasiveness and drug resistance reversion of Ery, we have enriched a fraction of GSCs from the parental cell line U373 by exposing the cells to environmental stress conditions [17], GSC selected, overexpressed surface markers such as CDC133, Oct3/4 and CD44low, proteins that play a crucial role in the maintenance of GSCs and the resistance to chemo and radiotherapy under nutrient deprivation and hypoxia conditions. Yusuf et al. reported that glucose deprivation induces the survival of GSCs with higher clonogenic and invasive potential through the upregulation of Wnt/β-Catenin signaling pathway [26], whereas LGK974 (Wnt inhibitor) sensitizes GSCs to the cell death under glucose starvation conditions. The authors suggest that glucose starvation increases the invasive capacity in GSCs by inducing a mesenchymal transition via the Wnt/β-Catenin pathway [26]. When the mitochondria have a greater fusion, an elongated morphology can be observed as was seen in GSC (Fig. 1e) [27] as indicative of a higher invasiveness rate and OxPhos (oxidative phosphorylation) activity [28]. Thus, GSC obtained with this protocol presented characteristics related to a stem-like phenotype. It has been reported that cancer cells have a greater mitochondrial fusion to maintain proliferation, promoting cell survival, metabolism re-programming and invasion under stress conditions, such as exposition to chemotherapeutics drugs or glucose deprivation [29]. Wang et al. demonstrated that Krüppel-like factor 4 (KLF4), a transcriptional factor promotes mitochondrial fusion in GBM cells under serum and nutrition deprivation, inducing a protection in cells from death, and increasing respiratory capacity and ROS generation. The authors suggest that mitochondrial fusion induced by KLF4 in GBM cells can support a dependence on oxidative phosphorylation (OXPHOS), a stem cell-like phenotype which may contribute to tumor recurrence and resistance to cell death of GSC, possibly through mitochondrial fusion [30].
Phosphorylated NLK is secreted from GSC and stimulates the progression of GBM
Invasiveness is induced by external factors coming from the microenvironment of accessory cells and autocrine signals of self-CSC [31], allowing them a drug resistance and the relapse and favoring metastasis. One of these factors is the NLK a moonlight protein that plays a distinct role, when it is localized in the cytosol participates as a catalytic enzyme in the gluconeogenesis–glycolysis pathways and when is secreted act as a cytokine that interacts with its receptor, gp78/AMFR, inducing apoptotic resistance, matrix metalloproteases secretion, angiogenesis and cell motility (Fig. 5) [32]. It has been demonstrated that hypoxia induces the propagation of GSC as well as NLK expression and secretion, leading at the NLK/AMFR signaling pathway activation [33]. In our study, we observed that both GSC and its parental cell line contained intracellular NLK (HPI/AMF). However, GSC showed an increased NLK phosphorylation in serine and threonine residues correlating with a higher secretion of NLK in the supernatant of GSC as well as a significant increment in the expression of gp78 (NLK receptor), and a higher association of this receptor to NLK in comparison with U373 cells. Our results suggest that NLK is secreted from GSCs and stimulates the invasiveness in an autocrine signaling. NLK is further upregulated by hypoxia, consistent with the known regulation of the enzyme by HIF-1α. Also, our results suggest that NLK is phosphorylated in GSC, which promotes its extracellular release and formation of NLK/gp78 complex, promoting cell invasion. The mechanisms that induce the extracellular secretion of NLK are unknown; however, it has been proposed that AMF/HPI phosphorylation is a potential regulator of its secretion. In this sense, it has been demonstrated that NLK is phosphorylated in serine residues by CKII serine/threonine kinase in response to EGF and subsequently secreted in a higher concentration by a canonical endoplasmic reticulum/Golgi pathway in human breast cancer cells [34]. Inhibition of invasion in cancer cells is through the inactivation of PI3K/AKT signaling pathway and blocking of the transcriptional function of β-catenin/TCF4 and activating protein 1 (AP-1) [34]. AP-1 composed of the c-jun/c-fos regulator complex, promotes the expression of matrix metalloproteinases (MMP), such as MMP1 and MMP3, required for basement membrane and ECM degradation. In addition, it has been reported that human AMF/HPI is phosphorylated at serine 185 by casein kinase II (CK2) and conformational changes induced by phosphorylation may be associated with its secretion [35]. In this sense, NLK can act as an autocrine cytokine due to its interaction with the receptor gp78 for metastasis progression, and activating an intracellular pathway that induces Akt and ERK and then NF-κB and Wnt/β-catenin (Gallardo-Pérez et al. manuscript in preparation) (Fig. 5). Also, AMFR positively regulates cell motility through Rho-associated protein kinase 2 (ROCK2)/cofilin signaling. In addition, NLK upregulation mediates EMT [36] by SNAIL transcriptional factor overexpression and decreases E-cadherin and β-Catenin levels [36]. In human GBM biopsies, NLK and AMFR are upregulated in hypoxic pseudopalisades cells, which is associated with poorer survival in patients with GBM [37]. In addition to its glycolytic and mitogenic effects, NLK can mediate the differentiation of myeloid cells and antiproliferation effects in HeLa, A-549, MCF-7 or other cell lines [38]. Hence, the pleiotropic effects of NLK depend on cell type and microenvironmental conditions, or the status of phosphorylation, which can be a key to elucidating the preferred effect in each case.
For the subsequent studies, Ery was evaluated on GSC and an IC50 of viability at relevant concentrations was obtained (345 nM). Previous findings in breast CSC indicated an IC50 of Ery of approximately 24 nM [17], indicating the sensitivity of CSC to this phytodrug (while other drugs had an effect in range in micromolar) and non-tumor cancer cells were insensitive to Ery with viability higher than 95% [17].
The phosphorylation status of NLK (HPI/AMF) was suggested in tyrosine residues [14], but other sites of phosphorylation has not been reported. This work evaluated the status of NLK phosphorylation in P-Ser and P-Thr. We observed that these sites were preferentially phosphorylated in GSC than in its parental cell line correlating with a higher secretion of the NLK in the supernatant of cells. These findings agree with the notion that cell signaling molecules must to be phosphorylated in different residues to induce its release [39]. In addition, it is reported that NLK is internalized into the cells by means of a PI3K and dynamin-dependent raft endocytic pathway, regulating inclusive mitochondrial fusion and dynamics and endoplasmic reticulum stress and apoptosis. However, NLK has showed unwanted growth stimulatory effects in some GBM models [16] or antiproliferation in HeLa, A-549, MCF-7 or other cell lines [38]. The status of phosphorylation again, can be a key to elucidate the preferred effect in each case. Either way, inhibition of NLK (HPI/AMF) by Ery has shown to be efficient against CSC population.
Ery as adjuvant therapy for GSC inhibition
Some strategies to inhibit NLK has been evaluated including siRNAs or DNA antisense, but precautions must be taken because these strategies can alter the general protective role of NLK in cell death control on neuronal cells [40]. Therefore, novel strategies to NLK (HPI/AMF) inhibition should involve safe drugs or inhibitors. Some of them include compounds from plants widely consumed by the general population. This is the case of Ery which comes from edible specimens of rhubarb roots which have been inclusive proposed as modulator of cardiovascular risk [41] preventing obesity and diabetes.
Drug resistance is a serious complication during GBM treatment. Particularly, during treatment with TMZ many GBM cells remains viable and because Ery has, besides anti-invasive properties, antiproliferative effects, we rush to evaluate if Ery prevent recurrence or regrowth after the first (initial) treatment with TMZ. Previously, in a rat model of colon cancer, Ery markedly diminished the growth of ascites and induced apoptosis, suggesting that Ery may be more effective in combination with anticancer drugs [22]. In effect, Ery was capable of overcome drug resistance of GSC to TMZ at relevant concentrations of nanomolar at single doses. However, this treatment allowed the subsequent growth of the remaining cells; growth that was inhibited by the combination of TMZ (single doses) and the daily addition of Ery to the GSC in culture (Fig. 4a). It is probable that Ery act as an enhancer of the TMZ. In a previous study, combination of Herceptin (anti-Her-2 monoclonal antibody) and erythrose-4-phosphate (E4P) or D-mannose-6-phosphate caused an interestingly diminution of breast cancer growth and invasiveness compared with each agent by separately [42]. The novelty of our treatment includes the daily addition of a natural compound that have no effect on cell viability of non-tumor cells but that increases the rate of death on the GSC in such a way that at 120 h of the initial treatment with TMZ, GSC completely succumbed (Fig. 4c). Above remains an interesting topic because several growth factors are involved in the activation of NLK (HPI/AMF) as heregulin [43] and it may be that this activation is inhibited by the constant presence of Ery in the medium of GSC.
We observed in this study that the administration of Ery (345 nM) on the enriched fraction of GSC inhibited the growth and then significantly decreased the invasiveness as well as the levels (10 to 96.6%) of the stemness and invasiveness markers such as CDC133, β-catenin, Notch, vimentin, MMP-1, and MMP-9 with respect at GSC without Ery treatment. These markers are associated with the induction of proliferation, EMT, invasiveness, and chemo and radio resistance. Thus, it seems in our study the Ery blocks the NLK/AMFR cellular receptor, diminishing the activation of the transduction pathways associated with the onset of EMT/migration/invasion in CSC from GBM (Fig. 5). Previously was reported that E4P inhibits the cell migration and chemotactic effect of NLK in GBM stem-like cell in vitro [16]. The cells were growth in serum-free Neurobasal medium or in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum [16]. On the other hand, Gallardo-Perez et al. demonstrated that NLK inhibition with E4P (5–250 nM) in the breast cancer MCF-7 stem cells (BCSC), growth in hypoxia/hypoglycemia; plus: taxol and adriamycin conditions, decreased the levels of stemness (ALDH13, CD44), pluripotency (Oct3/4, β-catenin, p38 MAPK) and EMT (SNAIL, vimentin, MMP-1) markers as well as the suppression of invasiveness potential, mammospheres formation capacity and HPI/AMF expression in Breast CSC [17]. These authors also observed that BCSC mammospheres in the presence of E4P induce a downregulation of pro-apoptotic protein (Bcl-2, XIAP, cIAP1) [17]. It has been established that NLK/ AMFR signaling in cancer cells induced an anti-apoptotic effect by decreasing the Apaf-1 and caspase-9 mRNA levels generating resistance to drug-induced apoptosis [44]. Apaf-1 and caspase-9 proteins induce the apoptotic cell death pathway via mitochondria [45].
Also, it has been demonstrated that co-treatment of shNLK more bevacizumab at xenografts nude mice with GSCs present a reduced perivascular invasion [16]. These results suggest that phytodrugs such as NLK can potentiate the anti-proliferative, anti-angiogenic, anti-invasive, or pro-apoptotic effects and overcome the chemo-resistance of antineoplastic drugs.
The novelty of our treatment includes the daily addition of a natural compound that does not affect cell viability of non-tumor cells, but that increases the rate of death on the GSC in such a way that at 120 h of the initial treatment with TMZ, GSCs completely succumbed (Fig. 4c). The above remains an interesting topic because several growth factors are involved in the activation of NLK (HPI/AMF) as heregulin [43], and it may be that this activation is inhibited by the constant presence of Ery in the medium of GSCs.
Conclusion
Phytodrugs as Ery can be used in the treatment of recurrent and invasive GSC and can be proposed as an adjuvant therapy due to its innocuousness on non-tumor cells and its capacity to enhance the chemotherapy.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CSC:
-
Cancer stem cells
- GBM:
-
Glioblastoma
- GSC:
-
Glioblastoma cancer stem cells
- E4P:
-
Erythrose 4-phosphate
- EMT:
-
Epithelial-mesenchyme transition
- Ery:
-
Erythrose
- HPI/AMF:
-
Hexose phosphate isomerase/autocrine motility factor
- IC50 :
-
Drug concentration required to inhibit 50% of cellular viability
- MMPs:
-
Matrix metalloproteinases
- NLK:
-
Neuroleukin
- P-Ser:
-
Phosphor-serine
- P-Thr:
-
Phosphor-threonine
- TMZ:
-
Temozolomide
References
Lin D, Wang M, Chen Y, Gong J, Chen L, Shi X, Lan F, Chen Z, Xiong T, Sun H, Wan S. Trends in intracranial glioma incidence and mortality in the United States, 1975–2018. Front Oncol. 2021;11:748061. https://doi.org/10.3389/fonc.2021.748061.
Back MF, Ang EL, Ng WH, See SJ, Lim CC, Chan SP, Yeo TT. Improved median survival for glioblastoma multiforme following introduction of adjuvant temozolomide chemotherapy. Ann Acad Med Singap. 2007;36(5):338–42.
Virga J, Szivos L, Hortobágyi T, Chalsaraei MK, Zahuczky G, Steiner L, Tóth J, Reményi-Puskár J, Bognár L, Klekner A. Extracellular matrix differences in glioblastoma patients with different prognoses. Oncol Lett. 2017;17(1):797–806. https://doi.org/10.3892/ol.2018.9649.
Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. https://doi.org/10.1186/s13046-022-02349-7.
Arora A, Somasundaram K. Glioblastoma vs temozolomide: can the red queen race be won? Cancer Biol Ther. 2019;20(8):1083–90. https://doi.org/10.1080/15384047.2019.1599662.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. https://doi.org/10.1016/S1470-2045(09)70025-7.
Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–17. https://doi.org/10.1101/gad.261982.115.
Alfonso J, Talkenberger K, Seifert M, Klink B, Hawkins-Daarud A, Swanson KR, Hatzikirou H, Deutsch A. The biology and mathematical modelling of glioma invasion: a review. J R Soc Interface. 2017;14(136):20170490. https://doi.org/10.1098/rsif.2017.0490.
Inoue A, Takahashi H, Harada H, Kohno S, Ohue S, Kobayashi K, Yano H, Tanaka J, Ohnishi T. Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int J Oncol. 2010;37(5):1121–31. https://doi.org/10.3892/ijo_00000764.
Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57–83. https://doi.org/10.1016/j.critrevonc.2019.02.010.
Hagemann C, Anacker J, Ernestus RI, Vince GH. A complete compilation of matrix metalloproteinase expression in human malignant gliomas. World J Clin Oncol. 2012;3(5):67–79. https://doi.org/10.5306/wjco.v3.i5.67.
Abdel-Hamid NM, Abass SA. Matrix metalloproteinase contribution in management of cancer proliferation, metastasis and drug targeting. Mol Biol Rep. 2021;48(9):6525–38. https://doi.org/10.1007/s11033-021-06635-z.
Liang W, Chen Y, Li X, Guo F, Sun J, Zhang X, Xu B, Gao W. Label-free proteomic analysis of smoke-drying and shade-drying processes of postharvest rhubarb: a comparative study. Front Plant Sci. 2021;12:663180. https://doi.org/10.3389/fpls.2021.663180.
Niinaka Y, Paku S, Haga A, Watanabe H, Raz A. Expression and secretion of neuroleukin/phosphohexose isomerase/maturation factor as autocrine motility factor by tumor cells. Cancer Res. 1998;58(12):2667–74.
Watanabe H, Carmi P, Hogan V, Raz T, Silletti S, Nabi IR, Raz A. Purification of human tumor cell autocrine motility factor and molecular cloning of its receptor. J Biol Chem. 1991;266(20):13442–8.
Kathagen-Buhmann A, Maire CL, Weller J, Schulte A, Matschke J, Holz M, Ligon KL, Glatzel M, Westphal M, Lamszus K. The secreted glycolytic enzyme GPI/AMF stimulates glioblastoma cell migration and invasion in an autocrine fashion but can have anti-proliferative effects. Neuro Oncol. 2018;20(12):1594–605. https://doi.org/10.1093/neuonc/noy117.
Gallardo-Pérez JC, Adán-Ladrón de Guevara A, Marín-Hernández A, Moreno-Sánchez R, Rodríguez-Enríquez S. HPI/AMF inhibition halts the development of the aggressive phenotype of breast cancer stem cells. Biochim Biophys Acta Mol Cell Res. 2017;1864(10):1679–90. https://doi.org/10.1016/j.bbamcr.2017.06.015.
Manuel Iglesias J, Beloqui I, Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon A, Menendez JA, Dopazo J, Martin AG. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS ONE. 2013;8(10):e77281. https://doi.org/10.1371/journal.pone.0077281.
Gallardo-Pérez JC, Rivero-Segura NA, Marín-Hernández A, Moreno-Sánchez R, Rodríguez-Enríquez S. GPI/AMF inhibition blocks the development of the metastatic phenotype of mature multi-cellular tumor spheroids. Biochim Biophys Acta. 2014;1843(6):1043–53. https://doi.org/10.1016/j.bbamcr.2014.01.013.
Jiménez-García LF, Segura-Valdez ML. Visualizing nuclear structure in situ by atomic force microscopy. Methods Mol Biol. 2004;242:191–9. https://doi.org/10.1385/1-59259-647-9:191.
Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32(40):4814–24. https://doi.org/10.1038/onc.2012.494.
Liu LL, Yi T, Zhao X. Antitumor effect of d-erythrose in an abdominal metastatic model of colon carcinoma. Oncol Lett. 2015;9(2):769–73. https://doi.org/10.3892/ol.2014.2764.
Pandey V, Ranjan N, Narne P, Babu PP. Roscovitine effectively enhances antitumor activity of temozolomide in vitro and in vivo mediated by increased autophagy and Caspase-3 dependent apoptosis. Sci Rep. 2019;9(1):5012. https://doi.org/10.1038/s41598-019-41380-1.
Oliver L, Lalier L, Salaud C, Heymann D, Cartron PF, Vallette FM. Drug resistance in glioblastoma: are persisters the key to therapy? Cancer Drug Resist. 2020;3(3):287–301. https://doi.org/10.20517/cdr.2020.29.
Stylli SS. Novel treatment strategies for glioblastoma-a summary. Cancers (Basel). 2021;13(22):5868. https://doi.org/10.3390/cancers13225868.
Yusuf S, Aretz P, Nickel AC, Westhoff P, Sharma A, Qin N, Remke M, Steiger HJ, Hänggi D, Liu H, Liu H, Neumann S, Reifenberger G, Maciaczyk J. WNT/β-catenin-mediated resistance to glucose deprivation in glioblastoma stem-like cells. Cancers. 2022;14(13):3165. https://doi.org/10.3390/cancers14133165.
Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166(3):555–66. https://doi.org/10.1016/j.cell.2016.07.002.
Gallardo-Pérez JC, de Guevara AA, García-Amezcua MA, Robledo-Cadena DX, Pacheco-Velázquez SC, Belmont-Díaz JA, Vargas-Navarro JL, Moreno-Sánchez R, Rodríguez-Enríquez S. Celecoxib and dimethylcelecoxib block oxidative phosphorylation, epithelial–mesenchymal transition and invasiveness in breast cancer stem cells. Curr Med Chem. 2022;29(15):2719–35. https://doi.org/10.2174/0929867328666211005124015.
Bordi M, Nazio F, Campello S. The close interconnection between mitochondrial dynamics and mitophagy in cancer. Front Oncol. 2017;7:81. https://doi.org/10.3389/fonc.2017.00081.
Wang S, Shi X, Wei S, Ma D, Oyinlade O, Lv SQ, Ying M, Zhang YA, Claypool SM, Watkins P, Xia S. Krüppel-like factor 4 (KLF4) induces mitochondrial fusion and increases spare respiratory capacity of human glioblastoma cells. J Biol Chem. 2018;293(17):6544–55. https://doi.org/10.1074/jbc.RA117.001323.
López de Andrés J, Griñán-Lisón C, Jiménez G, Marchal JA. Cancer stem cell secretome in the tumor microenvironment: a key point for an effective personalized cancer treatment. J Hematol Oncol. 2020;13(1):136. https://doi.org/10.1186/s13045-020-00966-3.
Li Y, Wei Z, Dong B, Lian Z, Xu Y. Silencing of phosphoglucose isomerase/autocrine motility factor decreases U87 human glioblastoma cell migration. Int J Mol Med. 2016;37(4):998–1004. https://doi.org/10.3892/ijmm.2016.2500.
Funasaka T, Yanagawa T, Hogan V, Raz A. Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia. FASEB J. 2005;19(11):1422–30. https://doi.org/10.1096/fj.05-3699com.
Kho DH, Zhang T, Balan V, Wang Y, Ha SW, Xie Y, Raz A. Autocrine motility factor modulates EGF-mediated invasion signaling. Can Res. 2014;74(8):2229–37. https://doi.org/10.1158/0008-5472.CAN-13-2937.
Haga A, Niinaka Y, Raz A. Phosphohexose isomerase/autocrine motility factor/neuroleukin/maturation factor is a multifunctional phosphoprotein. Biochim Biophys Acta. 2000;1480(1–2):235–44. https://doi.org/10.1016/s0167-4838(00)00075-3.
Funasaka T, Hogan V, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Can Res. 2009;69(13):5349–56. https://doi.org/10.1158/0008-5472.CAN-09-0488.
Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, Cloughesy TF, DeGroot JF, Galanis E, Gilbert MR, Hegi ME, Horbinski C, Huang RY, Lassman AB, Le Rhun E, Lim M, van den Bent MJ. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–113. https://doi.org/10.1093/neuonc/noaa106.
Park HS, Jeoung NH. Autocrine motility factor secreted by HeLa cells inhibits the growth of many cancer cells by regulating AKT/ERK signaling. Biochem Biophys Res Commun. 2020;525(3):557–62. https://doi.org/10.1016/j.bbrc.2020.02.135.
Yang Y, Lian S, Meng L, Qu L, Shou C. Antibody array revealed PRL-3 affects protein phosphorylation and cytokine secretion. PLoS ONE. 2017;12(1):e0169665. https://doi.org/10.1371/journal.pone.0169665.
Romagnoli A, Oliverio S, Evangelisti C, Iannicola C, Ippolito G, Piacentini M. Neuroleukin inhibition sensitises neuronal cells to caspase-dependent apoptosis. Biochem Biophys Res Commun. 2003;302(3):448–53. https://doi.org/10.1016/s0006-291x(03)00188-8.
Liudvytska O, Kolodziejczyk-Czepas J. A review on rhubarb-derived substances as modulators of cardiovascular risk factors-a special emphasis on anti-obesity action. Nutrients. 2022;14(10):2053. https://doi.org/10.3390/nu14102053.
Talukder AH, Bagheri-Yarmand R, Williams RR, Ragoussis J, Kumar R, Raz A. Antihuman epidermal growth factor receptor 2 antibody herceptin inhibits autocrine motility factor (AMF) expression and potentiates antitumor effects of AMF inhibitors. Clin Cancer Res. 2002;8(10):3285–9. https://doi.org/10.1074/jbc.ra117.001323.
Talukder AH, Adam L, Raz A, Kumar R. Heregulin regulation of autocrine motility factor expression in human tumor cells. Cancer Res. 2000;60(2):474–80.
Haga A, Funasaka T, Niinaka Y, Raz A, Nagase H. Autocrine motility factor signaling induces tumor apoptotic resistance by regulations Apaf-1 and Caspase-9 apoptosome expression. Int J Cancer. 2003;107(5):707–14. https://doi.org/10.1002/ijc.11449.
Trejo-Solís C, Serrano-Garcia N, Escamilla-Ramírez Á, Castillo-Rodríguez RA, Jimenez-Farfan D, Palencia G, Calvillo M, Alvarez-Lemus MA, Flores-Nájera A, Cruz-Salgado A, Sotelo J. Autophagic and apoptotic pathways as targets for chemotherapy in glioblastoma. Int J Mol Sci. 2018;19(12):3773. https://doi.org/10.3390/ijms19123773.
Acknowledgements
The present work was partially supported by grants from CONACyT-México (No. 243249 to JCGP). Authors want to thanks to students Monserrat Vazquez-Bautista, Erika Montserrat Navarro-Araujo and Andrea Torrero-Díaz for their technical assistance.
Funding
The present work was partially supported by Grants from CONACyT-México (No. 243249 to JCGP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [JCGP], [MCTS], [LTAM], [RLM], [LFJG] and [DXRC]. The first draft of the manuscript was written by [JCGP] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gallardo-Pérez, J.C., Trejo-Solís, M.C., Robledo-Cadena, D.X. et al. Erythrose inhibits the progression to invasiveness and reverts drug resistance of cancer stem cells of glioblastoma. Med Oncol 40, 104 (2023). https://doi.org/10.1007/s12032-023-01969-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01969-z