Abstract
Nab-paclitaxel plus gemcitabine (Nab-Gem) represents one of the standard regimen for first-line treatment of metastatic pancreatic adenocarcinoma (mPDAC). However, few data are available in mPDAC relapsed after gemcitabine as adjuvant treatment. Our study aims to evaluate the efficacy and feasibility of Nab-Gem as first-line treatment for mPDAC patients previously treated with adjuvant treatment. We retrospectively analyzed the safety and efficacy data of 36 patients, who received first-line Nab-Gem after gemcitabine as adjuvant treatment. All patients received gemcitabine after radical surgery. Median disease-free survival was 12 months (95% CI 9.7–14.3); at relapse, all patients received Nab-Gem. We observed an objective response rate and disease control rate of 11.1% and 63.9%, respectively. With a median follow-up of 47 months, median progression-free survival was 5 months (95% CI 1.0–9.0), whereas median overall survival (OS) was 13 months (95% CI 5.5–20.5). Median OS was higher in patients with a relapse ≥ 7 months after the end of adjuvant treatment than in patients relapsed < 7 months (14 vs. 8 months, respectively, p: 0.52). Our results show that first-line Nab-Gem is feasible and effective in patients previously treated with gemcitabine as adjuvant treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related deaths worldwide, with 45,750 estimated deaths in 2019 [1]. Surgery remains the only curative treatment for PDAC, but only 20% of patients undergo to surgical radical resection [2], due to the late presentation of symptoms, showing an unresectable or metastatic disease at diagnosis. However, despite of curative resection, the majority of patients shows a postoperative tumor recurrence [3]. Based on this fact, several trials have been conducted to improve the outcome for these patients by using adjuvant strategies, including chemotherapy and/or radiotherapy.
One of the landmark trial regarding the use of adjuvant chemotherapy is CONKO-001 study [4], in which 368 patients affected by PDAC was randomized to receive six cycle of adjuvant gemcitabine after complete surgical resection or surgery alone. This trial showed that gemcitabine significantly improved median disease-free survival (DFS: 13.4 vs. 6.7 months, p: < 0.001) and overall survival (OS) with a 5- and 10-year survival rate of 20.7% and 10.4%, respectively (vs. 7.7% in the observational arm, hazard ratio (HR) 0.76; p: 0.01).
Later, polichemotherapy regimen has been studied in the adjuvant setting in the recent ESPAC-4 trial, that compared a combination of gemcitabine and capecitabine versus gemcitabine alone, showing a significant OS benefit in the combination arm [5]. However, its results were considered controversial due to the large proportion of patients underwent to R1 resections and/or with node-positive, high level of postoperative Ca 19.9 and for the absence of postoperative restaging by computed tomography (CT) scan. Therefore, despite of the indication in the current international guidelines [6], many oncological centers do not consider the ESPAC-4 trial schedule as the standard of care for PDAC adjuvant treatment due to the high number of bias.
Recently, the landscape of adjuvant treatment for PDAC has been modified by the PRODIGE 24-PA6 trial [7] in which polichemotherapy with 5-fluorouracil/leucovorin, oxaliplatin and irinotecan (mFOLFIRINOX) showed to improve median DFS and median OS if compared to gemcitabine, even if with high grade of toxicities.
Moreover, the role of combined chemoradiotherapy (CRT) in this setting has been evaluated over the last years in several trials which investigated different techniques of radiotherapy [8,9,10], showing conflicting results. Taking into account that these trials had some bias [e.g. lack of quality assurance of radiotherapy techniques, use of split course radiotherapy or low dose (40 Gy)], they showed that the addition of radiotherapy to adjuvant chemotherapy did not improve the outcomes for PDAC. In addiction, in the ESPAC-1 trial radiotherapy seems to have a detrimental effect on survival [11]. However, the metanalysis available in this field suggest that CRT could have a role in patients with margin-positive (R1 or R2 resection) [12] as well as in node-positive tumors [13].
Despite of the adjuvant treatment, unfortunately almost 80% of patients treated for PDAC show a relapse or a progression of disease (PD) in the 5-years follow-up period [14]. In metastatic setting, palliative chemotherapy remains the standard of care and the recent development of new combination of chemotherapy regimens, such as FOLFIRINOX [15] and nab-paclitaxel plus gemcitabine (Nab-Gem) [16] has significantly improved the outcomes of these patients. Therefore, according to the guidelines [6], FOLFIRINOX or Nab-Gem represent the standard of care in the first-line treatment of patients affected by metastatic PDAC (mPDAC) with good performance status (PS). Among these schedules, Nab-Gem showed to be feasible and effective also in the real life population [17]. However, few data on its safety and efficacy are available in patients relapsed after gemcitabine adjuvant treatment. Furthermore, this population is underrepresented in the MPACT trial (only 4% of pts have received a previous therapy, and without cytotoxic dosage) [16].
Based on these evidences, the aim of our study is to evaluate the efficacy and feasibility of Nab-Gem as first-line treatment for mPDAC in patients previously treated with gemcitabine as adjuvant treatment for resectable PDAC.
Materials and methods
Patients
We performed a retrospective review of clinical data for PDAC patients treated at two Italian center (Division of Medical Oncology of the University of Campania “Luigi Vanvitelli” and Medical Oncology-Hospital “A. Cardarelli” in Naples). Patient aged 18 years or older with histologically confirmed PDAC, receiving at least one cycle of first-line chemotherapy with Nab-Gem at time of relapse and previously treated with adjuvant gemcitabine as single agent after curative surgery were considered eligible for our analysis. Patients who received adjuvant treatment others than gemcitabine, previous anticancer treatments in the first-line setting, randomized in clinical trial or with incomplete laboratory reports were excluded from this study. The institutional board at the center approved the protocol and the study was done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent at the time of therapy about the use of their data for future medical research. The analysis was carried out with the approval of Ethics Committee of AOU “Luigi Vanvitelli”—AORN Ospedali dei Colli, of our institution.
The following clinical and pathological variables were recorded from patients’ history: gender, age, Eastern Cooperative Oncology Group performance status at relapse (ECOG PS criteria on a 5-point scale, with 0 indicating no symptoms and higher numbers indicating greater disability up to 5 for dead [18]), tumor site, presence of biliary stent, tumor marker at diagnosis and at relapse (carbohydrate antigen 19-9: CA 19.9), type of surgery, pathological disease’s stage, tumor histotype, data about adjuvant treatment (start and stop date, number of cycles and adverse events), sites and number of metastasis at time of PD.
Treatment and follow-up
Adjuvant treatment
All patients received adjuvant chemotherapy according to the ECOG PS and the clinical practice guidelines for non-metastatic PDAC [6] within 8 weeks after curative resection. A radiological and biochemical assessment with total body CT scan and Ca 19.9 determination was performed before starting adjuvant treatment as clinical practice. Gemcitabine was administered intravenously for six cycles, according to the CONKO-001 schedule [4]. No patients received gemcitabine in combination with capecitabine. Dose reductions were applied in cases of grade 3/4 toxicities and in case of persistent grade 2 toxicities at discretion of the physician, according to the Common Terminology Criteria for Adverse Events (CTCAE) Scale [19]. After the treatment, all patients were followed-up every 3 months for the first 2 years, every 6 months from 3rd to 5th year, according to the guidelines [6].
First-line treatment
A combination of Nab-Gem was administered as first line treatment according to the MPACT schedule [16] until death, PD, unacceptable toxicity or patient refusal in all metastatic patients. Recombinant human granulocyte colony-stimulating factor (G-CSF) and erythropoietin were administered as needed by physician. Dose reductions were applied like in the adjuvant treatment and according to CTCAE [19]. A radiological and biochemical assessment with CT scan and Ca 19.9 determination was performed every 2 months in all patients to evaluate the response, according to RECIST 1.1 criteria [20]. Magnetic resonance imaging, brain CT scan or fluorodeoxyglucose positron emission tomography were performed as needed in addition to CT evaluation in controversial cases. After the end of the first-line, patients that did not show PD (defined as at least a 20% increase in the sum of the longest diameter of target lesions or the appearance of new lesions [20]) were followed-up with a total body CT scan and CA 19.9 every 2 months until evidence of PD or death for any reason.
Statistical analysis
SPSS software was used for statistical analysis (version 21.00; SPSS, Chicago, IL). A significant level of 0.05 was chosen to assess the statistical significance. Survival distribution was estimated by the Kaplan–Meier method with 95% confidence interval (CI). We considered OS and progression-free survival (PFS) as the time from the start of first-line treatment to the date of death from any cause and PD, respectively, and DFS as the time from the diagnosis until PD. Moreover, we evaluated the time from the completion of adjuvant treatment to PD. The differences in survival were evaluated by the log-rank test and described by the Kaplan–Meier method. The median follow-up time was calculated from the Kaplan–Meier curve with reversed censoring. Cox proportional-hazards model was applied to the univariate and multivariate survival analysis.
Results
Patients’ characteristics
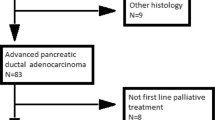
We revised the data of 141 patients affected by mPDAC and treated at our institutions from November 2011 to April 2019. Among these, we selected patients who relapsed after curative resection and adjuvant treatment and, in total, 36 patients were included in the analysis. Patients received adjuvant treatment at our institution from October 2009. The last follow-up time was May, 2nd, 2019.
Patients’ characteristics are summarized in Table 1. The median age was 65 years old with a range of 35–79; males: 19 (52.8%); ECOG PS 0/1/2 at relapse: 19.4%/55.6%/25%; primary location: head 66.7%; biliary stent: 5 (13.9%); type of surgery: Whipple 31 (86.1%), distal pancreatectomy: 5 (13.9%). All 36 patients were assessable for toxicity, survival and radiological response using RECIST 1.1 criteria [20].
Treatment and toxicities
First-line treatment
Patients received a median of 3.5 cycles (range 1–25) of Nab-Gem as first-line chemotherapy and treatment was still ongoing in 4 patients (11.1%) at time of our analysis. Hematological and non-hematological toxicities were collected from patients’ history as reported in Table 2.
The most frequent toxicities were alopecia (58.3%), anemia (13.9%), neuropathy (13.9%), neutropenia, fatigue, nausea and hypertransaminasemia (each of them reported in 8.3% of patients). The global incidence of grade 3 toxicity was 16.6% and no grade 4 were recorded. Concerning treatment administration, 15 patients received a reduced dose (41.6%) and 12 patients (33.3%) had a delay in dose administration (these doses were not re-administered). G-CSF and erythropoietin were administered in 4 (11.1%) and (8.3%) patients, respectively. No patients stopped treatment do to unacceptable toxicity or refusal.
Adjuvant treatment
Regarding the previous adjuvant treatment, patients received a median of 6 cycles of gemcitabine (range 2–7) with radiotherapy adjuvant treatment in addition to chemotherapy (concomitant chemoradiotherapy) in 4 patients (11.1%). The radiotherapy schedule comprised a total of 45 Gy. The most common toxicities were: grade 1 fatigue, anemia and nausea, grade 2 thrombocytopenia and diarrhea [each of them reported in one patients (2.8%)]. Thirty-three patients (91.6%) completed treatment without toxicities. No grade 3 and 4 toxicities were reported and no patients stopped treatment due to adverse event.
Objective tumor response and survival
The assessment of the best tumor response at first-line treatment according to RECIST 1.1 criteria [20] showed no complete responses, partial response (PR) in 4 patients (11.1%), stable disease (SD) in 19 patients (52.8%) and PD in 13 patients (36.1%). Therefore, the objective response rate (ORR) was 11.1% and the disease control rate (DCR) was 63.9%.
After a median follow-up of 47 months from the diagnosis (95% CI 42.4–51.6 months), median DFS was 12 months (95% CI 9.7–14.3 months) and median time between the completion of adjuvant treatment and PD was 7 months (range: 1–66 months). Patients showed a median PFS of 5 months (95% CI 1.0–9.0 months) (Fig. 1), whereas median OS was 13 months (95% CI 5.5–20.5 months) (Fig. 2). After stratification according to the median time between the completion of adjuvant treatment and relapse, patients with a relapse ≥ 7 months showed a higher median OS if compared with pts relapsed < 7 months (14 vs. 8 months, respectively, p: 0.52).
The majority of patients died due to the disease (77.7%, n: 28), whereas 8 patients (22.3%) are still alive at the time of data cut-off. Regarding PFS, 5 patients (13.8%) did not progress (died without PD or treatment still ongoing), whereas 31 patients (86.1%) showed PD. Of these, 16 patients (51.6% of progressed patients) received at least one cycle of second line treatment, according to the following schedules: Capecitabine plus oxaliplatin (Xelox: 25%); Liposomal irinotecan (Naliri) plus 5-fluorouracil (after the publication of NAPOLI-1 trial results [21] as compassionate use allowed only in the University of Campania center): 18.7%; De Gramont schedule: 18.7%; capecitabine (12.5%); 5fluorouracil plus irinotecan (Folfiri: 12.5%); and 5-fluorouracil plus oxaliplatin (Folfox 4-mod: 12.5%).
To evaluate the prognostic value of clinicopathologic variables, we performed a univariate analysis for OS and PFS, as shown in Table 3.
ECOG PS (HR 0.28, 95% CI 0.08–0.96, p: 0.04) and the presence of liver metastasis (HR 0.36, 95% CI 0.15–0.84, p: 0.02) showed to be independent prognostic factor for OS at univariate analysis, whereas gender, age, site of primary tumor, number and sites of metastasis and presence of biliary stent were not linked to survival. However, only the metastatic liver involvement showed to be statistically significantly related to survival at multivariate analysis for OS (HR 0.41, 95% CI 0.17–0.95; p: 0.04). The univariate analysis for PFS found that, among all the clinicopathological variables, only ECOG PS was independently related to progression (HR 0.29, 95% CI 0.08–0.96, p: 0.04).
Discussion
Our retrospective analysis adds new significant data regarding the use of first-line Nab-Gem regimen in mPDAC patients already treated with gemcitabine as adjuvant therapy. In fact, although there are many studies in literature (both perspective and retrospective) that investigated different types of regimens and schedules, no data are available about the evaluation of response to Nab-Gem in mPDAC patients already pre-treated with adjuvant gemcitabine.
Assuming that our objective was not to propose a new standard of care for the “adjuvant—first-line sequence” but rather to evaluate the feasibility and efficacy of the sequence itself, this information could be relevant, since ESPAC-4 [5] and, mostly, PRODIGE24-PA6 [7] trials were published very recently. Based on this reason, the majority of patients in the current clinical practice received gemcitabine as adjuvant treatment. Moreover, although the ESPAC-4 trial showed better results for adjuvant combination of gemcitabine plus capecitabine versus gemcitabine alone [5], this study had many biases, which, at least in part, limit its use in clinical practice in many centers. For example, it showed (as supplemental materials) that there was no differences in relapse-free survival (RFS) between arms. Moreover, a significant number of patients included in the analysis had elevated postoperative Ca19.9, leading to a heterogeneous sample of patients made up of a part with possible micro-metastatic disease. Therefore, based on these and other limits, many authors criticize the ESPAC-4 study, and, even after its publication, they continue to use gemcitabine as single agent in the adjuvant setting.
On the other hand, the very recently published PRODIGE24-PA6 trial [7], which compares adjuvant mFOLFIRINOX versus gemcitabine alone after radical PDAC resection, presents a much more solid design with unequivocal results in favor of mFOLFIRINOX itself. In fact, according to international guidelines [6], mFOLFIRINOX becomes a new standard for resected PDAC patients, although at the price of more G3/4 adverse events (75.9% vs. 52.9%). Therefore, due to the safety profile, the use of mFOLFIRINOX should be avoided in patients with suboptimal PS.
Today, new PDAC biomarkers of poor prognosis and possible relapse after surgery and adjuvant treatment are emerging beyond classical Ca19.9, like ctDNA: a recent Australian study [22] clearly showed that PDAC patients with detectable circulating KRAS mutations after surgery all relapsed, despite adjuvant gemcitabine treatment.
In this context, we consider our analysis about efficacy and feasibility of first-line Nab-Gem after adjuvant gemcitabine as a relevant issue, at least for two reasons: first, considering the relatively long period of median DFS of patients receiving adjuvant treatment and the very recent publication of PRODIGE24 [7], the majority of patient in every day clinical practice relapsed after an adjuvant treatment received gemcitabine after surgery. Second, gemcitabine could be still considered a good option in patients unfit for adjuvant mFOLFIRINOX and not candidate to ESPAC-4 schedule [5]. Moreover, all patients involved in our analysis were treated in the adjuvant setting prior to publication of ESPAC-4 [5] and PRODIGE24 trials [7] and many of them had a suboptimal PS at relapse (25% PS 2 and 56% PS 1, according to ECOG scale) or were elderly patients (55.5% were ≥ 65 years old and 19.4% ≥ 70 years old). For all these reasons, first-line Nab-Gem appeared to be feasible and, at least, a good choice in our population.
Regarding the efficacy, our results showed that Nab-Gem is effective, with a first-line median PFS of 5 months, comparable to that of MPACT trial (5.5 months), and a median OS of 13 months, slightly higher than in the MPACT trial itself (8.5 months) [16] or in our real life experience previously published [17]. Moreover, we reported an ORR of 11.1% (even if without complete responses) and a 63.9% of DCR (52.8% SD), once again overlapping with the MPACT results (23% of ORR and 48% of DCR) [16].
To note, both the MPACT [16] and PRODIGE4 [15] studies excluded patients already treated with cytotoxic doses of gemcitabine or any other regimen in the adjuvant setting, making this patient population virtually absent in these two pivotal clinical trials. However, our analysis suggests that Nab-Gem combination can be feasible and effective also in patients pre-exposed to Gemcitabine and, therefore, considered as potentially resistant to this compound. This observation clinically supports the experimental evidences that Nab-Paclitaxel could deplete the desmoplastic stroma (a hallmark of PDAC microenvironment), increasing and dilating blood vessels, and that it could increase of many fold the intratumoral concentration of gemcitabine, overcoming a possible resistance [23]. For this reason, Nab-Gem as first-line and used after adjuvant gemcitabine should not be considered as a simple rechallenge, because the Nab-Gem combination (with its strong synergistic effect) is completely different from the gemcitabine alone. On the other hand, many “rechallenge examples” exist in literature that showed to be effective at relapse, although in other cancers and different setting, such as the cetuximab-based therapy in metastatic colorectal cancer [24].
Regarding toxicity and adherence to treatment, our data are in line with the literature [16], showing that only 27.8% of patients received a dose reduction due to toxicity (versus 29% in MPACT) and the most frequent grade 3 adverse events were neutropenia (8.3%)—less frequent than in the pivotal trial (38%)—and peripheral neuropathy (5.5%).
Noteworthily, our study patients with quick relapse (< 7 months) had worse median OS compared to those who relapsed later (8 vs. 14 months, respectively), suggesting that time to relapse might be a useful prognostic factor. However, it is important to note that this difference is not statistically significant in our analysis and the curves cross many times, probably due to the small size of our sample.
Regarding the factors that could influence the outcome, in the univariate analysis for OS, the ECOG PS and the presence of liver metastasis showed to be independent prognostic factors, whereas in the multivariate analysis only the metastatic liver involvement is significantly related to survival, making it the unique independent prognostic factor for OS in our cohort. On the other hand, only ECOG PS showed to be related to PFS at univariate analysis, underlying the importance of PS in mPDAC.
Finally, it is important to consider that our analysis had some limitations: first, the retrospective design, with negative implications about collection and report of some clinical data (for example the number of metastatic lesions within different metastatic site that could be define an oligometastatic disease), also generating a possible selection bias for the patients included. Second, the small simple size, although we must consider that: (I) this study represents a unique experience in the literature, with no previous work in this field; (II) the number of PDAC patients that could undergo to surgery and subsequently adjuvant treatment is overall low, and (III) this experience raised as a joint effort of only two clinical Italian sites.
Conclusions
Our analysis showed that Nab-Gem regimen is safe and effective also in patients previously exposed to gemcitabine in the adjuvant setting, underlying that the combination of the two molecules is much more than the simple sum of the drugs. This fact could give rise to a strong synergic effect to overcome the possible resistance to gemcitabine alone.
However, wider experiences (possibly multicenter, perspective and with a larger sample of patients) would be necessary to explore much in detail the role of the combination in patients already treated with gemcitabine.
References
Siegel R, Miller K, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551 Epub 2019 Jan 8.
Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–705.
Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary managenement of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–47.
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines. Ann Oncol. 2015;26(suppl 5):v56–68.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX versus gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406.
Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–10.
Klinkenbijl J, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region. Ann Surg. 1999;230:776–82.
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10.
Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85.
Stocken DD, Büchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92(8):1372–81.
Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:2541–56.
Gbolahan OB, Tong Y, Sehdev A, O’Neil B, Shahda S. Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study. BMC Cancer. 2019;19(1):468. https://doi.org/10.1186/s12885-019-5630-4.
Conroy T, Desseigne F, Ychou M, et al. Groupe Tumeurs Digestives of Unicancer, PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
De Vita F, Ventriglia J, Febbraro A, Laterza MM, Fabozzi A, Savastano B, et al. NAB-paclitaxel and gemcitabine in metastatic pancreatic ductal adenocarcinoma (PDAC): from clinical trials to clinical practice. BMC Cancer. 2016;16(1):709. https://doi.org/10.1186/s12885-016-2671-9.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55.
CTCAE 4.03. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010). US Department of Health and Human Services. Nacional Cancer Institute.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wang-Gillam A, Li CP, Bodoky G, et al. NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–57.
Lee B, Lipton L, Cohen J, Tie J, Javed AA, Li L, et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localised pancreatic cancer. Ann Oncol. 2019. https://doi.org/10.1093/annonc/mdz200.
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–54. https://doi.org/10.1200/JCO.2011.36.5742 Epub 2011 Oct 3.
Kuczynski EA, Sargent DJ, Grothey A, et al. Drug rechallenge and treatment beyond progression—implications for drug resistance. Nat Rev Clin Oncol. 2013;10(10):571–87. https://doi.org/10.1038/nrclinonc.2013.158.
Acknowledgements
We thank all patients and their families for the participation in this study.
Funding
This research received no external funding
Author information
Authors and Affiliations
Contributions
Conceptualization, FDV and APe; methodology and formal analysis, APe; investigation, APe, APa, LP, GT, FCa, MML, MC, AV, MO, GC, CM, MB; resources, APa, LP; data curation, APa, FCa; writing—original draft preparation, APe, APa, LP; writing—review and editing, APe, APa, LP, FCi, FDV; supervision, FCi, MB, FDV.
Corresponding authors
Ethics declarations
Conflict of interest
A.Pe: honoraria from Lilly; M.O: Honoraria from Italfarmaco, EISAI, epionpharma, Roche; F.Ci: Advisory Boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Celgene, Lilly; Institutional Research Grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Takeda; F.D.V: Advisory Boards: Roche, Amgen, Celgene, Lilly. The authors declare that all these conflicts of interest are not connected with the issue of this paper. The other authors have no conflicts of interest to declare.
Ethical approval
All patients, at the time of therapy, signed informed consent about the use of their data for future medical research. The analysis was carried out with the approval of Ethics Committee of AOU “Luigi Vanvitelli”—AORN Ospedali dei Colli, of our institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Petrillo, A., Pappalardo, A., Pompella, L. et al. Nab-paclitaxel plus gemcitabine as first line therapy in metastatic pancreatic cancer patients relapsed after gemcitabine adjuvant treatment. Med Oncol 36, 83 (2019). https://doi.org/10.1007/s12032-019-1306-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1306-9