Abstract
Cigarette smoking is directly associated with lung cancer. Non-small cell lung carcinoma (NSCLC) represents approximately 80% from all types of lung cancer. This latter is hard to diagnose and to treat due to the lack of symptoms in early stages of the disease. The aim of this study was to evaluate ADA activity and the expression of P2X7, A1, and A2A receptors and in lymphocytes. In addition, the profile of pro-inflammatory and anti-inflammatory cytokines serum levels of patients with lung cancer in advanced stage was evaluated. Patients (n = 13) previously treated for lung cancer at stage IV (UICC) with chemotherapy had their blood collected. Cancer patients showed a decrease in ADA activity and an increase in A1 receptor expression in lymphocytes when compared to the control group. Moreover, patients exhibited an increase in IL-6 and TNF-α, while IL-17 and INF-ϒ serum levels were lower in patients with lung cancer. The decreased ADA activity and the increase in A1 receptor expression may contribute to adenosine pro-tumor effects by increasing IL-6 and TNF-α and decreasing IL-17 and INF-γ serum levels. Our data show an indirect evidence that purinergic signaling may have a role in promoting a profile of cytokines levels that favors tumor progression.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Lung cancer is the second most prevalent cancer in men and women, being the main cause of cancer death in the population [1]. Approximately 80% of lung cancer is non-small cell lung carcinoma (NSCLC), and these patients often have the diagnosis delayed due to the lack of symptoms in the early phases of this disease [2].
When the disease is detected, 34% of patients with lung cancer already present metastases, which increases the risk of treatment failure [3]. Cigarette smoking is the best-known risk factor for lung cancer since its related carcinogenic substances promote oxidative stress and inflammation [4]. In 1863, Rudolf Virchow observed that immune cells were presented in tumor tissues, hypothesizing a direct association between inflammation and cancer [5].
It is estimated that chronic inflammation is responsible for at least 15% of human cancers [6]. Currently, the concept of tumor microenvironment where normal cells (such as fibroblasts, immune cells, endothelial cells), genetically modified cells, and other mediators locally produced or derived from blood has been much more satisfactorily recognized regarding tumor development [7]. The inflammatory response is tightly regulated by cytokines, a class of signaling molecules that have autocrine, paracrine, and endocrine effects. Although different cytokines may elicit several biological responses in target tissues, they are often classified based on their action on inflammation, i.e., pro-inflammatory or anti-inflammatory. For instance, IL-6, TNF-α, IL-17, INF-γ, IL-12 are well-known cytokines which effects are microenvironment dependent. It is known that tumor cells are able to increase pro-inflammatory cytokines [8] and there are evidences between the serum cytokine concentrations and lung cancer [9, 10].
In the purinergic system, ATP and adenosine act as extracellular signaling molecules and they have emerged as novel mediators of tumor microenvironment since they play a role in the control of immune and inflammatory process [11]. For the first time, Rapaport reported in 1983 the anti-neoplastic actions of ATP [12]. His work demonstrated that ATP addition to cancer cells led to the inhibition of cell growth due to cell cycle arrest in the S phase in both pancreatic and colon tumor models. In contrast, adenosine seems to promote tumor growth [13]. Once released, the nucleotides interact with specific purinergic receptors, P1 (adenosine) and P2 receptors.
P2 receptors are divided into G protein-coupled (P2Y) and ion channel-linked (P2X) receptors [14, 15]. The subtype P2X7 receptor is one of the most studied purinergic receptors due to its particular ability to undergo a gradual change in its shape according to the ATP levels in extracellular milieu, which can lead to the formation of a non-selective pore in the membrane, increasing the influx of calcium into the cell and promoting cell apoptosis [16] [17].
When ATP is hydrolyzed by, for example, NTPDase 1 (CD39/nucleoside triphosphate diphosphohydrolase-1) and 5′-nucleotidase (CD73), adenosine is generated in the extracellular milieu [18]. Adenosine concentrations increase when metabolic demand is high, such as in hypoxia. Therefore, hypoxia induced by tumor development may increase the ATP breakdown and the generation of adenosine [19, 20]. The extracellular levels of adenosine are regulated by adenosine deaminase (ADA), an enzyme found in the plasmatic membrane of different cell types and also in a soluble form in serum.
Adenosine P1 receptors (A1, A2A, A2B, and A3) are widely expressed in immune cells of the myeloid and lymphoid lineage. The role of A1 and A3 receptors remains poorly understood, while there are some evidences that A2A and A2B receptors are involved in the regulation of inflammation [21].
Since lung cancer remains a highly lethal disease, the alarming data on the incidence and mortality of lung cancer challenge researchers to understand the mechanisms underlying the disease development. Once chronic inflammation is intimately related to the onset of tumors and purinergic signaling modulates this process, the objective of this study was to evaluate the ADA activity, P2X7, A1, and A2A receptor expression in lymphocyte and serum levels of different cytokines, such as interleukin (IL) IL-6, IL-17, IL-4, IL-2, IL-10, Tumor Necrosis Factor α (TNF-α) and Interferon-γ (INF-γ) from patients with lung cancer.
Methods
Patient selection
Thirteen patients included in this study were diagnosed with lung cancer of non-small cell—NSCLC—at the University Hospital of Santa Maria. Using Union for International Cancer Control (UICC) criteria, patients were included in the stage IV of the disease. These patients were previously treated with anti-neoplastic cisplatin and gemcitabine. The blood sample was collected in vacutainer tubes without anticoagulant for serum separation in University Hospital of Santa Maria division of Hematology/Oncology. The control group was composed of 13 healthy individuals with the same age range of the group of patients. Individuals with chronical or infectious disease and those who smoke were excluded from the control group. This work has been approved by Human Ethics Committee of the Health Science Center from the Federal University of Santa Maria (0061.0.243.000-10) and all individuals provided a written consent form.
Isolation of mononuclear cells from human blood
Mononuclear leukocytes were isolated from human blood collected with EDTA and separated on Ficoll-Histopaque density gradients as described by Böyum [22].
Western blotting analysis to detect purinergic receptor content
Western blot was used to analyze the immunoreactivity for P2X7, A1, and A2A receptors. Protein was determined by the Coomassie blue method according to Bradford [23] using bovine serum albumin as standard. 80 μg of protein was loaded in a 10% SDS. After transfer to the nitrocellulose membrane (0.45 μm, Bio-rad), 5% non-fat milk blocking solution was used. Membranes were incubated overnight at 4 °C with rabbit anti-A1 R (1:1000; Millipore, São Paulo, Brazil) and mouse anti-A2A (1:1000; Millipore, São Paulo, Brazil). The density of actin was used as protein loading control, using the rabbit-anti-actin antibody (1:5000, Cell Signaling). After primary antibody incubation, membranes were washed and incubated with anti-mouse or anti-rabbit secondary antibodies conjugated with horseradish peroxidase (1:5000, Bio-Rad Laboratories, Hercules, CA, USA) for 1 h at room temperature. Protein detection by chemiluminescence was captured with Amersham Imager 600 (GE healthcare life sciences). Densitometric analysis was performed with Image J (NIH, Bethesda, MD, USA) software for Windows.
Determination of cytokine levels in the serum
Blood was centrifuged at 5000 rpm for 10 min. Precipitate was discarded and serum was used to determine the cytokine levels. The cytokines IL-6, IL-17, IL-4, IL-2, IL-10, TNF, and INF-γ were determined according to the method Cytometric Bead Array (CBA), using specific kits to this method as described by Morgan et al. [24].
ADA activity in lymphocyte and erythrocyte
Adenosine deaminase (ADA) activity was determined according to Guisti and Galanti [28]. Briefly, 50 µL of serum was added to 21 mmol/L of adenosine, pH 6.5, and the reaction was incubated at 37 °C for 60 min. This method evaluates the production of ammonia when ADA acts in excess of adenosine. Results were expressed in units per liter (U/L). One unit (1 U) of ADA is the amount of enzyme required to release 1 mmol of ammonia per minute from adenosine at standard assay conditions.
Statistical analysis
T-student test was used for the statistical values. Results were considered significant at P ≤ 0.05 and were expressed as mean ± standard error of mean (SEM).
Results
Patient characteristics
The classification of patients with lung cancer enrolled in this study regarding gender, age, anti-neoplastic drugs, classification of disease stage, and data related to the smoking habits are shown in Table 1. Most of the patients were male (77%), over 60 years old; all patients were cigarette smokers for at least 40 years. All patients were diagnosed at stage IV of lung cancer, according to the criteria of the Union for International Cancer Control (UICC). The control group individuals had the same age range of the group of patients, had no disease, and were not smokers (data not shown). Additional clinical data, such as erythrocyte count, hemoglobin levels, total leukocyte count, and platelet count of the patients involved in this research, are presented in Table 2.
ADA activity in lymphocyte and erythrocyte and P2X7, A1, and A2A receptor expression in lymphocytes
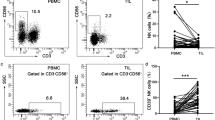
Patients with lung cancer exhibited a decrease in ADA activity in both lymphocyte and erythrocyte, as seen in Fig. 1a. P2X7 density receptors in lymphocytes are shown in Fig. 1b. No statistical difference between groups was observed. A1 and A2A density receptors in lymphocytes are shown in Fig. 1c, d, respectively. An increase in A1 density receptors was observed in lung cancer patients when compared with the control group (P ≤ 0.005). A2A density receptor was not statistically different between groups.
a ADA activity in lymphocytes and erythrocytes from patients with lung cancer. Results are expressed as mean ± standard deviation of mean (n = 13). b P2X7, c A1 and d A2A density receptor (R) in lymphocytes from patients with lung cancer. Densitometry analysis are normalized to controls and a representative immunoblot is below the graph. Results are expressed as mean ± standard deviation of mean (n = 13). *P ≤ 0.005
Cytokine serum levels
Cytokines values are summarized in Table 3. No changes were seen in IL-2, IL-4, and IL-10 in lung cancer patients when compared to control (Fig. 2). Lung cancer patients exhibited a decrease in INF-γ and IL-17 serum levels (P ≤ 0.05). On the other hand, IL-6 and TNF-α serum levels were increased in lung cancer patients when compared to control group (P ≤ 0.005), as can be seen in Fig. 2.
Discussion
Lung cancer is one of the main types of cancer among men and women worldwide regarding incidence and mortality [25]. The incidence of lung cancer in Brazil has increased in past few decades, similar to what is seen in the rest of the world [26]. Smoking is closely related to the development of lung cancer and epidemiological evidence indicates that 1 billion men and 250 million women around the world smoke every day [25]. This close correlation between smoking and the development of lung cancer can also be identified in our study, since all patients had been smoking large quantity of cigarettes for at least 40 years.
This characteristic has also been observed in previous studies from our group, which showed that the great majority of patients who had lung cancer had smoked for a long time [27,28,29]. In this context, the literature reports that approximately 87% of lung cancer cases are tobacco exposure-related and the relative risk of developing lung cancer is 24 times higher among smokers than among non-smokers [30]. In addition, leukocytosis and anemia are often associated with lung cancer, and these parameters may be associated with unfavorable prognostic [31,32,33,34]. The increase in leukocytes in lung disease may be associated with the production of hematopoietic growth factors by tumor cells [32]. The anemic state in this disease may be related to the chemotherapy regimen [34]. In our study, we have observed that patients with lung cancer at stage IV had both anemia and leukocytosis.
In the present study, we have shown that lung cancer patients exhibited an increase in IL-6 and TNF-α and a decrease in IL-17 and INF-γ serum levels when compared to control. In addition, lung cancer patients presented a decrease in ADA activity in lymphocytes and erythrocytes associated with an increase in A1A receptor expression in lymphocyte.
Several studies have shown that A1 receptors (A1R) may have a pro-tumoral action. A1R have been associated to carcinogenesis in previous investigations, such as colorectal adenocarcinomas, human leukemia, and human melanoma, where it plays a role in increasing the chemotaxis of tumor cells [35,36,37]. Herein, we have seen an increase in A1 receptor (A1R) expression in mononuclear cells from patients with lung cancer in an advanced stage of the disease. In addition, we have shown that ADA activity was decreased in erythrocytes and lymphocytes.
Schiedel et al. (2013) have shown that A1R-selective agonist (CPA) showed a clear anti-proliferative property in peripheral blood lymphocytes [38]. It is known that adenosine and 2′-deoxyadenosine accumulation, due to the lack of ADA activity, in extracellular and intracellular is lymphotoxic and promotes severe combined immunodeficiency (SCID) [39, 40], which is characterized by a loss of function and depletion of T and B lymphocytes [41]. In our study, ADA activity was decreased in erythrocyte and lymphocyte and this result may suggest that less adenosine is being converted to inosine, leading to an accumulation of this nucleoside in tumor microenvironment and/or an increase in its availability to lymphocytes.
CD73, an enzyme that catalyzes 5′-AMP to adenosine, is found to be upregulated in various types of cancer [42, 43]. In hypoxemic tumors, adenosine accumulates and initiates a range of tissue responses, including regulation of angiogenesis [44]. Angiogenic activation of blood vessels observed in CD73-Knockout mice with melanoma was shown to be mediated mainly through A1AR, despite permissive role of A2R and A3R [45].
In addition, it is known that A1R activation by adenosine promotes interleukin-6 (IL-6) synthesis [46], which is secreted by lymphocytes and has tumorigenic action [47]. IL-6 is one of the best-characterized pro-tumorigenic cytokines [48]. Chen et al. [47] have shown that immune cells invade tumor microenvironment in a process mediated by pro-inflammatory cytokines. In the present study, it has been shown that IL-6 serum levels were increased in patients with lung cancer.
On the other hand, adenosine action on A2A receptors remains controversial, since literature describes promoting tumor functions as well as antitumor actions. In our study, no changes were observed in the expression of A2A receptors in lymphocytes from patients with lung cancer compared to healthy patients. However, the hypoxic condition generated by tumor environment increases adenosine as a consequence of high rate of ATP hydrolysis and decreased activity of ADA [49]. This latter effect was seen in our study as well. A2AR activation by a specific agonist (CGS) strongly reduces production of IL-2 and TNF-α from Tc1 to Tc2 cells, but does not affect IFN-γ secretion [50]. When given in vivo or in culture of antigen presenting T cells, A2A agonists inhibit production of IL-6 and enhance production of IL-10. Herein, we have seen increased serum levels of TNF-α and IL-6 and decreased levels of IFN-γ. Taken together, we might hypothesize that adenosine accumulation in tumor microenvironment is acting through A1R on different lymphocyte populations which may explain the cytokines levels seen in our work.
About 60 carcinogenic substances have been identified in tobacco smoke [25]. These substances may promote damage to the DNA by activating pro-carcinogenic compounds, inactivating tumor suppressor genes, as well as promoting angiogenesis and significant pro-inflammatory effects [51]. In this study, we have shown that lymphocytes from lung cancer patients exhibited a decrease in adenosine removal through decreased ADA activity. In addition, we have observed a remarkable increase in A1 receptor expression in those cells which is consistent with the cytokines profile we have seen, i.e., increased IL-6 and TNF-α and decreased IFN-γ. It is known that these serum cytokine concentrations are associated with reduced lung cancer survival [52]. Therefore, understanding the mechanism by which cytokines are regulated in lung cancer, especially the role of purinergic system, may offer new strategies that can be developed to prevent disease progression.
Conclusions
For the first time, it has been demonstrated here that patients with NSCLC at stage IV of disease exhibited an increase in the density of A1 receptor and a decrease in ADA in lymphocytes, which is related with IL-6 and TNF-α increase in serum which may contribute to the inflammatory process associated with tumorigenesis.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available since the consent form protects patients’ individual data, but are available from the corresponding author on reasonable request.
Abbreviations
- ADA:
-
Adenosine deaminase
- A1R:
-
A1 receptor
- CD73:
-
Ecto-5′-nucleotidase
- NSCLC:
-
Non-small cell lung carcinoma
- NTPDase 1:
-
Nucleoside triphosphate diphosphohydrolase-1
- SCID:
-
Severe combined immunodeficiency
- UICC:
-
Union for international cancer control
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA. 2016;66(1):7–30. https://doi.org/10.3322/caac.21332.
CP SBW. World Cancer Report. IARC Press. 2014:350-2.
Dziedzic DA, Rudzinski P, Langfort R, Orlowski T. Risk factors for local and distant recurrence after surgical treatment in patients with non-small-cell lung cancer. Clin Lung Cancer. 2016;2:25. https://doi.org/10.1016/j.cllc.2015.12.013.
Roca M, Roca IC, Mihaescu T. Lung cancer—a comorbidity in chronic obstructive pulmonary disease. Rev Med Chir Soc Med Nat Iasi. 2012;116(4):1055–62.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. https://doi.org/10.1016/S0140-6736(00)04046-0.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. https://doi.org/10.1038/nature01322.
Onuchic AC, Chammas R. Câncer e o microambiente tumoral. Rev Med (São Paulo). 2010;89(1):21–31.
Fukuyama T, Ichiki Y, Yamada S, Shigematsu Y, Baba T, Nagata Y, et al. Cytokine production of lung cancer cell lines: correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci. 2007;98(7):1048–54. https://doi.org/10.1111/j.1349-7006.2007.00507.x.
Yamaguchi T, Yamamoto Y, Yokota S, Nakagawa M, Ito M, Ogura T. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998;28(12):740–4.
De Vita F, Orditura M, Auriemma A, Infusino S, Catalano G. Serum concentrations of proinflammatory cytokines in advanced non small cell lung cancer patients. J Exp Clin Cancer Res. 1998;17(4):413–7.
Kanthi YM, Sutton NR, Pinsky DJ. CD39: interface between vascular thrombosis and inflammation. Curr Atheroscler Rep. 2014;16(7):425. https://doi.org/10.1007/s11883-014-0425-1.
Rapaport E. Treatment of human tumor cells with ADP or ATP yields arrest of growth in the S phase of the cell cycle. J Cell Physiol. 1983;114(3):279–83. https://doi.org/10.1002/jcp.1041140305.
Spychala J. Tumor-promoting functions of adenosine. Pharmacol Therap. 2000;87(2–3):161–73.
Burnstock G. Purinergic signalling—an overview. Novartis Foundation symposium. 2006;276:26–48; discussion -57, 275–81.
Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. https://doi.org/10.1152/physrev.00043.2006.
Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7(7):575–90. https://doi.org/10.1038/nrd2605.
Roger S, Jelassi B, Couillin I, Pelegrin P, Besson P, Jiang LH. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochem Biophys Acta. 2015;1848(10 Pt B):2584–602. https://doi.org/10.1016/j.bbamem.2014.10.029.
Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362(4–5):299–309.
Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochem Biophys Acta. 2011;1808(5):1400–12. https://doi.org/10.1016/j.bbamem.2010.09.020.
Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81(2):154–64.
Di Virgilio F, Vuerich M. Purinergic signaling in the immune system. Auton Neurosci. 2015;191:117–23. https://doi.org/10.1016/j.autneu.2015.04.011.
Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig Suppl. 1968;97:77–89.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110(3):252–66. https://doi.org/10.1016/j.clim.2003.11.017.
Yu Y, Liu H, Zheng S, Ding Z, Chen Z, Jin W, et al. Gender susceptibility for cigarette smoking-attributable lung cancer: a systematic review and meta-analysis. Lung Cancer. 2014;85(3):351–60. https://doi.org/10.1016/j.lungcan.2014.07.004.
INCA. Instituto Nacional do Câncer José Alencar Gomes da Silva. 2016.
Zanini D, Pelinson LP, Schmatz R, Belmonte Pereira L, Curry Martins C, Baldissareli J, et al. δ-Aminolevulinate dehydratase activity in lung cancer patients and its relationship with oxidative stress. Biomed Pharmacother. 2014;68(5):603–9. https://doi.org/10.1016/j.biopha.2014.04.005.
Zanini D, Schmatz R, Pelinson LP, Pimentel VC, da Costa P, Cardoso AM, et al. Ectoenzymes and cholinesterase activity and biomarkers of oxidative stress in patients with lung cancer. Mol Cell Biochem. 2013;374(1–2):137–48. https://doi.org/10.1007/s11010-012-1513-6.
Zanini D, Schmatz R, Pimentel VC, Gutierres JM, Maldonado PA, Thome GR, et al. Lung cancer alters the hydrolysis of nucleotides and nucleosides in platelets. Biomed Pharmacother. 2012;66(1):40–5. https://doi.org/10.1016/j.biopha.2011.09.003.
Duarte RL, Paschoal ME. Molecular markers in lung cancer: prognostic role and relationship to smoking. Jornal brasileiro de pneumologia: publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2006;32(1):56–65.
Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009;115(17):3919–23. https://doi.org/10.1002/cncr.24480.
Shoenfeld Y, Tal A, Berliner S, Pinkhas J. Leukocytosis in non hematological malignancies—a possible tumor-associated marker. J Cancer Res Clin Oncol. 1986;111(1):54–8.
Zhang T, Jiang Y, Qu X, Shen H, Liu Q, Du J. Evaluation of preoperative hematologic markers as prognostic factors and establishment of novel risk stratification in resected pN0 non-small-cell lung cancer. PLoS ONE. 2014;9(10):e111494. https://doi.org/10.1371/journal.pone.0111494.
Langer CJ, Choy H, Glaspy JA, Colowick A. Standards of care for anemia management in oncology: focus on lung carcinoma. Cancer. 2002;95(3):613–23. https://doi.org/10.1002/cncr.10712.
Khoo HE, Ho CL, Chhatwal VJ, Chan ST, Ngoi SS, Moochhala SM. Differential expression of adenosine A1 receptors in colorectal cancer and related mucosa. Cancer Lett. 1996;106(1):17–21.
Gessi S, Varani K, Merighi S, Morelli A, Ferrari D, Leung E, et al. Pharmacological and biochemical characterization of A3 adenosine receptors in Jurkat T cells. Br J Pharmacol. 2001;134(1):116–26. https://doi.org/10.1038/sj.bjp.0704254.
Merighi S, Varani K, Gessi S, Cattabriga E, Iannotta V, Ulouglu C, et al. Pharmacological and biochemical characterization of adenosine receptors in the human malignant melanoma A375 cell line. Br J Pharmacol. 2001;134(6):1215–26. https://doi.org/10.1038/sj.bjp.0704352.
Schiedel AC, Lacher SK, Linnemann C, Knolle PA, Müller CE. Antiproliferative effects of selective adenosine receptor agonists and antagonists on human lymphocytes: evidence for receptor-independent mechanisms. Purinergic Signal. 2013;9(3):351–65. https://doi.org/10.1007/s11302-013-9354-7.
Apasov SG, Sitkovsky MV. The extracellular versus intracellular mechanisms of inhibition of TCR-triggered activation in thymocytes by adenosine under conditions of inhibited adenosine deaminase. Int Immunol. 1999;11(2):179–89.
Desrosiers MD, Cembrola KM, Fakir MJ, Stephens LA, Jama FM, Shameli A, et al. Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol (Baltimore). 2007;179(3):1884–92.
Gelfand EW, Lee JJ, Dosch HM. Selective toxicity of purine deoxynucleosides for human lymphocyte growth and function. Proc Natl Acad Sci USA. 1979;76(4):1998–2002.
Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2014;134(6):1466–73. https://doi.org/10.1002/ijc.28456.
Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. https://doi.org/10.1007/s11302-012-9309-4.
Auchampach JA. Adenosine receptors and angiogenesis. Circ Res. 2007;101(11):1075–7. https://doi.org/10.1161/CIRCRESAHA.107.165761.
Koszalka P, Golunska M, Urban A, Stasilojc G, Stanislawowski M, Majewski M, et al. Specific activation of A3, A2A and A1 adenosine receptors in CD73-knockout mice affects B16F10 melanoma growth, neovascularization, angiogenesis and macrophage infiltration. PLoS ONE. 2016;11(3):e0151420. https://doi.org/10.1371/journal.pone.0151420.
Zhou Y, Schneider DJ, Blackburn MR. Adenosine signaling and the regulation of chronic lung disease. Pharmacol Therap. 2009;123(1):105–16. https://doi.org/10.1016/j.pharmthera.2009.04.003.
Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll–the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008;27(2):225–33. https://doi.org/10.1038/sj.onc.1210907.
Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54–74. https://doi.org/10.1016/j.smim.2014.01.001.
Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72(21):5441–7. https://doi.org/10.1158/0008-5472.CAN-12-1600.
Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, et al. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105(12):4707–14. https://doi.org/10.1182/blood-2004-04-1407.
Huang RY, Chen GG. Cigarette smoking, cyclooxygenase-2 pathway and cancer. Biochem Biophys Acta. 2011;1815(2):158–69. https://doi.org/10.1016/j.bbcan.2010.11.005.
Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G, et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomark Prev. 2009;18(1):215–22. https://doi.org/10.1158/1055-9965.epi-08-0705.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS - PPSUS), and the Federal University of Santa Maria, RS, Brazil. The role of funding was to provide scholarship and essentials for lab maintenance.
Author information
Authors and Affiliations
Contributions
DZ undertook biochemical studies, blood collection, participated in design of the study, analyzed and interpreted data, and drafted the manuscript. LM analyzed and interpreted the data and drafted the manuscript. LPP, VCP, AMC, VCAG, CBS, JMG, VMM, and DBRL participated in biochemical and western blot experiments, blood collection, patient selection, and recruitment. MRCS participated in designing and coordinating the study, analyzed and interpreted all data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interests.
Ethics approval
This work has been approved by Human Ethics Committee of the Health Science Center from the Federal University of Santa Maria (0061.0.243.000-10) and all individuals provided a written consent form.
Informed consent
All patients have signed a consent form authorizing the use of their personal data for research purpose only.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zanini, D., Manfredi, L.H., Pelinson, L.P. et al. ADA activity is decreased in lymphocytes from patients with advanced stage of lung cancer. Med Oncol 36, 78 (2019). https://doi.org/10.1007/s12032-019-1301-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1301-1