Abstract
The efficacy of cisplatin (CIS) and 5-fluorouracil (5-FU) against squamous cell carcinomas of the head and neck (SCCHN) remains restricted due to their severe toxic side effects on non-cancer (normal) tissues. Recently, the broccoli extract sulforaphane (SF) was successfully tested as a combination therapy to target cancer cells. However, the effect of lower doses of CIS or 5-FU combined with SF on SCCHN remained unknown. This study tested the chemotherapeutic efficacies of SF combined with much lower doses of CIS or 5-FU against SCCHN cells aiming to reduce cytotoxicity to normal cells. Titrations of SF standalone or in combination with CIS and 5-FU were tested on SCCHN human cell lines (SCC12 and SCC38) and non-cancerous human cells (fibroblasts, gingival, and salivary cells). Concentrations of SF tested were comparable to those found in the plasma following ingestion of fresh broccoli sprouts. The treatment effects on cell viability, proliferation, DNA damage, apoptosis, and gene expression were measured. SF reduced SCCHN cell viability in a time- and dose-dependent manner. SF-combined treatment increased the cytotoxic activity of CIS by twofolds and of 5-FU by tenfolds against SCCHN, with no effect on non-cancerous cells. SF-combined treatment inhibited SCCHN cell clonogenicity and post-treatment DNA repair. SF increased SCCHN apoptosis and this mechanism was due to a down-regulation of BCL2 and up-regulation of BAX, leading to an up-regulation of Caspase3. In conclusion, combining SF with low doses of CIS or 5-FU increased cytotoxicity against SCCHN cells, while having minimal effects on normal cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is one of the most prevalent malignant neoplasms of the upper aerodigestive tract. SCCHN is now the seventh most common cancer worldwide, with over 500,000 new cases diagnosed and 380,000 deaths annually which is nearly 4.6% of all cancer cases [1, 2]. Despite the improvements in treatment modalities, the 5-year survival rate for SCCHN patients has remained unchanged at about 50% over the past 30 years [3, 4] as 40–60% of SCCHN survivors suffer from relapse in the form of recurrences or metastases [5, 6].
Resistance to standard surgical, radiation, and chemical therapies continues to be a limiting factor in the treatment of SCCHN. One major factor in cancer treatment failure is because the efficacy of current standard chemotherapy, such as cisplatin (CIS) and 5-fluorouracil (5-FU), is restricted partly due to their severe toxic side effects. CIS forms DNA adducts which lead to induction of apoptosis in cancer cells [7], while 5-FU inhibits the thymidylate synthase enzyme through its metabolite to inhibit cancer cells division [8]. These mechanisms have non-specific chemotherapeutic effects and thus affect both cancer and non-cancer (normal) cells. The toxic side effects of CIS are dose-dependent and can cause nephrotoxicity, bone marrow suppression with hemolytic anemia, and neurotoxicity [9,10,11]. Similarly, the side effects of 5-FU include dermatologic effects, hand and foot syndrome, neurotoxicity, and cardiotoxicity [8]. Incidence of 5-FU associated cardiotoxicity is 7.6% with a mortality rate between 2.2 and 13% [8]. Reducing the chemotherapeutic dose while maintaining its efficacy is critical to improve the treatment outcome of cancers and to decrease morbidity and mortality rates.
Recently, studies have highlighted the potentials of phytochemicals as a source of therapeutics for certain forms of cancers [12]. Sulforaphane (SF) is the most characterized isothiocyanate compound and is found in high concentrations in cruciferous vegetables, such as in broccoli [13]. It has been demonstrated that SF has multiple biological activities such as anti-inflammatory, anti-oxidant, and anti-cancerous [14,15,16]. In addition, SF has low toxicity [17], making it an interesting candidate as a chemotherapeutic agent. SF has been shown to target multiple pathways involved in the functions of cancer cells when used in combination with other anti-cancer compounds. Specifically, SF increased the effect of imatinib and gemcitabine against chronic myeloid leukemia cells and pancreatic cancer cells, respectively [18, 19]. However, the anti-oxidant ability of SF induced the expression of phase 2 metabolic enzymes, which may protect cells from reactive oxygen species [20]. This is a concern for many chemotherapeutic agents as they function through free radicals, so SF combination may reduce the efficacy of these drugs. There are very few studies that examined the SF effect on head and neck squamous cell carcinomas and to our knowledge there are no studies regarding the effects of SF on the activity of conventionally used chemotherapy, CIS and 5-FU, as a combined treatment. We hypothesized that SF is a suitable agent to lower the doses of conventional chemotherapeutic drugs (such as CIS and 5-FU) without losing their efficacy. This would result in a reduction or even elimination of the severe toxic side effects associated with current chemotherapeutic drugs. This study examined the effects of combining SF with low-dose chemotherapy against human SCCHN for the first time. We also determined the underlying mechanism of action of the SF-combined chemotherapy.
Materials and methods
Cell culture
SCC12 and SCC38 cell lines were purchased from the University of Michigan and were used as models for SCCHN (Table 1). They were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, Massachusetts, United States) supplemented with 1% non-essential amino acids (Gibco). Primary fibroblasts (FB) were isolated from human salivary glands and cultured in RPMI medium (Thermo Fisher, Massachusetts, United States) [21]. Both media were supplemented with 10% fetal bovine serum and 1% Antibiotic–Antimycotic (Thermo Fisher). Gingival Epithelium Progenitors, Single Donor (HGEPs) were purchased from Cedar Lane Laboratories and were cultured in ready-to-use CnT-Prime medium (CELLnTEC, Switzerland) [22]. Immortalized normal human salivary gland acinar cell line (NS-SV-AC) was a gift from Dr. Azuma M (Tokushima University, Japan) and was cultured in KGM-2 (Lonza, Switzerland) supplemented with 2% Pen/Strep. All cell types were incubated in a humidified incubator at 37 °C with 5% CO2.
Cytotoxic agents
Sulforaphane (Cayman Chemical, Michigan, United States) was purchased as a solution in ethanol with purity ≥ 98% and stored at − 20 °C. Cisplatin (Cayman Chemical) was prepared in phosphate-buffered saline to a 0.3 mg/ml stock and kept at 4 °C protected from light. 5-Fluorouracil (Sigma Aldrich, Missouri, United States) was prepared in dimethyl sulfoxide (DMSO) to 50 mg/ml stock. Final concentrations of the solvents in the working solution medium were 0.1% or less.
MTT assay
1–3 × 103 cells were seeded in 96-well plates according to cell type. Twenty-four hours later, they were treated with different concentrations of SF and/or chemotherapeutic agents and further incubated for 72 h. The medium was then removed and 10% solution of 5 mg/ml MTT in medium (Sigma Aldrich (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) was added to each well and incubated at 37 °C for 2 h. The medium was removed and formazan was dissolved by adding DMSO to each well. The optical density was measured at 562/540 nm in a EL800 Microplate Reader (BIO-TEK Instruments, Vermont, United States). The assay was done in triplicates.
Colony-forming assay
Tumor cells were seeded at 1 × 105 cells per well in a 6-well tissue culture plates. Twenty-four hours later, the cultures were treated with SF 3.5 µM with or without CIS 0.5 µg/ml or 5-FU 0.13 µg/ml and incubated for 72 h. The cells were trypsinized, plated at a density of 400 living cells per well in 6-well tissue culture plates, and incubated for 10 days (changing the medium every 3 days). To determine colony formation, culture medium was removed and colonies were fixed and stained with 1% crystal violet, 50% methanol in DDH2O for 1 h. The number of colonies with > 50 cells were counted under an inverted microscope and the percentage of cell survival was calculated.
To assess the cells ability to repair DNA, the previous technique was used but the cultures were treated with sub-lethal doses of SF (0.875 µM) and/or CIS (0.02 µg/ml), 5-FU (0.2 ng/ml) for 72 h.
Annexin V apoptosis detection
Post-treatment apoptosis was measured by using the PE-Annexin V Apoptosis Detection Kit (BD Bioscience, Ontario, Canada). Briefly, 1.5 × 105 cells were seeded per well in a 6-well plate for 24 h and then treated with SF and/or chemotherapeutic agents for 72 h. Cells were detached using Accutase (BioLegend, California, United States), washed with annexin binding buffer, and then stained with PE-Annexin V and 7-AAD for 15 min in the dark at room temperature. Cells were washed and resuspended in fresh buffer and analyzed by flow cytometry using a LSR Fortessa (BD Biosciences). Data analysis was performed using FlowJo vX (FlowJo LCC, Oregon, United States).
Evaluation of mRNA expression levels by quantitative real-time PCR (QPCR)
QPCR was used to detect changes in genes coding for BAX, Caspase3, and BCL2. Higher drug concentrations were used in the cells treatment to show the effect of treatment on the genetic level. Total RNA was extracted from SCCHN cells treated with SF 7 µM with or without CIS 2 µg/ml or 5-FU 13 µg/ml for 72 h using TRIzol (Thermo Fisher Scientific). The first-strand cDNA was synthesized from 1 µg total RNA using High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). For the quantification of gene amplification, QPCR was performed using StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific) in the presence of PowerUp SYBR Green Master Mix (Thermo Fisher Scientific). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous expression standard. Target sequences were amplified at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60–65 °C annealing temperature for each gene for 1 min. The following gene-specific primers were used:
GAPDH: (5′- GAGAAGGCTGGGGCTCATTT-3′, 5′- AGTGATGGCATGGACTGTGG − 3′), BCL2: (5′-CTGCACCTGACGCCCTTCACC-3′, 5′-CACATGACCCCACCGAACTCAAAGA-3′), BAX: (5′-CGGGTTGTCGCCCTTTTCTA-3′, 5′-TGGTTCTGATCAGTTCCGGC-3′), Caspase3: (5′-CTCGGTCTGGTACAGATGTCGA-3′, 5′-CATGGCTCAGAAGCACACAAAC-3′). All assays were performed in triplicate and the expression was calculated on the basis of ΔΔCt method. The n-fold change in mRNAs expression was determined according to the method of 2− ΔΔCT.
Statistical analysis
Data were presented as the means ± standard deviation (SD) of three independent experiments with comparable results. Student’s t test and one-way analysis of variance (ANOVA) were used to assess significant differences between groups; P values < 0.05 were considered statistically significant. GraphPad prism 6 software was used (GraphPad Software, California, United States).
Results
SF inhibited the growth of SCCHN cells
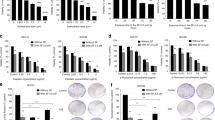
SCC12 and SCC38 cell lines were treated with various concentrations of SF alone. We found that SF inhibited the viability of both SCCHN cell lines to a similar extent (Fig. 1a). The IC50 of SF was 3.81 µM and 3.87 µM for SCC12 and SCC38, respectively. Morphological changes indicating early apoptosis as cellular swelling, pyknosis, and formation of apoptotic bodies in cancer cells were observed at a concentration of 3.5 µM and it was more noticeable with 7 µM SF concentration (Fig. S1a). These inhibitory effects of SF increased over time as demonstrated by the MTT assay (Fig. 1b). These results indicated that SF inhibited SCCHN cell growth in a dose- and time-dependent manner.
Sulforaphane affected SCCHN cell viability in a time- and dose-dependent manner. a SCC12 and SCC38 cells were treated with 0, 0.875, 1.75, 3.5, 7, and 14 µM of SF for 72 h. Cellular viability was measured in triplicate in three independent experiments by MTT assay. Data are presented as mean ± SD (“a” significance relative to 0 µM, “b” significance relative to 0.875 µM, “c” significance relative to 1.75 µM, “d” significance relative to 3.5 µM, “e” significance relative to 7 µM. P < 0.05). b SCC12 and SCC38 were treated with 3.5 µM of SF for the indicated times (“a” significance relative to 0 h, “b” significance relative to 24 h. P < 0.05)
SF increased the effects of chemotherapeutic drugs against SCCHN cells
SCC12 and SCC38 cells were treated with SF in combination with CIS or 5-FU; cell viability was analyzed by morphological inspection and MTT assay after 72 h. The addition of SF to CIS more than doubled the cytotoxic effect on SCCHN cells, as compared to CIS alone, as the combined SF treatment with 0.5 µg/ml CIS had similar or even more inhibitory effect of 1 µg/ml of CIS alone. This effect was even greater in the SF + 5-FU combined treatment as reduction in the cell viability was comparable to tenfold higher doses of 5-FU alone. The combined SF with 0.013 µg/ml 5-FU had similar effect of the 0.13 µg/ml 5-FU alone and the same with 0.13 dose (Fig. 2a, b). These results were observed in the both cell lines.
Sulforaphane synergized the effects of CIS and 5-FU against SCCHN cells. a SCC12 and b SCC38 cells were treated with 3.5 µM of SF with or without 0.1, 0.5, 1, 2 µg/ml of CIS or 0.013, 0.13, 1.3, 130 µg/ml of 5-FU for 72 h. Cellular viability was assessed using a MTT assay in triplicates in three independent experiments. Data are presented as mean ± SD (* P < 0.05 and ** P < 0.01 relative to treatment in the absence of SF, @@ P < 0.01 relative to control). c To verify the effects of SF on clonogenic cell division, SCC12 and SCC38 cells were pretreated with SF (3.5 µM) with or without CIS (0.5 µg/ml) or 5-FU (0.13 µg/ml) for 72 h before being seeded in 6-well plates for 10 days (400 cells/well). Fixed and stained colonies containing > 50 cells were counted under an inverted light microscope. Data are presented as mean ± SD (** P < 0.01 relative to treatment in the absence of SF, @@ P < 0.01 relative to control without treatment). Photographs of the fixed and stained colonies are presented on the (d) panel. e SCC12 and SCC38 cells were pretreated with sub-lethal doses of SF (0.875 µM) with or without CIS (0.02 µg/ml) or 5-FU (0.2 ng/ml) for 72 h and 400 cells per condition were seeded in 6-well plates for 10 days. Fixed and stained colonies containing > 50 cells were counted under an inverted light microscope. Data are presented as mean ± SD (** P < 0.01 relative to treatment in the absence of SF, @ P < 0.05 and @@ P < 0.01 relative to control without treatment). Photographs of the fixed and stained colonies are presented on the (f) panel
We found that the CIS treatment reduced the clonogenic ability of SCC12 and SCC38 to 64% and 60%, respectively, when compared to untreated (no drug) controls. SF reduced colony formation to 46% and 41% compared to untreated controls. The combined SF + CIS treatment further decreased colony formation to 25%. 5-FU also decreased the numbers of colonies formed to 50% and 38% in SCC12 and SCC38, respectively; however, the SF + 5-FU combination further reduced the clonogenicity to 7% compared to controls (Fig. 2c, d, Appendix Table 1).
Related results were obtained when we tested the effects of SF on DNA repair post- treatment. SF, CIS, and 5-FU were administered at a concentration of 0.875 µM, 0.02 µg/ml, and 0.2 ng/ml, respectively, based on dose–response experiments demonstrating that these concentrations were sub-lethal (Fig. S2a, b). CIS reduced clonogenicity to 75% and 77% for SCC12 and SCC38, respectively, while SF reduced colony formation to 71% and 69% when compared to untreated controls. When combined, CIS + SF showed an additive effect and reduced colony formation to 24% and 22%. We had comparable results with 5-FU which reduced the clonogenicity to 77% and 70% for SCC12 and SCC38, respectively, but when we used combined 5-FU + SF this reduction improved to 15% (Fig. 2e, f, Appendix table 2). Taken together, our data showed that SF increased the drug-mediated cytotoxic effects on cellular viability, clonogenic ability, and DNA damage in SCCHN tumors.
Sulforaphane has minimal cytotoxic effects on normal (non-cancerous) cells
We examined the toxicity of SF on non-cancerous cells. Human primary salivary fibroblasts (FB), human gingival epithelial progenitor cells (HGEPS), and a human salivary gland acinar cell line (NS-SV-AC) were treated with SF. Although SF had minimal toxic effect on FB and HGEPS, except when we used at a concentration 14 µM, with IC50 23.46 µM and 23.32 µM respectively, we found a stronger toxic effect on the NS-SV-AC cell line with IC50 6.36 µM but still higher than IC50 for SCCHN (Fig. 3a). The morphological appearance of the tested cells did not change when less than 14 µM of SF was added (Fig. S1b). Moreover, the difference between the combined treatment and the standalone effects of CIS or 5-FU on the tested cells, including NS-SV-AC revealed no statistical significance (Fig. 3b, c, d). This suggested that normal (non-cancerous) mesenchymal and epithelial cells were not negatively affected by SF, while the viability of immortalized or malignant cells was reduced.
Sulforaphane had minimal to no effect on non-cancerous human cells. a Primary fibroblasts, primary gingival epithelial cells, and a salivary acinar cell line were treated with 0, 0.875, 1.75, 3.5, 7, and 14 µM of SF for 72 h. The cell viability was evaluated in triplicate in three independent experiments by MTT assay. Data are presented as mean ± SD. b Primary fibroblasts, c primary gingival epithelial cells, and d a salivary acinar cell line were treated with 3.5 µM of SF in the presence or absence of 0.5 and 1 µg/ml of CIS or 0.13 and 1.3 µg/ml of 5-FU for 72 h, respectively. Viability was measured by a MTT assay in triplicates in three independent experiments. Data are presented as mean ± SD
Sulforaphane increased drug-mediated cytotoxicity by induction of apoptosis
We then aimed to verify the induction of apoptosis by SF on cancer cells. SCC12 and SCC38 cells were treated with CIS or 5-FU with or without SF for 72 h before being stained for annexin V and analyzed by flow cytometry. Single treatment with CIS induced early apoptosis in 12% and 8% of SCC12 and SCC38 cells, respectively. The combined treatment of SF + CIS increased the apoptosis to 20% (Fig. 4a). Similarly, 5-FU as a standalone treatment induced apoptosis in 15% and 12% of the SCC12 and SCC38 populations. The combined treatment of SF + 5-FU increased apoptosis to 20% and 24% (Fig. 4b). This suggested that sulforaphane could reduce SCCHN cell numbers through the induction of apoptosis (Fig. 4c, d).
Sulforaphane increased drug-mediated cytotoxicity by inducing apoptosis. a SCC12 and b SCC38 were treated with 3.5 µM of SF with or without 0.5 µg/ml of CIS or 0.13 µg/ml of 5-FU for 72 h. The induction of apoptosis was assessed in triplicates in three independent experiments using annexin V/7AAD staining and flow cytometry. The data presented are gated on single cells. c, d The percentage of early apoptotic cells is presented as mean ± SD (** P < 0.01 compared with treatment in the absence of SF, @ P < 0.05, and @@ P < 0.01 relative to control without treatment)
Sulforaphane affected the regulation of pro- and anti-apoptotic genes
To better understand the enhancement of apoptosis induction by chemotherapy in SCCHN cells through the addition of SF, we examined expressions of the genes that are critical for cell apoptosis in carcinoma. SCC12 and SCC38 cells were treated with SF, CIS, or 5-FU alone or in combination for 72 h, followed by QPCR for the expression of the selected genes. Compared to the control group, BAX and CASP3 expression was significantly increased while the BCL2 was significantly decreased when 7 µM of SF was used. Similarly, the expression of BAX and CASP3 was increased while BCL2 was decreased significantly in the CIS and 5-FU treatments. However, when we used the combined SF + CIS or SF + 5-FU treatments, it elevated the expression levels of BAX and CASP3 and reduced the expression level of BCL2 significantly when compared to CIS or 5-FU treatment alone (Fig. 5a, b).
Sulforaphane mediated the up-regulation of pro-apoptotic and down-regulation of anti-apoptotic genes. a SCC12 and b SCC38 were treated with 7 µM of SF with or without 2 µg/ml of CIS or 13 µg/ml of 5-FU for 72 h. The expression of BAX, CASP3, and BCL2 was measured by QPCR and normalized to GAPDH expression. All assays were performed in triplicate in three independent experiments and were calculated on the basis of ΔΔCt method. Data represent mean ± SD (* P < 0.05 and ** P < 0.01 compared with treatment in the absence of SF)
Discussion
Squamous cell carcinoma of the head and neck is one of the most common malignant neoplasms. 60% of the reported cases for treatment present with locally advanced tumors and require combined modality therapy including surgery, radiotherapy, and chemotherapy [32]. One major reason for cancer treatment failure is the limited efficacy of the conventional chemotherapy by its severe toxic side effects. In this study, we presented an approach to decrease the chemotherapeutic dose while maintaining therapeutic efficacy by combining CIS or 5-FU with the low-toxicity, natural product sulforaphane.
Numerous studies reported the anti-neoplastic effect of SF against solid tumors such as breast tumors, hepatic tumors, brain tumors, pancreatic tumors, prostate tumors, and skin tumors [13]. Recently, it was shown that SF has comparable cytotoxic effects on the squamous cell carcinoma of the head and neck [33,34,35]. Our results showed that SF decreased the SCCHN cell lines viability through increasing treatment dosage and duration. SF inhibitory effect on head and neck cancer cells is comparable to other types of cancers as the IC50 measured after 72 h of treatment for SCC12 and SCC38 were very close to acute lymphocytic leukemia [35, 36]. We used 3.5 µM SF dose for the rest of the experiment as this dose showed the first signs of apoptosis was relatively safe to non-cancerous healthy cells and expected to be achieved by simple ingestion of fresh broccoli sprouts. Clarke reported SF 2.5 µM/L plasma concentration after 3 h from ingestion of 40 g of fresh broccoli sprouts [37].
Our preliminary data suggests that SF can be used as a co-treatment to improve conventional chemotherapy against SCCHN. When we tested this hypothesis, we found that SF co-treatment decreased SCCHN cells viability twofold more than CIS alone, and tenfold more than 5-FU alone after 72 h (P < 0.05). This increase in cytotoxic effect can be used to reduce the conventional doses of CIS and 5-FU used in treatment and, in turn, reduce the dose-dependent side effects. The co-treatment with SF did not only affect the viability but also reduced the self-renewal ability of the SCCHN cells, as observed by measuring colony formation following a 72-h treatment. The co-treatment significantly reduced the number of colonies formed when compared to the single treatment of CIS or 5-FU. Our results were comparable to those obtained by using SF against other types of cancers such as gastric carcinoma, pancreas, and prostate cancers [19, 38].
One of the causes for treatment failure is due to the cancer cell’s ability to evade the damage caused by the chemotherapy [39]. However, the synergetic effect of SF with CIS or 5-FU was noticeable in the inhibition of DNA repair after treatment. This was observed after treating SCCHN cells with a sub-lethal dose of CIS or 5-FU with or without SF for 72 h, followed by a colony-forming assay for 10 days. The co-treatment significantly decreased the clonogenic ability of the cells when compared to a single treatment. This indicated that the cells were unable to repair their damaged DNA after chemotherapy termination when SF was introduced. Our data demonstrated—for the first time—that the anti-oxidant properties of SF did not affect chemotherapy efficacy but instead increased the cytotoxic effects of chemotherapy on SCCHN cells.
One of the important criteria that make SF a suitable candidate for chemotherapy is the low toxicity on non-cancerous cells. We tested this by applying different concentrations of SF on human primary fibroblasts, epithelial cells, and a salivary acinar cell line for 72 h followed by measuring cell viability. SF had minimal toxic effects on primary cells, except when administered in high doses. This was not the case with the acinar cell line which had a significantly lower IC50 when compared to the primary cells, but still higher than the SCCHN cells. This result could be because acinar cells were no longer normal (primary) cells as they were immortalized with the simian virus 40; this immortalization procedure likely led to expression of genes that were targeted by SF. We also tested the effects of the co-treatment on these cells which showed comparable results; the co-treatment had no significant difference when compared to CIS or 5-FU treatment alone. This was observed in all of the tested cell types, including the acinar cell line. This observation was also reported in primary fibroblasts, endothelial cells, and immortalized 293 Kidney cells [19] and with a human gastric epithelial cell line (GES-1) [38].
The decreased SCCHN cell viability after using sulforaphane seemed to be caused by an increased induction of apoptosis. By using the annexin V assay, we found that SF treatment significantly increased early apoptosis in treated cancer cells. The combined treatment of SF and low doses of CIS or 5-FU led to increased apoptosis compared to using a single drug as a treatment. This was in agreement with reports by other groups [19, 40].
It is suggested that various anti-cancer agents will stimulate different apoptotic pathways, including the death receptor-mediated pathway, the mitochondrial apoptotic pathway, and the endoplasmic reticulum pathway [41]. While those pathways have different initiation mechanisms, they all have the same final phase in which the executioner caspases become activated [42]. The BCL2 proteins family is the center of regulation for Caspase3—one of the executioner caspases. Cellular apoptotic susceptibility with chemotherapy is regulated by the ratio between anti-apoptotic gene BCL2 and pro-apoptotic genes BAX, Bid, and Bak [43]. In our study, QPCR results showed that SF increased chemotherapy-induced apoptosis utilizing the caspase-dependent pathway by increasing the expression of Caspase3 through the up-regulation of BAX and down-regulation of BCL2. The combined treatment almost doubled BAX expression when compared to the single treatment. Comparable results were obtained via Western blotting by others [40, 44]. Further investigations at the protein level changes should be made.
In summary, we demonstrated that SF did not decrease the cytotoxic effects of chemotherapy, but rather strongly enhanced their efficacy against SCCHN. The combined treatment efficiently increased apoptosis along with inhibiting clonogenicity and DNA repair without increasing the cytotoxicity in non-cancerous cells which will be of great clinical significance. The combined treatment may be of therapeutic benefit in clinical settings in reducing the toxic side effects of chemotherapy and increasing its effect. Our data, combined with the works of others, suggest that SF can be used with lower doses of chemotherapy as co-treatments to the benefits of the patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-86. https://doi.org/10.1002/ijc.29210.
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–48. https://doi.org/10.1001/jamaoncol.2016.5688.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. https://doi.org/10.3322/caac.20107.
Machiels JP, Lambrecht M, Hanin FX, Duprez T, Gregoire V, Schmitz S, et al. Advances in the management of squamous cell carcinoma of the head and neck. F1000prime Rep. 2014;6:44. https://doi.org/10.12703/p6-44.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. https://doi.org/10.3322/ca.2007.0010.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–79. https://doi.org/10.1038/sj.onc.1206933.
Thomas SA, Grami Z, Mehta S, K Patel. Adverse effects of 5-fluorouracil: focus on rare side effects. Cancer Cell Microenviron. 2016;https://doi.org/10.14800/ccm.1266.
Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. https://doi.org/10.1038/sj.ki.5002786.
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419–37. https://doi.org/10.3322/caac.21204.
De Jongh FE, van Veen RN, Veltman SJ, de Wit R, van der Burg ME, van den Bent MJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88(8):1199–206. https://doi.org/10.1038/sj.bjc.6600884.
Xiao Z, Morris-Natschke SL, Lee KH. Strategies for the optimization of natural leads to anticancer drugs or drug candidates. Med Res Rev. 2016;36(1):32–91. https://doi.org/10.1002/med.21377.
Lenzi M, Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Cancer Treat Res. 2014;159:207–23. https://doi.org/10.1007/978-3-642-38007-5_12.
Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280(20):19911–24. https://doi.org/10.1074/jbc.M412443200.
Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67(20):9937–44. https://doi.org/10.1158/0008-5472.can-07-1112.
Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, et al. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67(13):6409–16. https://doi.org/10.1158/0008-5472.can-07-0340.
Herman-Antosiewicz A, Xiao H, Lew KL, Singh SV. Induction of p21 protein protects against sulforaphane-induced mitotic arrest in LNCaP human prostate cancer cell line. Mol Cancer Ther. 2007;6(5):1673–81. https://doi.org/10.1158/1535-7163.mct-06-0807.
Lin LC, Yeh CT, Kuo CC, Lee CM, Yen GC, Wang LS, et al. Sulforaphane potentiates the efficacy of imatinib against chronic leukemia cancer stem cells through enhanced abrogation of Wnt/beta-catenin function. J Agric Food Chem. 2012;60(28):7031–9. https://doi.org/10.1021/jf301981n.
Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19(1):188–95. https://doi.org/10.1038/mt.2010.216.
Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3(10):1239–48.
Schwarz S, Rotter N. Human salivary gland stem cells: isolation, propagation, and characterization. Methods Mol Biol (Clifton NJ). 2012;879:403–42. https://doi.org/10.1007/978-1-61779-815-3_25.
Abdallah MN, Abdollahi S, Laurenti M, Fang D, Tran SD, Cerruti M, et al. Scaffolds for epithelial tissue engineering customized in elastomeric molds. J Biomed Mater Res Part B. 2017. https://doi.org/10.1002/jbm.b.33897.
Expasy.org. 2017. http://web.expasy.org/cellosaurus/. Accessed 13 Sept 2017.
Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32(4):417–26. https://doi.org/10.1002/hed.21198.
Grenman R, Carey TE, McClatchey KD, Wagner JG, Pekkola-Heino K, Schwartz DR, et al. In vitro radiation resistance among cell lines established from patients with squamous cell carcinoma of the head and neck. Cancer. 1991;67(11):2741–7.
Carey TE, Van Dyke DL, Worsham MJ. Nonrandom chromosome aberrations and clonal populations in head and neck cancer. Anticancer Res. 1993;13(6b):2561–7.
Nagel R, Martens-de Kemp SR, Buijze M, Jacobs G, Braakhuis BJ, Brakenhoff RH. Treatment response of HPV-positive and HPV-negative head and neck squamous cell carcinoma cell lines. Oral Oncol. 2013;49(6):560–6. https://doi.org/10.1016/j.oraloncology.2013.03.446.
Pekkola-Heino K, Joensuu H, Klemi P, Grenman R. Relation of DNA ploidy and proliferation rate to radiation sensitivity in squamous carcinoma cell lines. Arch Otolaryngol. 1994;120(7):750–4.
Bradford CR, Zhu S, Ogawa H, Ogawa T, Ubell M, Narayan A, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck. 2003;25(8):654–61. https://doi.org/10.1002/hed.10274.
Martens-de Kemp SR, Dalm SU, Wijnolts FM, Brink A, Honeywell RJ, Peters GJ, et al. DNA-bound platinum is the major determinant of cisplatin sensitivity in head and neck squamous carcinoma cells. PLoS ONE. 2013;8(4):e61555. https://doi.org/10.1371/journal.pone.0061555.
Wang L, Mosel AJ, Oakley GG, Peng A. Deficient DNA damage signaling leads to chemoresistance to cisplatin in oral cancer. Mol Cancer Ther. 2012;11(11):2401–9. https://doi.org/10.1158/1535-7163.mct-12-0448.
Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(Suppl 7):vii252–61. https://doi.org/10.1093/annonc/mdq453.
Liu CM, Peng CY, Liao YW, Lu MY, Tsai ML, Yeh JC, et al. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J Formos Med Assoc. 2017;116(1):41–8. https://doi.org/10.1016/j.jfma.2016.01.004.
Kim JH, Han Kwon K, Jung JY, Han HS, Hyun Shim J, Oh SJ, et al. Sulforaphane increases cyclin-dependent kinase inhibitor, p21 protein in human oral carcinoma cells and nude mouse animal model to induce G(2)/M cell cycle arrest. J Clin Biochem Nutr. 2010;46(1):60–7. https://doi.org/10.3164/jcbn.09-65.
Jee HG, Lee KE, Kim JB, Shin HK, Youn YK. Sulforaphane inhibits oral carcinoma cell migration and invasion in vitro. Phytother Res. 2011;25(11):1623–8. https://doi.org/10.1002/ptr.3397.
Suppipat K, Park CS, Shen Y, Zhu X, Lacorazza HD. Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS ONE. 2012;7(12):e51251. https://doi.org/10.1371/journal.pone.0051251.
Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, Stevens JF, et al. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res. 2011;64(5):456–63. https://doi.org/10.1016/j.phrs.2011.07.005.
Wang X, Li Y, Dai Y, Liu Q, Ning S, Liu J, et al. Sulforaphane improves chemotherapy efficacy by targeting cancer stem cell-like properties via the miR-124/IL-6R/STAT3 axis. Sci Rep. 2016;6:36796. https://doi.org/10.1038/srep36796.
Al-Dimassi S, Abou-Antoun T, El-Sibai M. Cancer cell resistance mechanisms: a mini review. Clin Transl Oncol. 2014;16(6):511–6. https://doi.org/10.1007/s12094-014-1162-1.
Cho NP, Han HS, Leem DH, Choi IS, Jung JY, Kim HJ, et al. Sulforaphane enhances caspase-dependent apoptosis through inhibition of cyclooxygenase-2 expression in human oral squamous carcinoma cells and nude mouse xenograft model. Oral Oncol. 2009;45(8):654–60. https://doi.org/10.1016/j.oraloncology.2008.07.003.
Ferreira CG, Epping M, Kruyt FA, Giaccone G. Apoptosis: target of cancer therapy. Clin Cancer Res. 2002;8(7):2024–34.
Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12(11):1551–70.
Min K-j, Kwon TK. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr Med Res. 2014;3(1):16–24. https://doi.org/10.1016/j.imr.2013.12.001.
Devi JR, Thangam EB. Mechanisms of anticancer activity of sulforaphane from Brassica oleracea in HEp-2 human epithelial carcinoma cell line. Asian Pac J Cancer Prevent. 2012;13(5):2095–100.
Acknowledgements
We would like to thank Murali Ramamoorthi, Andre Charbonneau, Mohamed Nur Abdallah, and Gulshan Sunavala-Dossabhoy for donating human SCC, fibroblasts, HGEPC, and NS-SV-AC, respectively. We also thank Younan Liu and Mohammed Bakkar for helpful discussion in setting up preliminary experiments.
Funding
This work was partly funded by Canadian Institutes of Health Research (CIHR Grant 119585), Natural Sciences and Engineering Research Council of Canada (NSERC Grant 05247), MJW Kim research fund, and the Ministry of Higher Education in Egypt (MOHE postgraduate studies funding).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elkashty, O.A., Ashry, R., Elghanam, G.A. et al. Broccoli extract improves chemotherapeutic drug efficacy against head–neck squamous cell carcinomas. Med Oncol 35, 124 (2018). https://doi.org/10.1007/s12032-018-1186-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1186-4