Abstract
Radiation therapy and immunotherapy are two highly evolving modalities for the treatment of solid tumors. Immunotherapeutic drugs can either stimulate the immune system via immunogenic pathways or target co-inhibitory checkpoints. An augmented tumor cell recognition by host immune cells can be achieved post-irradiation, as irradiated tissues can release chemical signals which are sensed by the immune system resulting in its activation. Different strategies combining both treatment modalities were tested in order to achieve a better therapeutic response and longer tumor control. Both regimens act synergistically to one another with complimentary mechanisms. In this review, we explore the scientific basis behind such a combination, starting initially with a brief historical overview behind utilizing radiation and immunotherapies for solid tumors, followed by the different types of these two modalities, and the biological concept behind their synergistic effect. We also shed light on the common side effects and toxicities associated with radiation and immunotherapy. Finally, we discuss previous clinical trials tackling this multimodality combination and highlight future ongoing research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Historical background
Immunotherapy has emerged as a promising venue in cancer therapy. It is based on complementation or stimulation of the immune system to mount a response against cancer cells [1]. With that approach, immunotherapy aims to establish a tumor specific response with minimal toxicity [2].
Breakthrough work in immunotherapy started more than 100 years ago, with Dr. William Colley. Colley, a surgeon, injected streptococcal cultures (Colley’s toxin) into cancer patients in an effort to induce tumor regression after he noticed that some cancer patients had remissions after they developed skin infections [3]. Colley’s toxin was thought to trigger anti-bacterial phagocytes that might kill bystander tumor cells [4]. A similar observation has been made with intravesical injection of Bacillus Calmette–Guérin (BCG) in non-muscle invasive bladder cancer, which was shown to prolong patient survival [3, 5].
In 1950, Burnet’s theory of immunosurveillance supported the view that immunotherapy is possible. He suggested that tumor-associated antigens (TAA) could provoke an effective immunological reaction that would eliminate developing cancers [6]. This concept was further supported by the identification of many TAA [7,8,9,10].
Another milestone occurred in the 1950s but this time in the field of radiotherapy. Mole et al. described a novel phenomenon: the abscopal effect. The abscopal effect is when systemic effects in non-irradiated area (out-of-field) occur after treatment with localized radiation [11].
Several mechanisms to explain what induces such an effect were proposed [12, 13]. Demaria et al. [12] were the first to propose that this phenomenon is immune-mediated. They suggested that radiation-induced cell death releases cytokines, chemokines, and inflammatory stimuli, which can promote the appropriate signals for dendritic cell activation [12]. This systemic secretion of specific cytokines and chemokines causes the immune system to mount a response that can lead to a distant effect [14]. It is thought that high-dose radiation can provoke the abscopal effect. High-dose radiation causes necrotic cell death, which releases TAA to activate the immune system [11, 15, 16].
Overview of different types of cancer immunotherapy
There is evidence that a competent immune system can defend the body against cancer cells and even eliminate them [17, 18]. On the other hand, tumors have developed mechanisms to evade the immune system [19, 20]. This interplay between the tumor and the host immune system has been the subject of interest and the target of immunotherapy.
T cell activation
To mount an immune response against foreign antigens, T cells need to be activated. This occurs when antigenic peptides (tumor or other origin) expressed on the cell surface via the major histocompatibility complex (MHC) engage with the T cell receptor (TCR) [21]. This constitutes the primary signal. The secondary signal occurs upon the interaction between costimulatory T cell surface molecule CD28 and its target cell ligand B7 [22]. After activation, T cells undergo clonal proliferation and expansion to initiate a cytolytic response in the tumor microenvironment [19].
Tumor evasion mechanisms
Tumors evade the immune system through several mechanisms with some even still unknown. They can lead to impaired antigen processing and recognition by inactivation of the cellular machinery involved in MHC complex [23,24,25,26]. Moreover, they create an immunosuppressive microenvironment by recruiting inhibitory Treg cells and myeloid-derived suppressor cells (MDSCs) that secrete inflammatory cytokines that inhibit the cytolytic activity of cytotoxic T lymphocytes (CTLs) [19, 27, 28]. Co-inhibitory signals that cause T cell downregulation are also upregulated. These signals include the interaction between T cell inhibitory receptors such as programmed death-1 receptor (PD-1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4) and their ligand on tumor cells, PD-L1 or B7, respectively [29]. These two pathways are targets of immunotherapy as their inhibition prevents the suppression of T cells and improves their anti-tumor effects [29].
Immunotherapeutic agents
Immunotherapeutic drugs can be broadly categorized into two broad categories. The first category targets immune tolerance of the tumor via co-inhibitory checkpoints: anti-CTLA-4, anti-PD-1/PD-L1. The second category directly stimulates immunogenic pathways: cytokines, CAR T cells.
The binding of CTLA-4 on T cells to its receptor B-7 on antigen-presenting cells downregulates T cell activation and proliferation leading to inhibition of the immune response [30, 31]. Ipilimumab, a CTLA-4 antibody, antagonizes CTLA-4’s inhibitory action on T cells which mobilizes them to mount a response against tumor cells [32, 33].
T cell proliferation and functions are inhibited by the PD-1 signaling pathway [34]. In addition, the binding of PD-1 to its receptor (PD-L1) also induces cell cytolysis [34]. Thus, targeting this pathway via PD-1 or PD-L1 inhibitors leads to activation of T cells.
Cytokines are small molecules that play a role in cell signaling and regulation of both the innate and adaptive immune system [1]. Currently, two cytokines, interleukin 2 (IL-2) and interferon (IFN), are approved by the Food and Drug Administration (FDA) for the treatment of certain cancers. In particular, IL-2 has been approved for the treatment of renal cell carcinoma, leukemia and lymphoma [35, 36], while IFNs have been approved as adjuvant treatment in melanoma [19].
IL-2 primarily functions as a T cell growth factor and central regulator of immune function [37]. Moreover, stimulation of IL-2 induces proliferation and enhanced cytotoxicity of natural killer (NK) cells and promotes differentiation of B-cells [38]. Similarly, IFNs activate effector T cells, NK cells, induce direct tumor apoptosis, and alter tumor vasculature [39]. Nonetheless, these cytokines were associated with only modest clinical effects and significant side effects rendering their use to be further compromised [40, 41].
Another promising approach for cancer immunotherapy involves chimeric antigen receptor CAR-modified T (CAR T) cells. Chimeric antigen receptors are proteins expressed on the surface of T cells. CAR T cells contain an antigen-binding moiety, a hinge region, a transmembrane domain, and an intracellular costimulatory domain resulting in T cell activation subsequent to antigen-binding [42]. Moreover, CAR T cells have the potential to manipulate cytokine secretion and other parameters to improve passive and active immunity [43]. While the use of CAR T cells was associated with great results in the treatment of leukemia, its utility in solid malignancies is still debatable [43]. In the case of solid tumors, the number of infiltrating CAR T cells needs to reach a certain threshold to be effective. This can be accomplished when there is sufficient T cell extravasation and tumor-induced immunosuppression is deactivated. Tumor irradiation has been linked to increasing tumor infiltration by T cells in addition to eliminating immunosuppressive cell populations [44, 45], thus pointing toward a potential of combination between CAR T cells and radiation. Future studies are needed to determine the efficacy of this combination.
Evolution of modern radiation therapy
Medical applications of radiation originated prior to the turn of the twentieth century, with the discovery of X-rays by Roentgen. Characterized by the emanation and irreversible propagation of energy away from a source, radiation consists of various compositions including electromagnetic waves and particles [46]. Utilization of X-rays for purposes of therapy was initiated imminently following their discovered role in imaging. Such administration could be classified into three general categories depending on the source: external beam radiation therapy (EBRT/teletherapy), sealed-source radiotherapy (brachytherapy) or unsealed-source radiotherapy (molecular radiotherapy).
In France, Victor Despeignes delivered the first X-ray treatment to an oncologic patient 5 months after their discovery [47]. In subsequent years, Dr. Regaud another French pioneer in radiation introduced the concept of fractionation. This notion was based upon the observation that repeated administration of lower radiation doses sterilized a male ram with significantly less skin toxicity and necrosis relative to a sufficiently large single dose [47].
Following further scientific investigations and the advent of awareness toward the biologically harmful effects of ionizing radiation, increased efforts were undertaken to accurately deliver controlled quantities of radiation [48]. EBRT experienced a significant enhancement with the development of linear particle accelerators (Linacs) capable of reproducibly generating megavoltage X-rays for treatment of internal tumors [49]. Invention of computed tomography (CT) technology by Hounsfield and Cormack permitted the next radical transformation of EBRT via three-dimensional conformal radiation therapy (3DCRT). This 3D image-based treatment planning methodology allowed delineation of target volumes and susceptible structures via slice-based contours on CT sequences in contrast to marking beam portals on radiographs [50]. This compounded a significantly augmented precision in treatment with a reduction in delivery of dosage to organs at risk. A relatively recent refinement of this method, applicable to certain tumors, is intensity modulated radiation therapy (IMRT). Through computer-aided inverse planning, a series of intensity modulated beamlets are generated in constructing a set of beams delivered from various gantry positions to optimize a tuple of dosing constraints implemented by the oncologist. While encompassing particular challenges, IMRT exhibits significant potential for improving the therapeutic ratio in certain tumors [50, 51]. Of the most current advances in EBRT, stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT/SABR) focus on the administration of high radiation doses in a substantially accelerated or hypofractionated manner. Through this high accuracy and dose conformational delivery, the involved radiobiology diverges from that portrayed in conventional fractionation and permits attaining previously unfeasible biological equivalent doses [52]. This relatively novel modality displays some degrees of promise in therapy toward disease states as CNS metastases, pancreatic, oligometastatic tumors, and others lacking an orthodox solution [53–56].
Evolution of image guidance
As mentioned, technological advancements in radiological imaging modalities exhibited very prominent and positive impacts beyond their role for diagnostic purposes. Such developments extended suitable applications in improving pretreatment simulations in addition to in situ treatment localization and guidance. The latter function is termed image-guided radiation therapy (IGRT). Successful implementation of IGRT has allowed for various beneficial alterations secondary to the accurate delivery of radiation including toxicity reduction, dose escalation, hypofractionation, voxelization, and adaptation [57].
IGRT is a broad term encompassing utilization of a diverse array of modalities capable of measuring external and internal structural positioning in patients. A set of imaging techniques involve the attachment of a kilovoltage (kV) X-ray tube source and opposing flat-panel detector on an axis orthogonal to that of the port and beam on a Linac gantry. This permits the acquisition of two-dimensional planar radiographs and fluoroscopic images [58]. These may then be compared with a digitally reconstructed radiograph (DRR) of the original CT for matching patient positioning. An advanced operation of this system termed cone beam CT (CBCT) involves the time-series acquisition of multiple kV radiographs traversing a complete revolution of the gantry and a filtered back-projection algorithm to reconstruct a volumetric image [59]. A significant benefit to this form of kV imaging is excellent spatial resolution of soft tissue at reasonable radiation dosages [59]. On some devices, the Linac port may further be a source of megavoltage (MV) imaging [60]. This image is acquired in alignment with the axis of therapy and uses energies of higher orders of magnitude to better estimate and verify the denser (typically bony) landmarks [58, 60].
In accordance with the significantly improved soft tissue delineation obtained via magnetic resonance imaging (MRI), the underlying principles of nuclear magnetic resonance are employed in the next generation of IGRT. Implementation of MRI-guidance is not limited to EBRT, but has demonstrated growth and quality outcomes in brachytherapy [61, 62]. MR-guided RT is the latest tool in cancer therapy and provides oncologists with continuous high-resolution imaging for verification of both internal structures and tumor positioning [63]. One issue arises since magnetic fields are known to impose perpendicular forces to each point on the trajectories of charged particles [46]. A ramification of this influence is distortion of the original trajectory. Thus, most current MR-guided RT machines employ radioactive isotope gamma-ray sources as Cobalt-60 in the avoidance of charged particle acceleration. However, with works demonstrating progress in combining MRI-IGRT with a Linac source, MR-guided RT may harbor significant impacts to improving cancer treatments in the upcoming future [63].
Biological basis of combination of immunotherapy and radiation
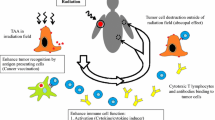
Tumors develop several mechanisms to evade the host’s immune system. However, recent findings have shown that a better tumor immune recognition can be initiated after irradiation as irradiated tissues can release “danger signals” that can be sensed by the host’s immune system [64–67].
In fact, one of the major reactions of the immune system toward tumors is a cytotoxic response called the immunogenic cell death (ICD) [68]. This process is very dependent on both the intrinsic characteristics of the tumor and the immune status of the patient [69]. RT is thought to trigger the ICD pathway by activating key steps involved in this process. This results in the translocation of the cytosolic chaperon protein (CRT) to the cell surface (which is an “eat me signal”) but also the release of HMBG-1 and ATP “danger signals”, also known as damage-associated molecular patterns (DAMPs), that can initiate pro-inflammatory events. HMBG-1 is a DNA-binding protein and TLR4-mediated dendritic cell (DC) activator, while ATP is an activator of the purinergic receptor P2RX7 [67, 70–74]. They act by promoting CD8+ T cell anticancer response. These cells have an essential role in eliminating cancer cells from the tumor bed and at distant sites of the disease. Thus, radio-induced cell death can lead to powerful anti-tumor immune response.
Moreover, radiation-induced DNA damage or loss of ataxia-telangiectasia mutated (ATM, a protein involved in DNA repair) leads to the release of nuclear DNA in the cytoplasm (cytosolic DNA) [75]. After sensing cytosolic DNA, STING (stimulator of interferon genes) adaptor protein will bind to Tank-binding kinase 1 (TBK1), which will be followed by the activation of the transcription of IFN regulatory factor 3 (IFN3) to induce type I interferon (IFN) [76–78]. Type I interferon (IFN) is thought to be the main link between innate and adaptive immunity [79]. In fact, T cells are promoted by DC that is activated by type I IFN signaling after irradiation. This makes type I IFN play a major role in the anticancer immune response.
In fact, type I IFN was reported to be secreted in high amounts only after fractionated irradiation [80, 81]. This is mainly due to the fact that fractionated RT induces the accumulation of double-stranded DNA in the cytoplasm, leading to the activation of the STING pathway. On the other hand, when a single high dose is applied, an exonuclease called TREX1 known to mediate anti-inflammatory effects becomes upregulated [82]. This leads to a lower type I IFN secretion, and therefore a less effective adaptive immune response. The difference between a single dose and fractionated RT was observed in vitro on TSA cultured cell lines, but also in vivo with murine models of breast and colorectal carcinoma [80, 82, 83]. The dose threshold at which TREX1 induces the inhibition of type I IFN secretion is thought to be dependent on cancer types and patients [83]. Understanding this phenomenon will lead to treatments with the dose per fraction that is likely to work the best to elicit an immune response.
In addition to its direct effect on ICD, RT was shown to upregulate other molecules that are involved in anti-tumor immune response are also upregulated by RT. One example is the increased cell surface expression of major histocompatibility complex (MHC) class I molecules [84–88]. These molecules have endogenous peptides to cytotoxic T lymphocytes leading to the recognition of tumor cells [89]. This upregulation was shown to be dose dependent, with a minimal dose of 4 Gy, but other studies are still required to assess the effect of multiple doses irradiations fractionation [86]. The alteration of the tumor bed by RT also supports the expression of pro-inflammatory chemokines like CXCL16 and endothelial adhesion factors VCAM and ICAM-1 that recruit immune cells to the site of disease, which also plays an important role in the immune response [90, 91].
On the other hand, RT can create an immunosuppressive environment by activating the expression of two T cell surface proteins, CTLA-4 and PD-1. These two immune checkpoints are associated with dysfunctional CD8+ T cells and immunosuppressive environment once they interact with their cognate ligands [92–94]. There is also increased evidence that ligands for CTLA-4 and PD-1 are upregulated during cancer development, which facilitates tumor growth [92, 95].
The abscopal effect
The effect of RT can potentially extend beyond the targeted site of the disease. In fact, complete regression of metastases at different sites was observed in some cancer patients treated by a combination of anti-CTLA-4 and RT [96, 97]. This abscopal effect is the subject of many recent preclinical and clinical studies recently that helped reveal its mechanism [66, 96–104]. DNA damages induced by RT lead to the death of the tumor cells. Tumor antigens are released in the microenvironment of the disease and activate the immune response subsequently [68, 105, 106].
Demaria and colleagues have investigated the abscopal effect extensively over the past decade [107]. In their study published in 2004, cell growth factor FlT3-L was used on mice with mammary carcinoma in both flanks (67NR). After irradiating the tumor in one flank, a significant tumor regression was observed on the untreated tumor. They also showed that the abscopal effect is tumor specific by irradiating the same mice with both mammary carcinoma and A20 lymphoma. No significant regression was observed on the A20 untreated lymphomas. Furthermore, CD8+ cytotoxic T cells were shown to have an important role in the abscopal effect: T cell-deficient nude mice presented a negligible abscopal effect, which means that a good immune system is required to observe this effect [107].
However, due to the escape mechanisms adopted by the tumor, the abscopal effect of RT alone is rarely observed in clinical cases. The focus was then shifted to combinational therapies, involving RT and IT, to enhance the immune direct mediated and abscopal anticancer response.
Principle of RT and IT combination
RT and IT are showing a synergistic effect able to improve the therapeutic ratio and with a longer tumor response. Different strategies combining RT and IT were studied recently in several preclinical tumor models [88, 101, 108, 109]. Using immune checkpoint inhibitors along with RT was shown to be one of the most promising strategies [110]. In 2005, Demaria’s team found that anti-CTLA-4 antibody can induce an abscopal anti-tumor response when combined with RT during the treatment of a metastatic 4T1 breast cancer model [111]. Other studies have pointed out that, generally, poorly immunogenic tumors might need RT for the anti-tumor immune effect to be observed [111, 112]. In another study on EL4 lymphoma cells and Lewis lung carcinoma (LL/C) cells on a mice model, an anti-CTLA-4 antibody significantly increased the anti-tumor activity of radiotherapy evidenced by tumor growth delay change from 13.1 to 19.5 days [113, 114]. The same effect can be found when anti-PD-1 was associated with RT. In a study conducted by Zeng et al., anti-PD-1 and RT were either used alone or together on glioblastoma mice model. As expected, the dual therapy had a better median survival (53 days) than either therapy alone (28 days for RT and 27 days for IT) [115].
A dual checkpoint blockade (anti-PD-1 and anti-CTLA-4), along with RT, is also showing promising results in a preclinical model. In a study conducted in 2015 on a melanoma mice model, the PD-L1 allowed the tumor to escape anti-CTLA-4-based therapy. A tri-therapy, combining RT, anti-PD-L1 and anti-CTLA-4 showed an overall better response when compared with dual or mono therapies, with more than 80% of mice showing a complete response to the treatment [116]. These preclinical data suggest that combined therapy may have a better outcome than monotherapies. However, additional studies and clinical trials are required to assess the outcome of such strategies.
Clinical trials tackling the combination of RT with immunotherapy
Previous clinical trials involving immunotherapy and radiation therapy
In accruing an eligible list of prior clinical trials involving the combination of immunotherapy and radiation therapy, we utilized the international database ClinicalTrials.gov [64]. A search criterion was composed using the general keyword “Immunotherapy” in addition to the interventional specifier “Radiation” encompassing both treatments listed under “Radiation” and “Procedure.” The search was modified to filter for closed interventional studies with results. In acquiring further trials regarding the utilization of SBRT/SABR in conjunction with immunotherapy agents or techniques, prior reviews were consulted [65, 66]. Published results and relevant NCT number are consolidated in a summary table (Table 1).
Applications of this dual-regimen toward oncological therapy span various clinical sites. Immunological modalities involved include biological agents for targeted regulation of immune control mechanisms, vaccines with respective adjuvants and immune cell transplantations among others. These techniques permit the introduction or relative suppression or augmentation of certain immune components to either mount a systemic response against tumor cells or inhibit tumor-induced suppression of such an immune response. Radiation modalities may include traditional fractionation or SBRT/SABR high-dose schemes. This combination of local and systemic treatments may enhance the anti-tumor effect secondary to an active interplay between the immune system and ionizing radiation on the body.
Owing to its high immunogenicity, melanoma has been at the forefront of immunotherapy clinical trials. In particular, a focus on biological agents targeting immune-suppressing cellular receptors has been displayed. A trial studying ipilimumab, a monoclonal antibody targeting cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), aimed to assess RT influence on systemic anti-melanoma immune responses [7]. Twenty-two patients harboring stage IV melanoma were treated with palliative RT and ipilimumab for 4 cycles. RT to 1–2 disease sites was initiated within 5 days after starting ipilimumab. Beneficial clinical outcomes, in the form of either disease response or stability, was demonstrated in 11 (50%) of patients at 55 weeks [7]. A separate trial assessed the efficacy of ipilimumab in conjunction with SRS in the context of metastatic melanoma to the brain [8]. A total of 113 total brain metastases (BM), across 46 melanoma patients receiving ipilimumab, were treated with single-fraction SRS to a median dose of 21 Gy. Fifteen patients obtained their radiation concurrently with ipilimumab, 19 received SRS prior, and 12 received SRS after. OS was significantly associated with the timing of SRS/ipilimumab (P = 0.035). The patients whom were treated with SRS at a point prior to the completion of immunotherapy (including jointly) portrayed improved regional recurrence and OS relative to the post-immunotherapy regimen (1-year OS 65 vs. 56 vs. 40%, P = 0.008; 1-year regional recurrence 69 vs. 64 vs. 92%, P = 0.003). A tendency toward diminished local recurrence was displayed with concurrent treatment as opposed to SRS prior or after (1-year local recurrence 0 vs. 13 vs. 11%, P = 0.21). Grade 3–4 toxicities were observed in 20% of patients [8]. Another monoclonal antibody nivolumab, an anti-programmed cell death protein 1 (PD-1) molecule, was investigated in combination with SRS in melanoma metastasis to brain [9]. Twenty-six patients with a total of 73 BMs treated over 30 sessions were analyzed. Kaplan–Meier estimates for local BM control following radiation at 6 and 12 months were 91 and 85%, respectively. Median OS from the date of SRS and nivolumab initiation was 11.8 and 12.0 months, respectively, in patients receiving nivolumab for un-resected disease. Overall, OS and metastatic control portrayed improvement relative to current standards [9].
In the setting of glioblastoma multiforme (GBM), a severe and fatal intrinsic brain tumor composed of glial cells, vaccine-based immunotherapy has provided improvement over certain end points. Cervical intra-nodal vaccination with autologous tumor lysate-loaded dendritic cells (DCs) in patients with GBM was assessed after radiation therapy and temozolomide (TMZ) [67]. A number of 10 vaccine participants were analyzed, yielding a median PFS of 9.5 months and an OS of 28 months [67]. This suggests addition of DC vaccines to radiation therapy and TMZ in the treatment of GBM to be safe and feasible in the potential induction of immune responses. A second trial evaluating the use of a vaccine in GBM employed an epidermal growth factor receptor variant III-peptide (PEP-3) vaccine in conjunction with RT and TMZ to promote immunogenicity [68]. At 6 months, PFS rate after intervention was 67% (95% CI 40–83%) and median OS displayed was 26.0 months (95% CI 21.0–47.7 months). OS of the experimental arm was greater than the control matched for eligibility criteria, prognostic factors, and TMZ treatment upon adjustment for age and KPS (hazard ratio, 5.3; P = 0.0013; n = 17) [68]. These results suggest further investigation in a potential phase III trial of the PEP-3 vaccine in the setting of GBM.
Metastatic prostate cancer has also been the subject of investigation as to the administration of immunotherapy and RT combinations. Ipilimumab was studied in patients with metastatic castration-resistant (androgen-insensitive) prostate cancer progression following therapy with docetaxel [69]. A total of 799 patients were randomly assigned to receive bone-directed radiotherapy (8 Gy in a single fraction) then to ipilimumab or placebo. Median OS was 11.2 months with ipilimumab versus 10.0 months in the control arm (HR 0.85, 0.72–1.00; P = 0.053) and not deemed statistically significant. The most common grade 3–4 adverse events were immune-related, being diarrhea, fatigue, anemia and colitis, and occurring in 101 (26%) patients in the ipilimumab group and 11 (3%) of patients in the placebo group [69]. Similarly, in the case of castration-resistant metastatic prostate cancer, immunotherapy in the form of a prostate specific antigen (PSA)-TRI ad of COstimulatory Molecules (B7-1, ICAM-1 and LFA-3) (TRICOM) was studied in combination with radiation from Samarium-153-ethylene diamine tetramethylene phosphonate (Sm-153-EDTMP), a beta-emitter with affinity for osteoblastic bone lesions [70]. Median PFS was 1.7 versus 3.7 months in the Sm-153-EDTMP exclusive and combination arms (P = 0.041, HR = 0.51, P = 0.046). No patient in the Sm-153-EDTMP exclusive arm attained PSA decline >30% compared with four patients (out of 21) in the combination arm. Meanwhile, comparable toxicities were observed between the arms [70]. These studies warrant further investigation in order to identify patients with metastatic prostate cancer who could potentially benefit from the combined approach.
Outside of these specific settings, clinical trials investigating the role of combination therapy have demonstrated results in other sites. SBRT was evaluated in combination with ipilimumab in the context of advanced solid tumors, particularly in liver and lung [71]. Thirty-five patients initiated ipilimumab, among whom 2 experienced dose-limiting toxicities and 12 (34%) grade 3 toxicities. Thirty-one patients could be evaluated for response external to the radiation fields. Three patients (10%) exhibited partial response and 7 (23%) demonstrated clinical benefit (defined as partial response or stable disease lasting ≥6 months). Clinical benefit was found to be associated with increases in peripheral CD8+ T cells, CD8+/CD4+ T cell ratio, and proportion of CD8+ T cells expressing markers CD137 and PD-1. Liver (vs. lung) irradiation produced greater T cell activation. Therefore, combining ipilimumab with SBRT/SABR was shown to be safe with signs of efficacy [71]. Finally, a neoadjuvant DC vaccine was studied in combination with RT for effects in a cohort of 18 newly diagnosed high-risk soft tissue sarcoma patients [72]. Neoadjuvant treatment was 50 Gy in 25 fractions of EBRT, combined with four intra-tumoral injections of DCs followed by complete resection. A total of 12 out of 18 (67%) patients were alive, among which 11/18 (61%) were alive without systemic recurrence over 2–8 years. There were no unexpected toxicities, and favorable immunological responses correlated with clinical responses in some cases. This provides evidence for potential effectiveness of DC vaccines in conjunction with RT in soft tissue sarcomas as well [72].
Toxicity associated with combination
Both immunotherapy and radiation therapy have their own toxicity profiles. Interestingly though, some adverse effects of both treatments overlap. This stems from activation of the immune system which can lead to its overstimulation resulting in immune-mediated toxicities [76]. Perhaps the most relevant adverse effect is pneumonitis. Radiation pneumonitis is thought to reflect an immune-mediated inflammatory reaction to radiation-induced lung damage [77, 78]. Patients receiving immune check point inhibitors are also at risk of developing pneumonitis even in the absence of radiotherapy [79]. It is still unclear if the incidence of pneumonitis from radiation therapy to the thorax is higher if delivered concurrently or in close proximity to immune check point inhibitors and if pneumonitis symptoms would be more severe with the treatment combination [80]. A secondary analysis of the KEYNOTE-001 trial provided insight on the pulmonary toxicity in patients with non-small cell lung cancer who received radiotherapy before pembrolizumab [81]. A total of 15 (63%) of 24 patients who had previously received thoracic radiotherapy had any recorded pulmonary toxicity versus 29 (40%) of 73 patients with no previous thoracic radiotherapy [81]. Three (13%) patients with previous thoracic radiotherapy had treatment-related pulmonary toxicity compared with one (1%) of those without; frequency of grade 3 or worse treatment-related pulmonary toxicities was similar [81]. A longer progression-free survival and overall survival were obtained while maintaining an acceptable safety profile.
Similarly, radiation to the abdomen causes changes in the mucosa of the colon and rectum resulting in enterocolitis [82]. Colitis is also reported with check point inhibitors which cause T cell activation within the gastrointestinal tract [83].
A phase I study was conducted to determine feasibility, dose-limiting toxicities and maximum tolerated SBRT fraction when given in conjunction with ipilimumab to patients with metastatic melanoma [84]. Fifteen patients (68%) developed different grade 3 toxicities with anemia being the most common; no grade 4 toxicities were observed [84]. Nonetheless, Barker et al. [85] reviewed the records of melanoma patients treated with ipilimumab and radiotherapy. The frequency of grade 3 or 4 adverse events in irradiated organs was 15% for the combination [85]. One grade 4 event was noted in a previously irradiated organ that was re-irradiated [85]. So, concurrent ipilimumab and radiation was not associated with higher than expected rates of adverse events, nor did it abrogate palliative effects of RT or survival benefits of ipilimumab [85].
Although clinical data on combining immunotherapy and radiation are still lacking, some trials are revealing positive results of this combination. Tang et al. [71] showed the safety and efficacy of combining SBRT with ipilimumab, where only 2 out of 35 (5.7%) patients developed dose-limiting toxicities and twelve (34%) developed grade 3 toxicities. Slovin et al. [86], examined the combination of ipilimumab and radiotherapy in metastatic castration-resistant prostate cancer. The most common reported adverse events in that trial were diarrhea (54%), colitis (22%), rash (32%), and pruritus (20%); grade 3/4 immune-related adverse events included colitis (16%) and hepatitis (10%) [86]. Thus, this combination resulted in clinical anti-tumor activity with disease control and manageable adverse events.
Trials examining the combination of immunotherapy with radiation therapy must be conducted with prudence, as it is yet to be determined whether the frequency and severity of immunotoxicities increase with such a regimen.
Assessing response to combination
The introduction of immunotherapy with its unique anticancer mechanisms not only prompted discussions about its efficacy but about means to assess the response of patients receiving such a therapy. Predictors of response to immunotherapy are currently under investigation.
Pretreatment tumor PD-L1 expression correlates with response to anti-PD-1 therapy [87]. Taube et al. [88] analyzed different tumor specimens obtained before treatment with anti-PD-1 therapy to determine whether PD-1 expression can be used as an indicator of anti-tumor immune response. They concluded that the single factor most closely correlated with response to anti-PD-1 blockade and with clinical benefit is tumor PD-L1 expression [88]. Similarly, Tumeh et al. [89] examined pretreatment melanoma samples. Patients who responded to anti-PD-1 treatment had higher numbers of CD8, PD-1, and PD-L1 expressing cells at the invasive tumor margin and inside tumors [89].
A higher mutational load was associated with better responses to CTLA-4 blockade and to anti-PD-1 therapy [90]. Somatic mutations and candidate neoantigens generated from these mutations were characterized for 64 patients with melanoma by Snyder et al. [91]. Mutational load was associated with the degree of clinical benefit [91]. In two independent cohorts, higher nonsynonymous mutation burden in tumors was associated with improved objective response, durable clinical benefit, and progression-free survival in non-small-cell lung cancers treated with anti-PD-1 [92].
Traditionally, response patterns to chemotherapeutic agents are defined according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria [93]. It is based on the concept of an overall assessment of tumor burden by summing the products of bidimensional lesion measurements and determines response to therapy by evaluation of change from baseline while on treatment [94]. Nonetheless, immunotherapy treatments display different kinetics in comparison with cytotoxic agents [95]. They may activate the immune system inducing a cellular response before affecting tumor burden or patient survival [95, 96]. Thus, responses seen with immunotherapeutic agents, whether complete or partial, can occur after an increase in tumor burden characterized as progressive disease according to RECIST criteria [93, 97]. To accommodate these new patterns of responses, the immune-related response criteria (irRC) were developed by Wolchok et al. irRC were developed after a series of large multinational studies of patients with advanced melanoma who received ipilimumab [93, 95]. Four distinct response patterns were seen [93]:
-
irCR complete disappearance of all lesions (whether measurable or not, and no new lesions) confirmation by a repeat, consecutive assessment no less than 4 weeks from the date first documented.
-
irPR decrease in tumor burden ≥50% relative to baseline confirmed by a consecutive assessment at least 4 weeks after first documentation.
-
irSD not meeting criteria for irCR or irPR, in absence of irPD.
-
irPD increase in tumor burden ≥25% relative to nadir (minimum recorded tumor burden) confirmation by a repeat, consecutive assessment no less than 4 weeks from the date first documented.
As such, tumor burden is assessed as a continuous variable which takes into account index lesions identified at baseline together with new lesions as they make occur after treatment commences [95]. Using irRC with agents that can cause tumor shrinkage such as ipilimumab allows a more comprehensive assessment of clinical activity and explain why patients with progressive disease by RECIST have a prolonged long term survival [20, 93]. However, the importance of adopting the irRC to monitor the response with agents that are less likely to cause tumor shrinkage such as cytokines and vaccines is yet to be determined and requires further prospective studies [93].
The use of irRC has implications on the continuation of treatment. With cytotoxic agents, evidence of progression (by RECIST) prompts the discontinuation of therapy. However, evidence of increase in tumor burden in patients receiving immunotherapy may not indicate cessation in the use of the immunotherapeutic agent.
As the combination of immunotherapy and radiation is novel, so are the appropriate clinical end points to monitor the response to such a regimen. As clinical trials examining such a combination emerge, they provide more data on the evaluation of response to this treatment. For instance, a phase I trial by Tang et al. [71] tested SBRT with ipilimumab in patients with advanced malignancies. Clinical benefit was associated with increases in peripheral CD8+ T cells, CD8+/CD4+ T cell ratio, and proportion of CD8+ T cells expressing PD-1 [71]. Other ongoing trials exploring the combination checkpoint inhibitors with radiation are also using T cell counts to monitor the response to treatment. They are measuring absolute lymphocyte counts, Treg counts, and PD-1 expression [98]. Other biomarkers are expected in the future as we learn more about the mechanism of immune stimulation by radiation and the synergistic action of immunotherapy.
Future research
Although significant progress has been made, several questions remain to be addressed. First, the ideal patient population who benefit from targeting the immune system remains to be elucidated. In most studies, it is estimated that only 10–20% of patients derive benefit. Some have proposed predictive biomarkers as PDL-1 expression [99], IFNγ response [100, 101] and absolute lymphocyte count [102]. Another question pertains to tumor sites that are amenable to this therapy. For example, in a patient with metastatic cancer to bones, liver and lung, it would be prudent to select the anatomical site with highest immunogenicity for targeting with SBRT. A third question relates to the sequence and number of cycles of immunotherapy in relation to SBRT. The ultimate time gap between SBRT and immunotherapy remains unknown. These questions should be addressed in future prospective studies exploring the combination of immunotherapy and radiation therapy.
Conclusion
The fields of radiotherapy and immunotherapy have immensely evolved since their establishment. The combination of RT and IT provides a synergistic effect to induce a longer tumor response. The combination is best studied within clinical trials as we learn more about patient selection, efficacy and toxicity. The novel approach has the potential of transforming the role radiotherapy from mere local control to systemic effect. The appropriate clinical end points to monitor and assess the response to this combination are still being studied. The use of radiotherapy and immunotherapy together in the treatment of malignancies constitutes a major breakthrough in oncology and can potentially improve clinical outcomes in patients with dismal prognosis.
References
Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J. 2006;1(2):138–47.
Helmy KY, et al. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 2013;4(10):1307–20.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9.
Schwartz RS. Book review. N Engl J Med. 1997;337(16):1178–9.
Sylvester RJ. Bacillus Calmette–Guérin treatment of non-muscle invasive bladder cancer. Int J Urol. 2011;18(2):113–20.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48.
Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10(3):281–7.
Boon T, et al. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65.
Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183(3):725–9.
Urbanski M, Cone RE. Appearance of T lymphocyte-derived proteins specific for the immunizing antigen in serum during a humoral immune response. J Immunol. 1992;148(9):2840–4.
Sologuren I, Rodríguez-Gallego C, Lara PC. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res. 2014;3(1):18–31.
Demaria S, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–70.
Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012;2:191.
Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178(6):505–23.
Tesniere A, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15(1):3–12.
Lumniczky K, Safrany G. Cancer gene therapy: combination with radiation therapy and the role of bystander cell killing in the anti-tumor effect. Pathol Oncol Res. 2006;12(2):118–24.
Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437(7055):141–6.
Dunn GP, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6(7):722–9.
Velcheti V, Schalper K. Basic overview of current immunotherapy approaches in cancer. Am Soc Clin Oncol Educ Book. 2016;35:298–308.
Vatner RE, et al. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325.
Thaxton JE, Li Z. To affinity and beyond: harnessing the T cell receptor for cancer immunotherapy. Hum Vaccines Immunother. 2014;10(11):3313–21.
Willemsen RA, et al. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol. 2005;174(12):7853–8.
Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. 2008;57(11):1719–26.
Aptsiauri N, et al. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–31.
Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60(8):1161–71.
Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(Suppl 4):A40–5.
Gajewski TF, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22.
Dany M, et al. Advances in immunotherapy for melanoma management. Hum Vaccine Immunother. 2016;12(10):2501–11.
Camacho LH. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med. 2015;4(5):661–72.
Linsley PS, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4(6):535–43.
Michielin O, Hoeller C. Gaining momentum: new options and opportunities for the treatment of advanced melanoma. Cancer Treat Rev. 2015;41(8):660–70.
Trinh VA, Hagen B. Ipilimumab for advanced melanoma: a pharmacologic perspective. J Oncol Pharm Pract. 2013;19(3):195–201.
Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30.
Hurley KE, Chapman PB. Helping melanoma patients decide whether to choose adjuvant high-dose interferon-alpha2b. Oncologist. 2005;10(9):739–42.
Yang JC, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21(16):3127–32.
Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88(5):986–97.
Begley CG, et al. Human B lymphocytes express the p75 component of the interleukin 2 receptor. Leuk Res. 1990;14(3):263–71.
Wagner TC, et al. Interferon receptor expression regulates the antiproliferative effects of interferons on cancer cells and solid tumors. Int J Cancer. 2004;111(1):32–42.
Panelli MC, et al. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J Transl Med. 2004;2(1):17.
Markman M, et al. Phase 2 trial of interferon-beta as second-line treatment of ovarian cancer, fallopian tube cancer, or primary carcinoma of the peritoneum. Oncology. 2004;66(5):343–6.
Haji-Fatahaliha M, et al. CAR-modified T-cell therapy for cancer: an updated review. Artif Cells Nanomed Biotechnol. 2016;44(6):1339–49.
Eckert F, et al. Beyond checkpoint inhibition – Immunotherapeutical strategies in combination with radiation. Clin Transl Radiat Oncol. 2017;2:29–35.
Lugade AA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–23.
Matsumura S, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–107.
Griffiths DJ. Introduction to electrodynamics. 3rd ed. Upper Saddle River: Prentice Hall; 1999. p. xv.
Laugier A. The first century of radiotherapy in France. Bull Acad Natl Med. 1996;180(1):143–60.
Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4(9):737–47.
Thwaites DI, Tuohy JB. Back to the future: the history and development of the clinical linear accelerator. Phys Med Biol. 2006;51(13):R343–62.
Purdy JA. 3D treatment planning and intensity-modulated radiation therapy. Oncology (Williston Park). 1999;13(10 Suppl 5):155–68.
Galvin JM, et al. Implementing IMRT in clinical practice: a joint document of the American Society for Therapeutic Radiology and Oncology and the American Association of Physicists in Medicine. Int J Radiat Oncol Biol Phys. 2004;58(5):1616–34.
Macia IGM. Radiobiology of stereotactic body radiation therapy (SBRT). Rep Pract Oncol Radiother. 2017;22(2):86–95.
Bhattacharya IS, et al. Stereotactic body radiotherapy (SBRT) in the management of extracranial oligometastatic (OM) disease. Br J Radiol. 1048;2015(88):20140712.
Greco C, et al. Spinal metastases: from conventional fractionated radiotherapy to single-dose SBRT. Rep Pract Oncol Radiother. 2015;20(6):454–63.
Linskey ME, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68.
Trakul N, Koong AC, Chang DT. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin Radiat Oncol. 2014;24(2):140–7.
Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9(12):688–99.
Glide-Hurst CK, Chetty IJ. Improving radiotherapy planning, delivery accuracy, and normal tissue sparing using cutting edge technologies. J Thorac Dis. 2014;6(4):303–18.
Jaffray DA, et al. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53(5):1337–49.
Huntzinger C, et al. Dynamic targeting image-guided radiotherapy. Med Dosim. 2006;31(2):113–25.
Fields EC, Weiss E. A practical review of magnetic resonance imaging for the evaluation and management of cervical cancer. Radiat Oncol. 2016;11:15.
Tanderup K, et al. Magnetic resonance image guided brachytherapy. Semin Radiat Oncol. 2014;24(3):181–91.
Pollard JM, et al. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 1073;2017(90):20160667.
NIH, U.S. ClinicalTrials.gov. 2017.
Bernstein MB, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516–24.
Shabason JE, Minn AJ. Radiation and immune checkpoint blockade: from bench to clinic. Semin Radiat Oncol. 2017;27(3):289–98.
Fadul CE, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother. 2011;34(4):382–9.
Sampson JH, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–9.
Kwon ED, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12.
Heery CR, et al. Samarium-153-EDTMP (Quadramet(R)) with or without vaccine in metastatic castration-resistant prostate cancer: a randomized Phase 2 trial. Oncotarget. 2016;7(42):69014–23.
Tang C, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. 2017;23(6):1388–96.
Raj S, et al. Long-term clinical responses of neoadjuvant dendritic cell infusions and radiation in soft tissue sarcoma. Sarcoma. 2015;2015:614736.
Hiniker SM, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2016;96(3):578–88.
Kiess AP, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368–75.
Ahmed KA, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27(3):434–41.
Alatrash G, et al. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12(5):631–45.
Roberts CM, et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med. 1993;118(9):696–700.
Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995;31(2):361–9.
Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS ONE. 2016;11(7):e0160221.
Alley EW, et al. Immunotherapy and radiation therapy for malignant pleural mesothelioma. Transl Lung Cancer Res. 2017;6(2):212–9.
Shaverdian N, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903.
Qadeer MA, Vargo JJ. Approaches to the prevention and management of radiation colitis. Curr Gastroenterol Rep. 2008;10(5):507–13.
Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11(2):91–9.
Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7.
Barker CA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1(2):92–8.
Slovin SF, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–21.
Hiniker SM, Maecker HT, Knox SJ. Predictors of clinical response to immunotherapy with or without radiotherapy. J Radiat Oncol. 2015;4:339–45.
Taube JM, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74.
Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Yuan J, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3.
Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99.
Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8.
Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20.
Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Hoos A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–97.
Hoos A, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30(1):1–15.
Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004;22(22):4442–5.
Spiotto M, Fu Y-X, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol. 2016. doi:10.1126/sciimmunol.aag1266.
Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7.
Chow LQM, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–45.
Sheikh NA, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–47.
Ku GY, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–75.
Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40:10–24.
Postow MA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31.
Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–32.
Demaria S, Pilones KA, Vanpouille-Box C, Golden EB, Formenti SC. The optimal partnership of radiation and immunotherapy: From preclinical studies to clinical translation. Radiat Res. 2014;182:170–81.
Demaria S, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70.
Dewan MZ, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–88. doi:10.1158/1078-0432.CCR-09-0265.
Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1bb antibody (bms-469492) treatment. Anticancer Res. 2006;26:3445–53.
Hiniker SM, Knox SJ. Immunotherapy and radiation. Semin Oncol. 2014;41:702–13.
Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and ctla-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34.
Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst. 2013;105:256–65.
Ruocco MG, et al. Suppressing t cell motility induced by anti–ctla-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–30.
Yoshimoto Y, et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by ctla-4 blockade in a mouse model. PloS One. 2014;9:e92572.
Zeng J, et al. Anti-pd-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9.
Victor CT-S, et al. Radiation and dual checkpoint blockade activates non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent was obtained as no human subjects were involved in this review.
Rights and permissions
About this article
Cite this article
El Chediak, A., Shamseddine, A., Bodgi, L. et al. Optimizing tumor immune response through combination of radiation and immunotherapy. Med Oncol 34, 165 (2017). https://doi.org/10.1007/s12032-017-1025-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1025-z