Abstract
Ovarian cancer is often diagnosed at advanced stages, when poorly responsive to standard treatment. First-line treatment consists in schemes including cytoreductive surgery followed by adjuvant chemotherapy schemes with platinum and taxane derivatives. Second-line regimens are based on gemcitabine and liposomal doxorubicin. Third line is often not worthwhile because of the high toxicity with poor response to treatment. Previously, we showed that paclitaxel (PTX) carried in non-protein lipid core nanoparticles (LDE) resembling the chemical structure of LDL has remarkably reduced toxicity. Here, the hypothesis was tested whether PTX-LDE could safely benefit patients in third-line treatment setting. Fourteen women unresponsive to second-line chemotherapy for ovarian cancer, aged 61 ± 10 years, clinical stage IV and TqNqM1, were included. PTX-LDE was administered at 175 mg/m2, 3/3 week dose. Patients were submitted to clinical examinations before each chemotherapy cycle. Serum biochemistry and imaging examinations to monitor disease progression were performed. In total, 74 cycles of chemotherapy were done and, in all cycles, clinical or laboratorial toxicities were not observed. Median progression-free survival (PFS) was 3.0 months (95% CI 2.0–3.9). In four patients, PFS was >6 months and in 2 > 1 year. The unpreceded, striking absence of toxicity and consistently long PFS, compared to previous results, indicate that at least 4 among 14 patients had tumor arrest by the treatment and clear benefit of PTX-LDE at third-line setting. The absence of observable toxicity allows dose escalating to improve response to treatment, as perspective to be tested in the ensuing studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian carcinoma (EOC) is the third most common cancer in women [1] and one of the leading causes of gynecologic cancer death. The tumor rapidly propagates to the abdominal cavity, and patients are frequently diagnosed with advanced disease. Thus, the long-term survival prognosis is rather poor [2, 3]. Approximately 239,000 new ovarian cancer cases are diagnosed per year around the world, with yearly occurring 152,000 deaths from the disease [4].
The first-line standard therapy for ovarian cancer consists in debulking surgery followed by adjuvant combination chemotherapy based on carboplatin and taxanes. The response to treatment is high, but the recurrence rate during or after chemotherapy is over 75% [5, 6]. Second-line treatment is less standardized. Platinum drugs are reintroduced in platinum-sensitive patients, defined as those in whom the relapse occurred for more than 6 months after first-line finishing [7]. Non-platinum drugs such as paclitaxel (PTX), gemcitabine, cyclophosphamide, oral etoposide, liposomal doxorubicin, topotecan [8] and, more recently, bevacizumab [9] and PARP inhibitor drugs [10] have been used. The prognosis of platinum-refractory patients is pronouncedly worse than that of the platinum-responsive patients.
Few ovarian cancer patients appear in clinical conditions that could allow their undergoing third-line chemotherapy designed to control the disease progression [11]. Because of the high toxicity of the standard chemotherapy agents and their limited effectiveness in third-line schemes, the development of new therapeutic strategies is mandatory to offer to those patients consistently longer progression-free survival (PFS) [12].
Reduction in the toxicity of the anticancer drugs while increasing or at least preserving their pharmacological action can be achieved by designing vehicles capable of selectively targeting neoplastic cells and tissues where they deliver drugs while sparing the normal tissues [13, 14]. In this regard, the overexpression of lipoprotein receptors such as the low-density lipoprotein (LDL) receptors is a hallmark of cancer cell metabolism which clearly differentiates tumors from normal tissues. This offers an exceptional opportunity for acquisition of drug targeting systems [15]. The need for cholesterol and other lipids for building of new membranes allowing the accelerated mitosis rates determines the overexpression of the LDL receptors [16, 17]. As found by Maranhão et al. [15], after injection in the bloodstream, non-protein lipid nanoparticles termed LDE that resemble LDL lipid structure can bind to the LDL receptors and concentrate in the tumor tissues drugs associated with the nanoparticles. This was shown in patients with ovarian [18], breast [19] and other gynecologic carcinomas [20].
In experimental animals, including non-human primates [21], and, subsequently, in trials enrolling patients with advanced cancers, antineoplastic agents such as PTX [20, 22], etoposide [23, 24] and carmustine [25, 26] had the toxicity pronouncedly decreased when carried in LDE. In all tested LDE–drug formulations, the pharmacological action of the carried drugs was not abated by their association with LDE [20, 22,23,24,25].
Previously, we had determined the pharmacokinetics of PTX-LDE in patients with gynecological cancers [20] and shown that, after injection in patients before surgery for ovarian carcinoma, the LDE uptake by the tumor was tenfold higher than by the contralateral normal ovarian tissue [18]. Those findings prompted us to design this single-arm phase II study of PTX-LDE as treatment for patients with epithelial ovarian carcinoma refractory to platinum derivatives. The endpoints of the study were the PFS, the clinical and laboratorial toxicity under the PTX-LDE treatment. The presence in the tumor tissues of LDL and LRP-1 receptors that take up native LDL and LDE was also evaluated.
Materials and methods
Patients
Fourteen consecutive volunteers with epithelial ovarian carcinoma were selected for the study from the outpatient clinics of the Arnaldo Vieira de Carvalho Cancer Institute, in the city of São Paulo, Brazil, for a one-arm, non-randomized, open-label phase II study. Patients had been previously submitted to first- and second-line chemotherapy schemes. Their physical and clinical data and previous treatments are given in Table 1.
This study was approved by the Arnaldo Vieira de Carvalho Cancer Institute Research Ethics Committee, reference number 218/09.
Preparation of PTX oleate associated with LDE
To increase the stability and yield of the association with LDE, a derivatized PTX compound, PTX oleate, was synthesized, as previously described [27].
The PTX-LDE formulation was prepared from a lipid mixture composed of 135 mg cholesteryl oleate, 333 mg egg phosphatidylcholine, 132 mg Miglyol 812 N, 6 mg cholesterol and 60 mg of PTX and the aqueous phase comprised 100 mg of polysorbate 80 and 10 ml tris–HCl buffer, pH 8.05. A pre-emulsion was obtained by ultrasonic irradiation until complete drug dissolution. Emulsification of all lipids, functionalized drug and the aqueous phase was obtained by high-pressure homogenization using an Emulsiflex C5 homogenizer (Avestin, Ottawa, Canada). After homogenization cycles, the formed nanoparticle was centrifuged and the nanoparticles are sterilized by passage through 0.22-μm pore polycarbonate filter (Millipore, Darmstadt, Germany) and kept at 4 °C until it was used. The incorporation of PTX to LDE was confirmed by high-performance liquid chromatography (Shimadzu, Columbia, MD) developed in isocratic mode, mobile phase 100% methanol and UV–visible detector (234 nm).
Immunohistochemistry
Five-micron-thick sections of formalin-fixed paraffin-embedded ovarian cancer and adjacent tumor infiltrated tissue were routinely processed, before surgery and previous chemotherapy treatment. For immunohistochemical analysis, the anti-LDLR rabbit antimouse polyclonal antibody (LifeSpan Biosciences Inc., Seattle, WA) and the anti-LRP antimouse monoclonal antibody (Calbiochem Merck Millipore Co., Darmstadt, Germany) were used in this study.
For immunostaining for LRP-1, antigen retrieval was performed by heating with 10 mM citrate buffer, pH 6.0, for 30 min and cooling for 5 min at room temperature. For immunostaining for LDLR, antigen retrieval was not necessary. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide. Briefly, each tissue section was incubated in 10% fetal calf serum for 1 h at 42 °C. The slides were then incubated overnight at 4 °C with a 1:50 dilution of anti-LDLR antibody or with a 1:400 dilution of anti-LRP antibody. Next, the sections were incubated for 30 min at room temperature with a SuperPicture Polymer Detection System (Invitrogen, Carlsbad, CA). The sections were incubated with a 3,3′-diamino-benzidine (DAB) chromogen system (Dako America Inc., Carpinteria, CA) for 1.5 min at room temperature and then counterstained with hematoxylin. Between incubations, the slides were rinsed three times for 5 min with PBS.
Fields from each section were captured at 400X magnification by means of the Image Analysis System Quantimet 500+ (Leica Microsystems, Cambridge, UK).
Results
Under PTX-LDE treatment, none of the participant patients showed clinical or laboratorial toxicities that could be ascribed to the treatment. In all 74 performed chemotherapy cycles, myelotoxicity, documented by platelet, leukocyte and red blood cell counting, was absent. Hypersensitivity reactions typical of the standard, commercial PTX formulations, including vasomotor changes and pulmonary symptoms, as well as hepatic and renal toxicity and alopecia also did not appear. During the treatment, all patients showed well-being and performance status >80%. In all patients, treatment withdrawals were decided on the basis of disease progression and in none because of intolerability to treatment.
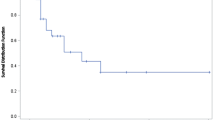
Table 1 shows the data of PFS of the fourteen study subjects submitted to the PTX-LDE treatment. Among them, 4 patients (28%) showed PFS higher than 6 months and two showed PFS higher than 1 year. The median PFS was 3.0 months (95% CI 2.0–3.9) (range 2–14 months).
Figure 1 shows images of immunohistochemistry stained LDL receptors in ovarian carcinoma, and adjacent tumor infiltration areas from tissues fragments excised in the former debulking surgery patients were submitted.
Immunohistochemistry images of LDL receptors (LDLR) and LDL receptor-related protein 1 (LRP-1) obtained in the primary ovarian carcinoma tissues and adjacent tumor infiltration areas. a: peritoneal tissue of patient 3. b: intestinal segment of patient 4. c–e: right ovary, right uterine tube and great epiploon, respectively, of patient 7. f–h: right ovary, right uterine tube and omentum, respectively, of patient 11. ×400
Discussion
In this study, we showed the intense presence of LDL receptors in the tumor sites, as obtained by immunohistochemistry from four of the study patients (patients number 3, 4, 7 and 11), confirming the basic mechanism of LDE uptake by the neoplastic cells that allows the concentration of the nanoparticles in the tumor sites. In fact, not only the LDL receptors but also de LRP-1 receptors were also intensively present; both receptors have the capability of internalizing native LDL [28, 29] and PTX-LDE. This indicates that the several-fold greater uptake of LDE by the tumor than by the normal ovarian tissue previously documented in women with ovarian carcinoma [18] was indeed due to the upregulation of the lipoprotein receptors in the neoplastic tissues. This study is the first to document the status of lipoprotein receptors in ovarian carcinoma.
The toxicity of the treatment is a major concern when considering third-line chemotherapy for ovarian carcinoma. At this stage, the feeble condition of the patients and the prospects of poor response to the currently available treatments do not encourage their use in view of the expected low cost–benefit. In regard to toxicity, the results of this study, in which PTX-LDE was used, were indeed unpreceded. As administered at the usual, 175 mg/m2 dose, to the 14 patients, totaling 74 chemotherapy cycles, this formulation presented no observable clinical or laboratorial toxicities. Therefore, the toxicity of PTX is no longer an important contraindication or limiting factor for its use when associated with LDE as third-line treatment.
The toxicities of standard PTX formulations, in which polyoxyethylated castor oil is used as vehicle, consist mainly in myelotoxicity, specially neutropenia [30], as well as mucositis [31] and cardiotoxicities such as ventricular arrhythmias, bradycardia and conduction blocks [32]. Hypersensitivity reactions such as dyspnea, urticaria and arterial hypotension are related to the vehicle rather to PTX [33]. Some other preparations have been developed and tested in clinical trials based on drug delivery systems such as liposomes [34], albumin macro-aggregates [35] and polymer nanoparticles [36]. Treatment of patients with those preparations resulted in diminution of the toxicities related to polyoxyethylated castor oil, but the toxicities related to PTX drug were still present [37, 38].
In respect to the toxicity of third-line chemotherapy schemes for ovarian cancer described in the literature, Sabbatini et al. [39] reported the results of 74 patients treated with PTX poliglumex. This formulation consists of PTX carried in polymeric nanoparticles and was administered at 175 mg/m2 and at 235 mg/m2 PTX dose levels. They observed grade III and IV neutropenia, gastrointestinal toxicity and neuropathy. Eleven patients were removed from the study for toxicity. Bodnar et al. [40] reported that among 11 patients treated with daily 800 mg oral sorafenib, a multitargeted kinase inhibitor, one showed grade III hematological toxicity. Grade I and II acne (7/11), mucositis (5/11), diarrhea (3/11), arthritis (1/11) and gingivorrhagia (1/11) were also observed, and hand–foot syndrome occurred in eight patients. Pignata et al. [41] observed 37 patients treated with the combination of daily pazopanib (800 mg) and PTX (80 mg/m2 every 28 days) and 36 patients receiving PTX alone (80 mg/m2 every 28 days). In the group treated with the combined scheme, grade III and IV hematological toxicity occurred in 11 patients as neutropenia, in four patients as leucopenia and in two patients as anemia. Non-hematological toxicity occurred in four patients as fatigue, in three patients as hypertension. Three patients had raised aspartate or alanine aminotransferase. In the group treated with the PTX alone scheme, hematological toxicity occurred in one patient as neutropenia and leucopenia, and in five patients as anemia. Non-hematological toxicity occurred only in two patients as fatigue. Hong et al. [42] tested gemcitabine and vinorelbine as second- or third-line therapy in 44 patients with either ovarian or primary peritoneal carcinomas. Grade III or IV neutropenia was observed in 22 patients and 15 needed immediate dose reduction. Two patients were excluded because of toxicity. Nausea and vomiting and fatigue were observed in roughly half of the patients. In some of the above-mentioned studies [39, 42], patients treated under second-line setting were also included, together with third-line treatments. This is of note since in second-line the toxicity would conceivably be lower than in third-line schemes.
Described as above, regarding the toxicity of the treatment, there was thus a clear-cut superiority of the PTX-LDE monotherapy over the previous treatments tested in the third-line setting. It is worthwhile to point out that treatment as in patients number 2, 5, 6 and 9 could be sustained for periods longer than the cumulative toxicities of the conventional chemotherapeutic agents could have allowed.
The remarkable reduction in toxicity obtained by the use of LDE as PTX carrier can be accounted not only for the concentration of the drug in the tumor sites while diminishing the uptake by the normal tissues. The association with LDE leads to profound change in biodistribution and pharmacokinetics of the drug [37]. Together with the sequestration of the drug into the nanoparticles that avoids contact with the blood and other circulating fluids, the new biodistribution is the major determinant of the toxicity reduction.
PFS obtained from the 14 patients enrolled in this study was 3 months, which is not essentially different from PFS obtained in the studies dealing with other third-line treatments [39, 40, 42]. The data of third-line of the study by Bodnar et al. [40] showed that the PFS of the eleven patients treated in third line with sorafenib was 2 months and in none of the patients PFS was longer than 6 months. In the study by Bruchim et al. [43] in which 63 patients were treated in third line with a multiplicity of chemotherapeutic agents and protocols, including topotecan, PTX, carboplatin and oral etoposide, the median PFS was 6 weeks. In the study by Hong et al. [42], in which 19 patients received third-line treatment with vinorelbine and gemcitabine, the median PFS was 3.3 months and no patients had PFS longer than 6 months. In the study by Sabbatini et al. [39] enrolling 49 patients treated in third line with PTX poliglumex, the median PFS was 2.8 months and also in that study none of the patients had PFS longer than 6 months. Thus, in none of those studies, PFS exceeded 6 months. In contrast, in the current study, in four of our 14 patients (28%) the PFS exceeded 6 months, which is a remarkable outcome, especially considering that in two patients PFS exceeded 1 year.
In the above-mentioned studies, drugs tested in third-line setting had not been included in the first- or second-line chemotherapy. It is thus worthwhile to point out that all the patients enrolled in the current study had already been treated with standard PTX at the first-line scheme. The subsequent progression of the disease suggests that they have become resistant to PTX therapy. In this respect, previous PTX exposure induces drug resistance by multidrug complex and also induces the rise of PTX-resistant tubulin mutants [44]. Therefore, the longer PFS observed in the four patients might suggest that PTX-LDE had somehow the ability to overcome PTX resistance. It is also possible that if PTX had not been used in the first- and second-line schemes, the PFS would be longer in our patients.
The absence of clinical and laboratorial toxicity observed here makes certainly possible to considerably increase the dose of PTX carried in LDE aiming to increase PFS with still good tolerability to the treatment. In this regard, in a study enrolling patients with advanced breast cancer, PTX-LDE at the 250 mg/m2/3/3 week dose lacked observable toxicity [unpublished results]. Of note was the fact that in the previous studies mentioned here [39, 42], some patients were withdrawn or had the drug dose reduced due to toxicity. In the current study, it was otherwise possible to continuously treat patients number 5 and 6 for more than 1 year without noticeable signs of cumulative toxicity.
In conclusion, our data clearly support the use of PTX-LDE as third-line monochemotherapy for ovarian cancer. The results also suggest that PTX-LDE can be eligible for clinical trials at first- or second-line setting in combined chemotherapy. The lack of observable toxicity at the usual, 175 mg/m2 dose, makes room for the use of higher doses of PTX-LDE aiming to further improve the anticancer action of the drug.
References
Siegel RL, Miller KD, Jemal A. Cancer statistis 2017. CA Cancer J Clin. 2017;67:7–30.
Engel J, Eckel R, Schubert-Fritschle G, Kerr J, Kuhn W, Diebold J, et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer. 2002;38:2435–45.
Heintz AP, Odicino F, Maisonneuve P, Beller U, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. J Epidemiol Biostat. 2001;6:107–38.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29.
Covens A, Carey M, Bryson P, Verma S, Fung Kee Fung M, Johnston M. Systematic review of first-line chemotherapy for newly diagnosed postoperative patients with stage II, III, or IV epithelial ovarian cancer. Gynecol Oncol. 2002;85:71–80.
Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106.
Latorre A, De Lena M, Catino A, Crucitta E, Sambiasi D, Guida M, et al. Epithelial ovarian cancer: second and third line chemotherapy. Int J Oncol. 2002;21:179–86.
Gubbi A, Kendrick JE, Finkler NJ. The role of bevacizumab in recurrent, platinum-sensitive ovarian cancer. Expert Rev Anticancer Ther. 2014;14:1105–13.
McLachlan J, Banerjee S. Olaparib for the treatment of epithelial ovarian cancer. Expert Opin Pharmacother. 2016;17:995–1003.
Chiyoda T, Tsuda H, Nomura H, Kataoka F, Tominaga E, Suzuki A, et al. Effects of third-line chemotherapy for women with recurrent ovarian cancer who received platinum/taxane regimens as first-line chemotherapy. Eur J Gynaecol Oncol. 2010;31:364–8.
Nishio S, Katsumata N, Matsumoto K, Tanabe H, Yonemori K, Kouno T, et al. Usefulness of third-line chemotherapy for women with recurrent ovarian, fallopian tube, and primary peritoneal cancer who receive platinum/taxane regimens as first-line therapy. J Cancer Res Clin Oncol. 2009;135:551–7.
Liang XJ, Chen C, Zhao Y, Wang PC. Circumventing tumor resistance to chemotherapy by nanotechnology. Methods Mol Biol. 2010;596:467–88.
Jabir NR, Tabrez S, Ashraf GM, Shakil S, Damanhouri GA, Kamal MA. Nanotechnology-based approaches in anticancer research. Int J Nanomed. 2012;7:4391–408.
Maranhão RC, Garicochea B, Silva EL, Llacer PD, Pileggi FJ, Chamone DA. Increased plasma removal of microemulsions resembling the lipid phase of low-density lipoproteins (LDL) in patients with acute myeloid leukemia: a possible new strategy for the treatment of the disease. Braz J Med Biol Res. 1992;25:1003–7.
Ho YK, Smith RG, Brown MS, Goldstein JL. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous cells. Blood. 1978;52:1099–114.
Brown MS, Goldstein JL. How LDL receptors influence cholesterol and atherosclerosis. Sci Am. 1984;251:58–66.
Ades A, Carvalho JP, Graziani SR, Amancio RF, Souen JS, Pinotti JA, et al. Uptake of a cholesterol-rich emulsion by neoplastic ovarian tissues. Gynecol Oncol. 2001;82:84–7.
Graziani SR, Igreja FA, Hegg R, Meneghetti C, Brandizzi LI, Barboza R, et al. Uptake of a cholesterol-rich emulsion by breast cancer. Gynecol Oncol. 2002;85:493–7.
Dias ML, Carvalho JP, Rodrigues DG, Graziani SR, Maranhão RC. Pharmacokinetics and tumor uptake of a derivatized form of paclitaxel associated to a cholesterol-rich nanoemulsion (LDE) in patients with gynecologic cancers. Cancer Chemother Pharmacol. 2007;59:105–11.
Feio DCA, de Oliveira NCL, Pereira ELR, Morikawa AT, Muniz JAPC, Montenegro RC, et al. Organic effects of associating paclitaxel with a lipid-based nanoparticle system on a nonhuman primate, Cebus apella. Int J Nanomed. 2017;12:3827–37.
Pires LA, Hegg R, Valduga CJ, Graziani SR, Rodrigues DG, Maranhão RC. Use of cholesterol-rich nanoparticles that bind to lipoprotein receptors as a vehicle to paclitaxel in the treatment of breast cancer: pharmacokinetics, tumor uptake and a pilot clinical study. Cancer Chemother Pharmacol. 2009;63:281–7.
Azevedo CH, Carvalho JP, Valduga CJ, Maranhão RC. Plasma kinetics and uptake by the tumor of a cholesterol-rich microemulsion (LDE) associated to etoposide oleate in patients with ovarian carcinoma. Gynecol Oncol. 2005;97:178–82.
Pinheiro KV, Hungria VT, Ficker ES, Valduga CJ, Mesquita CH, Maranhão RC. Plasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin’s and non-Hodgkin’s lymphoma and a preliminary study on the toxicity of etoposide associated with LDE. Cancer Chemother Pharmacol. 2006;57:624–30.
Maranhão RC, Graziani SR, Yamaguchi N, Melo RF, Latrilha MC, Rodrigues DG, et al. Association of carmustine with a lipid emulsion: in vitro, in vivo and preliminary studies in cancer patients. Cancer Chemother Pharmacol. 2002;49:487–98.
Hungria VT, Latrilha MC, Rodrigues DG, Bydlowski SP, Chiattone CS, Maranhão RC. Metabolism of a cholesterol-rich microemulsion (LDE) in patients with multiple myeloma and a preliminary clinical study of LDE as a drug vehicle for the treatment of the disease. Cancer Chemother Pharmacol. 2004;53:51–60.
Rodrigues DG, Maria DA, Fernandes DC, Valduga CJ, Couto RD, Ibañez OC, et al. Improvement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studies. Cancer Chemother Pharmacol. 2005;55:565–76.
Strickland DK, Kounnas MZ, Argraves WS. LDL receptor related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–8.
Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Investig. 2001;108:779–84.
Maier-Lenz H, Hauns B, Haering B, Koetting J, Mross K, Unger C, et al. Phase I study of paclitaxel administered as a 1-hour infusion: toxicity and pharmacokinetics. Semin Oncol. 1997;24(6 Suppl 19):S19-16–S19-19.
Raber-Durlacher JE, Weijl NI, Abu Saris M, de Koning B, Zwinderman AH, Osanto S. Oral mucositis in patients treated with chemotherapy for solid tumors: a retrospective analysis of 150 cases. Support Care Cancer. 2000;8:366–71.
Arbuck SG, Strauss H, Rowinsky E, Christian M, Suffness M, Adams J, et al. A reassessment of cardiac toxicity associated with Taxol. J Natl CancerInst Monogr. 1993;15:117–30.
Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from Taxol. J Clin Oncol. 1990;8:1263–8.
Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23:3319–29.
Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Deliv. 2015;12:793–812.
Krishnamurthy S, Vaiyapuri R, Zhang L, Chan JM. Lipid-coated polymeric nanoparticles for cancer drug delivery. Biomater Sci. 2015;3:923–36.
Maranhão RC, Vital CG, Tavoni TM, Graziani SR. Clinical experience with drug delivery systems as tools to decrease the toxicity of anticancer chemotherapeutic agents. Expert Opin Drug Deliv. 2017;1:1–10.
Waite CL, Roth CM. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit Rev Biomed Eng. 2012;40:21–41.
Sabbatini P, Sill MW, O’Malley D, Adler L, Secord AA. Gynecologic Oncology Group Study. A phase II trial of paclitaxel poliglumex in recurrent or persistent ovarian or primary peritoneal cancer (EOC): a Gynecologic Oncology Group Study. Gynecol Oncol. 2008;111:455–60.
Bodnar L, Górnas M, Szczylik C. Sorafenib as a third line therapy in patients with epithelial ovarian cancer or primary peritoneal cancer: a phase II study. Gynecol Oncol. 2011;123:33–6.
Pignata S, Lorusso D, Scambia G, Sambataro D, Tamberi S, Cinieri S, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16:561–8.
Hong SH, Lee S, Kim HG, Lee HJ, Jung KH, Lee SC, et al. Phase II study of gemcitabine and vinorelbine as second- or third-line therapy in patients with primary refractory or platinum-resistant recurrent ovarian and primary peritoneal cancer by the Korean Cancer Study Group (KCSG)_KCSG GY10-10. Gynecol Oncol. 2015;136:212–7.
Bruchim I, Jarchowsky-Dolberg O, Fishman A. Advanced (> second) line chemotherapy in the treatment of patients with recurrent epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2013;166:94–8.
Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–95.
Acknowledgements
Dr Maranhão has a 1A Research Career Award from the National Council for Scientific and Technological Development (CNPq, Brasília, Brazil). The authors are grateful to Ms. Debora F. Deus and Lucy A. de Almeida for their help with the experiments.
Funding
This study was funded by the State of São Paulo Research Support Foundation (FAPESP, São Paulo, Brazil) [Grant Number 2014/03742-0].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Graziani, S.R., Vital, C.G., Morikawa, A.T. et al. Phase II study of paclitaxel associated with lipid core nanoparticles (LDE) as third-line treatment of patients with epithelial ovarian carcinoma. Med Oncol 34, 151 (2017). https://doi.org/10.1007/s12032-017-1009-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1009-z