Abstract
Gemcitabine in combination with low-dose cisplatin has shown promising activity in pancreatic cancer with manageable toxicities. The purpose of this study is to assess the activity of a combination of gemcitabine and low-dose cisplatin in the first-line treatment of metastatic and locally advanced pancreatic cancer patients. We conducted a retrospective analysis of all patients diagnosed with metastatic and locally advanced pancreatic cancer who received a combination of gemcitabine and cisplatin in the first-line setting. Patients with baseline cytopenias and elevated liver function tests were included. Patients received cisplatin at 20 mg per square meter followed by gemcitabine at a dose of 1000 mg per square meter at fixed dose rate every 2 weeks. Patients were treated until disease progression or unacceptable toxicities. A total of 58 patients were included in the analysis. The median progression-free survival was 4.4 months [95 % confidence interval (CI) 3.6–6.4], and median overall survival was 6.7 months (95 % CI 4.4–10.9). Thirty-eight patients (66 %) experienced at least one grade 3 or 4 toxicity. The most common grade 3 or 4 toxicity was hematologic toxicity (25 patients, 43 %). Biweekly fixed dose rate gemcitabine combined with low-dose cisplatin shows interesting activity in advanced pancreatic cancer. This regimen is an acceptable alternative for patients ineligible for gemcitabine plus nab-paclitaxel (i.e., those with elevated bilirubin at baseline) or clinical trials. Additionally, this regimen should be considered as a first-line option for those patients with breast cancer susceptibility gene mutations (BRCA1 and/or BRCA2).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the USA with a 5-year survival of <5 % [1]. Until recently, the standard of care for patients with metastatic or locally advanced, unresectable disease was single-agent gemcitabine yielding a median overall survival (mOS) of 5.7 months and median progression-free survival (mPFS) of 3.7 months [2]. Several trials with gemcitabine-based combinations had failed to produce any significant benefit over gemcitabine alone [3, 4]. However, a meta-analysis by Heinemann et al. in 2008 reported survival advantage of certain gemcitabine combinations (namely gemcitabine–fluoropyrimidine or gemcitabine–platinum) over monotherapy in the subset of patients with a good performance status (PS) [3]. Based on this analysis, the standard of care for good PS patients with metastatic or unresectable pancreatic cancer at our institution became gemcitabine in combination with low-dose cisplatin.
In 2011, Conroy et al. published results from the phase III PRODIGE trial showing a significant survival advantage utilizing the three-drug combination of FOLFIRINOX (fluorouracil, irinotecan, oxaliplatin) when compared to single-agent gemcitabine (mOS = 11.1 months vs. 6.8 months, respectively). Grade 3 or 4 toxicities that were significantly higher with this three-drug combination compared to gemcitabine alone included neutropenia (45.7 %), thrombocytopenia (9.1 %), diarrhea (12.7 %) and sensory neuropathy (9 %) [5]. These toxicities limit the use of FOLFIRINOX at our institution to the neoadjuvant setting. Finally, phase III data from the MPACT trial, published in 2013, showed a significant increase in median OS using nab-paclitaxel in combination with gemcitabine versus gemcitabine alone (mOS = 8.5 months vs. 6.7 months, respectively, p < 0.001). These results have now established the combination of gemcitabine and nab-paclitaxel as the new standard of care for first-line treatment of metastatic pancreatic cancer [6].
One subset of patients of particular interest for the use of platinum and more specifically cisplatin includes those who harbor mutations in the breast cancer susceptibility genes (BRCA1 and/or BRCA2). These mutations are present in approximately 5 % of patients with pancreatic cancer [7]. The BRCA proteins are involved in the repair of DNA double-strand breaks, allowing treatment of pancreatic tumors to garner more benefit from DNA-damaging agents such as platinum-based chemotherapy [8, 9]. This hypothesis is supported by several studies [8–10].
The purpose of our study is to assess the efficacy and safety of gemcitabine and low-dose cisplatin in the first-line setting in patients with metastatic or locally advanced pancreatic cancer.
Materials and methods
Study design
This is a retrospective analysis to assess the mPFS in metastatic and locally advanced, unresectable pancreatic cancer patients treated with a biweekly combination of gemcitabine and cisplatin in the first-line setting between January 1, 2010, and December 31, 2012. All patients received treatment in our gastrointestinal (GI) oncology clinic at The Arthur G. James Cancer Hospital and Richard J. Solove Research Institute at The Ohio State University. The institutional review board approved the study. For this type of study, formal consent is not required.
Eligibility criteria
Patients were required to be between the ages of 18 and 89 years, have locally advanced, unresectable or metastatic pancreatic cancer and have received first-line combination chemotherapy with gemcitabine and cisplatin in either the inpatient or outpatient setting. Patients were excluded if they had received previous chemotherapy for metastatic pancreatic cancer (prior adjuvant gemcitabine was allowed).
Treatment
Due to the favorable toxicity profile and efficacy in the locally advanced setting, it has been the practice at The James to utilize gemcitabine as a fixed dose rate, biweekly infusion in combination with low-dose cisplatin [10]. The dosing of cisplatin and gemcitabine in our institutional regimen is as follows: cisplatin at 20 mg per square meter over 60 min followed by gemcitabine at a dose of 1000 mg per square meter over 100 min (fixed dose rate or FDR) every 2 weeks.
Study assessments
Patients were seen prior to each treatment, and toxicities were assessed based on the Common Terminology Criteria for Adverse Events (CTCAE, version 4) and recorded prospectively in the patients’ medical records by a chemotherapy-trained nurse. These were verified by a clinical oncology pharmacist and subsequently reviewed by a GI medical oncologist. Further, any grade 3 or higher toxicities were discussed prior to therapy with the GI medical oncologist. Patients were assessed for tumor response by computed tomography (CT scans) every 8 weeks by a GI diagnostic radiologist and reviewed by the GI medical oncologist according to Response Evaluation Criteria in Solid Tumours (RECIST) [15].
Sample size
We anticipated that our patient population would have had a 49 % increase in PFS versus the historical control of gemcitabine alone (5.5 vs. 3.7 months, respectively). Using a single-arm parametric exponential model with alpha equal to 0.025, 58 patients have 80 % power to detect this difference, should it exist.
Statistical analysis
Median PFS and OS (defined as the time from start of treatment to documented progression and death, respectively) are reported using the methods of Kaplan–Meier. Cox proportional hazard regression was used to determine whether any patient demographics or clinical characteristics were associated with PFS. These characteristics are summarized using frequencies and percentages for categorical variables, while means and standard deviations or medians and the interquartile range (IQR) summarize continuous variables depending on their distribution. In addition, summary statistics describe the safety and toxicity profile of this population. Data collection included: gender, age, race, baseline performance status, pancreatic primary location (head, body, tail), baseline site of metastases, number of metastatic sites, prior surgery (Whipple), biliary stent status, CA 19-9 levels (baseline and during treatment), chemotherapy regimen, duration of chemotherapy treatment, time to progression (days), second-line therapy, time to death (days), chemotherapy-related toxicities, dose reductions/delays and growth factor use. All analyses were performed using Stata 12.1 or a higher version, Stata Corporation, College Station, TX.
Results
Patient characteristics
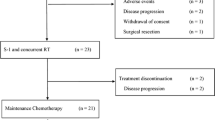
A total of 79 patients were identified that had received gemcitabine in combination with cisplatin for pancreatic cancer during the study period. A total of 58 patients met all inclusion criteria. The most common reasons for exclusion were receipt of previous chemotherapy for advanced disease (11 patients) and prior exposure to this regimen in the neoadjuvant setting (five patients). Baseline characteristics are presented in Table 1. Of note, 18 patients (31 %) had biliary stents and 11 patients (19 %) had received prior adjuvant treatment within 6 months. In addition, two patients (3 %) had baseline cytopenias (defined as ANC <1500, platelet count <100,000 and hemoglobin <9), while 13 patients (22 %) had baseline hepatic dysfunction (defined as ALT/AST >2.5 times upper limit of normal (ULN), alkaline phosphatase >5 times ULN or total bilirubin >ULN).
Efficacy
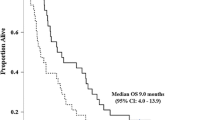
The survival analysis was based on 52 deaths (90 %) and 49 patients (84 %) with confirmed progression of disease. Median progression-free survival was 4.4 months (95 % CI 3.6–6.4) (Fig. 1a), and median overall survival was 6.7 months (95 % CI 4.4–10.9) (Fig. 2a). The median duration of treatment was 3.67 months, and patients received a median of four cycles of chemotherapy. Interestingly, one patient with known BRCA mutation received over 30 cycles of treatment and did not experience progression at the time of data cutoff over 3 years after starting treatment.
Kaplan–Meier curves for progression-free survival (PFS). a All patients; b patients that received no adjuvant treatment within 6 months, no baseline cytopenias (defined as ANC >1500, platelet count >100,000 and hemoglobin >9) and no baseline hepatic dysfunction (defined as ALT/AST <2.5 times upper limit of normal (ULN), alkaline phosphatase <5 times ULN and total bilirubin within normal limits); c patients that received adjuvant treatment within 6 months or the presence of baseline cytopenias or hepatic dysfunction; d patients that received adjuvant treatment within 6 months; e patients with baseline cytopenias or hepatic dysfunction
Kaplan–Meier curves for overall survival (OS). a All patients; b patients that received no adjuvant treatment within 6 months, no baseline cytopenias (defined as ANC >1500, platelet count >100,000 and hemoglobin >9) and no baseline hepatic dysfunction (defined as ALT/AST <2.5 times upper limit of normal (ULN), alkaline phosphatase <5 times ULN and total bilirubin within normal limits); c patients that received adjuvant treatment within 6 months or the presence of baseline cytopenias or hepatic dysfunction; d patients that received adjuvant treatment within 6 months; e patients with baseline cytopenias or hepatic dysfunction. NR not reached
Second-line therapy
Twenty-four patients (41 %) went on to receive second-line chemotherapy. The most common second-line chemotherapy was oxaliplatin plus a fluoropyrimidine (14 patients), while three patients received gemcitabine plus nab-paclitaxel following progression.
CA 19-9
CA 19-9 levels after two cycles of therapy were lower by 14 % at the median from baseline. Increases in CA 19-9 levels of ≥5 % after two cycles of chemotherapy have been previously shown to serve as a negative predictive marker [11]. Twenty-seven patients (47 %) had either a decrease in CA 19-9 levels with treatment or it remained stable (<5 % increase).
Safety
Thirty-eight patients (66 %) experienced at least one grade 3 or 4 toxicity. The most common grade 3 or 4 toxicity was hematologic toxicity (25 patients, 43 %) including 13 patients with grade 3 or 4 neutropenia, six patients with grade 3 or 4 thrombocytopenia and 17 patients with grade 3 or 4 anemia. The most common grade 3 or 4 non-hematologic toxicities were fatigue (15 patients, 26 %), decreased performance status (12 patients, 21 %) and elevations in liver function tests (LFTs) (10 patients, 17 %) including elevated ALT in four patients, elevated AST in three patients, elevated alkaline phosphatase in five patients and elevated bilirubin in eight patients. Five patients had grade 2 or higher anemia or thrombocytopenia at baseline, and four patients had grade 3 or higher LFT elevations at baseline. Eighteen patients (31 %) required dose reductions and 27 patients (47 %) required dose delays during treatment. Interestingly, none of the patients experienced grade 3 or 4 neuropathy during treatment.
Discussion
Gemcitabine monotherapy has long been the standard of care for patients with locally advanced or metastatic pancreatic cancer [2]. New data have now emerged demonstrating survival advantage with gemcitabine plus nab-paclitaxel, making it the new standard of care for these patients [6]. When assessing the stringent inclusion and exclusion criteria used in the MPACT trial, at least 40 % (23/58) of patients included in our study would never be candidates for gemcitabine plus nab-paclitaxel. Therefore, a regimen that maintains efficacy with acceptable toxicities is needed for these patients.
In the current study, efficacy was comparable to previously published trials of FDR gemcitabine plus cisplatin for metastatic pancreatic cancer [12–14]. Although our initial power calculations assumed a PFS of 5.5 months (similar to gemcitabine and nab-paclitaxel), we believe that given the baseline characteristics of our patient population, a mPFS of 4.4 months is encouraging and historically better than gemcitabine alone. In this palliative treatment setting, safety data were also found to be reasonable with mainly hematologic toxicities that have been manageable.
The patient population in our study included a number of patients with baseline hepatic dysfunction and/or cytopenias that otherwise would be ineligible for alternate established therapies. Additionally, eleven patients received adjuvant treatment within 6 months prior to treatment for advanced disease, a group that is typically excluded from large randomized trials because of their poor prognosis. These results support the benefits of such a regimen in patients who otherwise do not qualify for current established therapies.
Additionally, the toxicity profile of gemcitabine in combination with low-dose cisplatin is relatively favorable when accounting for our patient population and historical data [2, 5, 6].
Our data align with findings of the previously published meta-analysis by Heinemann et al. [3] in which gemcitabine-platinum combinations provided benefit in patients with good PS. Additionally, the efficacy found in our study is similar to that seen in the previously published trial of FDR gemcitabine in combination with low-dose cisplatin for advanced pancreatic cancer conducted by Ko et al. [13]. Furthermore, there is added convenience of our regimen compared to other established therapies given the biweekly administration schedule. This extra week off during each cycle allows cost savings ($134 for one cycle of FDR gemcitabine plus cisplatin vs. $183 for one cycle of gemcitabine alone).
Our study has a number of limitations and biases (including selection bias) given its retrospective nature. Additionally, this study included a relatively small sample size. Nonetheless, our results were consistent with historical controls.
Conclusions
Patients with locally advanced or metastatic pancreatic cancer have limited treatment options and an overall poor prognosis. Biweekly FDR gemcitabine plus low-dose cisplatin does show activity in this setting. It is encouraging that this regimen demonstrated similar survival endpoints across all subgroups and similar or less toxicities than other combination chemotherapy. While this is not an equally viable option for these patients, it is a complementary option for patients ineligible for clinical trials and gemcitabine plus nab-paclitaxel. Furthermore, this regimen should be considered for all patients with elevated bilirubin at baseline and/or mutated breast cancer susceptibility genes (BRCA1 and/or BRCA2).
References
American Cancer Society. Cancer facts & figures 2013. Atlanta: American Cancer Society; 2013.
Burris H, Moore M, Anderson J, et al. Improvements in survival and clinical benefit with gemcitabine as first line therapy for patients with advanced pancreas cancer. A randomized trial. J Clin Oncol. 1997;15:2403–13.
Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82.
Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma. Cancer. 2002;94:902–10.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Holter S, Borgida A, Dodd A, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol. 2015;33(28):3124–9.
Vyas O, Leung K, Ledbetter L, et al. Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation. Anticancer Drugs. 2015;26(2):224–6.
Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111(6):1132–8.
Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16(10):1397–402.
Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer. 2013;119(2):285–92.
Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21(18):3402–8.
Ko AH, Dito E, Schillinger B, et al. Phase II study of fixed dose rate gemcitabine with cisplatin for metastatic adenocarcinoma of the pancreas. J Clin Oncol. 2006;24(3):379–85.
Ko AH, Quivey JM, Venook AP, et al. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):809–16.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lundberg, J., Reardon, J., Blazer, M. et al. Biweekly gemcitabine and low-dose cisplatin in the treatment of locally advanced or metastatic pancreatic cancer patients: a single institute experience. Med Oncol 33, 4 (2016). https://doi.org/10.1007/s12032-015-0720-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0720-x