Abstract
Currently there is no predictor for survival after adjuvant sorafenib in patients with hepatocellular carcinoma (HCC) who have undergone curative resection. Thirty-eight patients who underwent curative resection of HCC received adjuvant sorafenib therapy between August 2009 and March 2012. Clinicopathological parameters including patient factors, tumor factors, liver background, and inflammatory factors (before surgery and dynamic changes after sorafenib therapy) were evaluated to identify predictors for overall survival (OS) and recurrence-free survival (RFS). The recurrence rate, mortality rate, and clinicopathological data were also compared. Increased NLR after sorafenib (HR = 3.199, 95 % CI 1.365–7.545, P = 0.008), increased GGT after sorafenib (HR = 3.204, 95 % CI 1.333–7.700, P = 0.009), and the presence of portal vein thrombosis (HR = 2.381, 95 % CI 1.064–5.328, P = 0.035) were risk factors related to RFS. By contrast, increased NLR after sorafenib was the only independent risk factor related to OS (HR = 4.647, 95 % CI 1.266–17.053, P = 0.021). Patients with increased NLR or increased GGT after sorafenib had a higher incidence of recurrence and death. Patients who had increased NLR tended to have higher preoperative levels of NLR and GGT. There were no differences in clinicopathological factors in patients with increased GGT and decreased GGT. In conclusion, increased NLR predicted a worse OS and RFS in patients with HCC who underwent curative resection with adjuvant sorafenib therapy. Increased GGT predicted a worse OS. NLR and GGT can be monitored dynamically before and after sorafenib therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the second most frequent cause of cancer-related death, and hepatocellular carcinoma (HCC) represents a major histological subtype, accounting for 70–85 % of the total liver cancer burden worldwide [1, 2]. Surgical resection is the main treatment modality for long-term survival, but the recurrence rate is very high and remains the main challenge to achieving long-term survival [3]. To date, there is no effective adjuvant therapy after liver resection. Sorafenib is the only systemic drug that has been proven to improve survival in patients with advanced HCC [4–7], but a interim analysis of a phase III randomized study evaluating the efficacy of adjuvant sorafenib after curative resection showed no survival benefit [8, 9]. The reason for the negative results of sorafenib is that about 30 % of patients in the sorafenib arm had discontinued treatment at 3 months, and almost half by 1 year, which translated into a shorter treatment duration and a lower actual daily dose in the sorafenib arm as compared with the placebo arm. We previously found that adjuvant sorafenib after curative resection of HCC prolonged overall survival (OS) [10]. However, there is no biomarker with which to predict the prognosis for patients with HCC who are receiving adjuvant sorafenib.
Parameters of the tumor and the background liver are mostly studied as predictors for survival, but increasing evidence has supported the role of systemic inflammation as a predictor of outcome in several human cancers including HCC [11]. As parameters of systemic inflammation, the Glasgow prognostic score [12], the inflammation-based index (IBI), the neutrophil-to-lymphocyte ratio (NLR) [13], C-reactive protein (CRP), and gamma-glutamyl transferase (GGT) are of value in determining the prognosis of different cancers [14, 15]. An ideal predictor should be easy to examine and allow dynamic monitoring, and a combination of parameters would have better prognostic value [16]. The current authors are interested in NLR and GGT because they are included in routine laboratory examinations of patients with HCC and they can be monitored dynamically. Preoperative NLR is reported to predict survival after liver resection in patients with HCC [17], and the preoperative level of NLR predicts survival for intermediate HCC treated with sorafenib and TACE [18]. Furthermore, several studies have found that increased postoperative changes in NLR, rather than preoperative NLR, predict a poorer survival in patients with HCC after curative resection [19, 20]. GGT activity has been widely used to evaluate the activity of chronic hepatitis, and recent studies have shown that GGT is an early marker of sub-clinical inflammation and oxidative stress in otherwise healthy individuals [21, 22]. Several studies of HBV or HCV-related HCC have suggested that high levels of GGT are related to a higher incidence of HCC recurrence and poor prognosis [14, 15]. Moreover, several studies have revealed that GGT is closely related to C-reactive protein, another parameter with which to evaluate systemic inflammation [23–25].
The current study aims to explore the predictors for survival, including dynamic changes in NLR and GGT, in patients who received adjuvant sorafenib after curative resection of HCC.
Materials and methods
Patients and follow-up
Subjects were 38 patients who had undergone curative resection at a single center (Tianjin Medical University Cancer Institute and Hospital) and who had received adjuvant sorafenib therapy after curative resection for HCC from August 1, 2009, to March 28, 2012. Curative resection was defined as complete removal of the tumor without microscopic residual tumors and a lack of tumors 1 month postoperatively. HCC was diagnosed by two independent pathologists (Cao WF and Zhan ZL). Relevant clinical data, including patient history, laboratory results, tumor characteristics, and follow-up data, were prospectively recorded. The primary end points were recurrence-free survival (RFS) and OS; the secondary end points were the recurrence rate and mortality rate. After surgery, 38 patients were found to have unfavorable pathological factors indicating a higher risk of recurrence (including multiple tumor nodules, presence of portal vein thrombosis, and presence of intrahepatic metastasis) received adjuvant sorafenib therapy. These patients were followed up at this clinic every month for the first year and then every 2 months for the rest of their lives. Liver function, alpha-fetoprotein (AFP), coagulation, routine blood results, and liver ultrasonography were routinely examined during patient follow-up. If recurrence was suspected, an enhanced magnetic resonance imaging (MRI) or enhanced computed tomography scan (CT) was immediately performed. Treatment with sorafenib was approved by the Ethics Committee of this Hospital.
Clinicopathological factors and definition of post-sorafenib changes in NLR/GGT
Clinicopathological factors included the following: age (<51 or ≥51 years, median age 51 years), gender (male or female), body surface area (<1.85 or ≥1.85 m2, median 1.85), preoperative AFP (<20 or ≥20 ng/ml), preoperative NLR (<2.24 or ≥2.24, mean value 2.24), preoperative GGT (≥40 vs. <40 μ/l, 40 μ/l is the upper limit for normal value of GGT), cirrhosis (yes or no), HBsAg (positive or negative), tumor size (<5 cm or ≥5 cm), number of tumor nodules (single or multiple), intrahepatic metastasis (yes or no), portal vein thrombosis (yes or no), and tumor differentiation (Edmondson’s classification I/II vs. III/IV). Systemic inflammatory factors included preoperative NLR, preoperative GGT, increased NLR, and increased GGT.
NLR and GGT were examined before surgery, 1 week before commencement of sorafenib, and 1 month after administration of sorafenib (Fig. 1). If post-sorafenib NLR minus pre-sorafenib NLR > 0, the change in NLR after sorafenib therapy was defined as “increased NLR.” If post-sorafenib NLR minus pre-sorafenib NLR < 0, the change in NLR after sorafenib was defined as “decreased NLR.” “Increased GGT” and “decreased GGT” after sorafenib therapy were defined in the same way. None of the patients had no changes whatsoever in NLR or GGT after sorafenib therapy.
Sorafenib therapy and evaluation
All the patients receiving sorafenib were given an initial dosage of 400 mg twice daily continuously, except in cases where the drug was discontinued due to death, tumor progression, or severe adverse effects. Blood pressure, stool characteristics, coagulation, liver function, and routine blood results were monitored every month. Neutrophils and lymphocytes were routinely examined in a routine blood test, while GGT was routinely examined in a liver function test. If adverse events classified as CTCAE grade 3 or higher occurred, the dosage of sorafenib was reduced or administration of sorafenib was discontinued.
Statistical analysis
Survival was calculated using the Kaplan–Meier method and the log-rank test for univariate analysis. Cox regression was used for multivariate analysis, and factors with P < 0.05 in univariate analysis were included in the multivariate analysis. OS was calculated from the date of surgery to the date of death or the date of the last follow-up. RFS was calculated from the date of surgery to the date of recurrence or the date of the last follow-up. The Chi-square test, Fisher’s exact probability, and Student’s t test were used to compare groups. Statistical analyses were performed with SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). A two-tailed P < 0.05 was considered to indicate a significant difference.
Results
Patient demographics, liver background, and tumor characteristics
The demographic data, information on liver background, and tumor characteristics of all patients are shown in (Supplementary Table 1 insert ‘in ESM’). The mean age was 51 years with a male/female ratio of 30:8. Twenty-six patients tested positive for HBsAg, and 12 tested negative. All patients had good Eastern Cooperative Oncology Group performance status scores (PS: 0–1) before commencement of sorafenib therapy, and all patients had liver function that was classified as Child–Pugh A. Thirteen patients had increased NLR and 25 patients had decreased NLR after sorafenib therapy, while 11 patients had increased GGT and 27 patients had decreased GGT after sorafenib therapy. Mean tumor size was 6.3 cm, and all patients had stage II/III tumors, with portal vein thrombosis in 12 patients and intrahepatic metastasis in 21 patients (Supplementary Table 1 insert ‘in ESM’). The median follow-up period was 28.6 months. For all the patients, the 1-, 2-, and 3-year OS was 86.5, 71, and 40 %, while the 1-, 2-, 3-year RFS was 73.7, 39.5, and 15 %, respectively.
Univariate and multivariate analyses of risk factors related to RFS and OS
Univariate analysis revealed that risk factors related to RFS included preoperative GGT ≥ 40, increased NLR after sorafenib, increased GGT after sorafenib, multiple tumors, and the presence of portal vein thrombosis. Preoperative NLR ≥ 2.24 was not a risk factor related to RFS in univariate analysis (P = 0.266). Multivariate analysis revealed that increased NLR after sorafenib (HR = 3.199, 95 % CI 1.365–7.545, P = 0.008), increased GGT after sorafenib (HR = 3.204, 95 % CI 1.333–7.700, P = 0.009), and the presence of portal vein thrombosis (HR = 2.381, 95 % CI 1.064–5.328, P = 0.035) were risk factors related to RFS (Table 1).
Univariate analysis revealed that risk factors related to OS included increased NLR after sorafenib, increased GGT after sorafenib, and the presence of cirrhosis. Neither preoperative NLR ≥ 2.24 nor preoperative GGT ≥ 40 were risk factors related to OS in univariate analysis (P = 0.416 and P = 0.165, respectively). Multivariate analysis revealed that only increased NLR after sorafenib was an independent risk factor related to OS (HR = 4.647, 95 % CI 1.266–17.053, P = 0.021). However, increased GGT after sorafenib therapy was only marginally significant (HR = 3.156, 95 % CI 0.964–10.336, P = 0.058; Table 2).
Comparisons of RFS for patients with increased NLR and patients with increased GGT
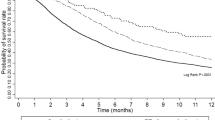
The 1- and 2-year RFS was 56 and 31.3 % for patients with decreased NLR and 15.4 and 0 % for patients with increased NLR, respectively. Median RFS was 6 months (95 % CI 4.4–7.6 months) for patients with increased NLR and 16 months (95 % CI 8.7–23.3 months) for the patients with decreased NLR (P < 0.001, Fig. 2a).
Survival based on changes in NLR and changes in GGT after sorafenib therapy. a, b Recurrence-free survival (RFS): a RFS for increased NLR and decreased NLR; b RFS for increased GGT and decreased GGT; c, d Overall survival (OS): c OS for increased NLR and decreased NLR; d OS for increased GGT and decreased GGT
The 1- and 2-year RFS was 55.6 and 21.4 % for patients with increased GGT and 0.91 and 0 % for patients with decreased GGT, respectively. Median RFS was 5 months (95 % CI 1.8–8.2 months) for patients with increased GGT and 14 months (95 % CI 6.1–21.9 months) for patients with decreased GGT (P < 0.001, Fig. 2b).
Comparisons of OS for patients with increased NLR and GGT
The 1-, 2-, and 3-year OS was 96, 86.8, and 52.6 % for patients with decreased NLR and 67.7, 38.7, and 0 % for patients with increased NLR, respectively. Median OS was 15 months (95 % CI 8.9–21.1 months) for patients with increased NLR and was greater than that for patients with decreased NLR (P = 0.001, Fig. 2c).
The 1-, 2-, and 3-year OS was 92.4, 80, and 62.5 % for patients with decreased GGT and 71.6, 47.7, and 23.9 % for patients with increased GGT, respectively. Median OS was 18 months (95 % CI 19.2–46.8 months) for patients with increased GGT and was greater than that for patients with decreased GGT (P = 0.019, Fig. 2d).
Mortality rate and recurrence rate in patients with increased NLR and GGT
Preoperative NLR ≥ 2.24 had no effect on the mortality rate (50 vs. 29.6 %, P = 0.221) and recurrence rate (90.9 vs. 74.1 %, P = 0.209). All patients with increased NLR had tumor recurrence with a recurrence rate of 100 %, but patients with decreased NLR had a recurrence rate of only 65 % (P = 0.022). The mortality rate also differed significantly between patients with increased NLR and patients with decreased NLR (61.5 vs. 20 %, P = 0.010; Table 3).
Similarly, all patients with increased GGT had tumor recurrence during follow-up, but patients with decreased GGT had a recurrence rate of only 70.3 % (P = 0.042). However, the difference in the mortality rate was only marginally significant (54.5 vs. 25.9 %, P = 0.092).
Comparison of clinicopathological factors in patients with increased NLR and patients with decreased NLR
Comparison of clinicopathological characteristics in terms of post-sorafenib changes in NLR revealed no significant difference in most clinicopathological factors for patients with increased NLR (or GGT) and decreased NLR (or GGT) after sorafenib therapy. The sole exception was a higher preoperative NLR and higher preoperative GGT between increased NLR and decreased NLR (Tables 4, 5).
Discussion
The current results indicate that OS and RFS were worse in patients who had increased NLR or increased GGT during adjuvant sorafenib therapy after curative resection for HCC, and the recurrence rate and mortality rate were also higher in these patients.
NLR has been studied as an indicator of prognosis in multiple cancers including HCC. However, most studies emphasized pretreatment NLR, whereas only a few focused on dynamic changes in NLR. Ohno et al. [27] reported that pre- and post-treatment NLR had a prognostic role in patients with non-metastatic and metastatic renal cell carcinoma (mRCC) who underwent radical nephrectomy and they also noted the association between postoperative NLR and RFS. Dynamic changes in NLR are of prognostic value in either resected small HCC or HCC treated with RFA [19, 20, 26]. Postoperative changes in NLR are hypothesized to reflect dynamic changes in the host’s inflammatory response and immune response before and after surgery. If NLR increases after RFA or resection, this indicates that the balance has tipped in favor of a pro-tumor inflammatory response; otherwise, the balance has tipped in favor of an anti-tumor immune response. In this regard, postoperative changes in NLR could affect survival differently. The clinical utility of such systemic inflammation-based predictors could be increased if they were shown to have prognostic value over time and after therapeutic intervention. Systemic reviews have pointed out that the cutoff point for preoperative NLR varies widely between different institutions, but a change in NLR can be measured by all institutions, allowing further studies to be compared. The present study is the first to explore the predictive value of post-sorafenib changes in NLR and changes in GGT in patients with HCC who are receiving adjuvant sorafenib therapy.
The relationship between NLR and changes in NLR and prognosis has also been reported in patients with kidney cancer undergoing molecular target therapy. Keizman et al. [28] have published the results of a retrospective analysis of patients with mRCC who were treated with sunitinib as a first-line therapy. In that study, a low NLR ≤ 3 (HR = 0.285, P = 0.001) was significantly associated with PFS, whereas a low NLR ≤ 3 was associated with OS (HR = 0.3, P = 0.043). Furthermore, Kobayashi et al. [29] revealed that changes in NLR during the early phase of targeted therapy may be a powerful signal of who will benefit from subsequent treatment with molecular-targeted therapy. They found that lower pretreatment NLR was associated with longer PFS in patients treated with sorafenib or sunitinib [29].
The current study found that increased post-sorafenib NLR was significantly associated with worse PFS and OS in patients with HCC who received adjuvant sorafenib therapy after curative resection. Many studies have shown that microenvironment factors are very important in tumor recurrence and metastasis after liver resection [30], and microenvironment factors are also one of the main reasons for sorafenib resistance [31–33]. Increased macrophages, which are a main component of the microenvironment of a tumor, usually predict a poor prognosis for HCC and other cancers. An elevated NLR has been associated with an increase in peritumoral infiltration of macrophages and an increase in interleukin-17 (IL-17) [34]. A preclinical study has found that sorafenib significantly induced increased expression of chemokines for monocytes/macrophages including CSF-1, VEGF, and SDF-1α and increased monocytes/macrophages in the tumor microenvironment as well as in the host, thus contributing to sorafenib resistance [35]. Furthermore, clinical studies have also shown that progression-free survival (PFS) following sorafenib therapy is significantly shorter in patients with high levels of chemokines for monocytes/macrophages including angiogenin-2, granulocyte colony-stimulating factor (G-CSF), and hepatocyte growth factor (HGF), and that the treatment response diminishes as the number of cytokines present at high concentrations increases [36]. In an adjuvant setting, elevation of NLR after sorafenib may reflect inflammation in the host and tumor microenvironment that may increase tumor metastasis. Furthermore, patients with a high risk of recurrence tend to have minimal residual disease after hepatectomy, so severe inflammation may facilitate a “metastatic niche” allowing the growth of residual or circulating tumor cells.
The clinical takeaway of the current study is that a patient’s inflammatory and immune status might be modulated by “re-educating” or reducing neutrophils or increasing lymphocytes. Recent research in a mouse model has indicated that modulating neutrophil functions from pro-tumorigenic to anti-tumorigenic can be accomplished with TGF-β inhibition [37]. This would provide the basis for interventions to treat systemic inflammation.
The current study has several limitations. First, it was a retrospective study at a single center. Prospective studies should be conducted to confirm the present findings in a larger population. Second, the OS and RFS were relatively worse because only patients with risk factors indicating susceptibility to recurrence received adjuvant sorafenib therapy. Finally, dynamic changes in other parameters such as PLR, Glasgow score, CRP, and albumin should be combined with NLR to further improve the predictive ability of these factors.
Conclusion
Increased NLR and increased GGT predicted worse OS and RFS in patients with HCC who underwent curative resection and later adjuvant sorafenib therapy. NLR and GGT should be monitored before and after adjuvant sorafenib therapy.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Tang ZY, Ye SL, Liu YK, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96.
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Chen J, Gao J. Advances in the study of molecularly targeted agents to treat hepatocellular carcinoma. Drug Discov Ther. 2014;8:154–64.
Zhong Y, Liu B, Deng M, Xu R. Adjuvant systemic drug therapy and recurrence of hepatocellular carcinoma following curative resection. Drug Discov Ther. 2013;7:164–6.
Printz C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM). Cancer. 2009;115:4646.
Wang Z, Zhang G, Wu J, Jia M. Adjuvant therapy for hepatocellular carcinoma: current situation and prospect. Drug Discov Ther. 2014;7:137–43.
Zhang W, Zhao G, Wei K, et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. BioScience Trends. 2014;8:333–8.
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–63.
Pinato DJ, Stebbing J, Ishizuka M, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012;57:1013–20.
Morimoto M, Numata K, Moriya S, et al. Inflammation-based prognostic score for hepatocellular carcinoma patients on sorafenib treatment. Anticancer Res. 2012;32:619–23.
Ju MJ, Qiu SJ, Fan J, et al. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for Child–Pugh A hepatocellular carcinoma after operation. J Gastroenterol. 2009;44:635–42.
Wang Z, Song P, Xia J, Inagaki Y, Tang W, Kokudo N. Can gamma-glutamyl transferase levels contribute to a better prognosis for patients with hepatocellular carcinoma? Drug Discov Ther. 2014;8:134–8.
Balta S, Demirkol S, Unlu M, Arslan Z, Celik T. Neutrophil to lymphocyte ratio may be predict of mortality in all conditions. Br J Cancer. 2013;109:3125–6.
Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–5.
Wei K, Wang M, Zhang W, Mu H, Song TQ. Neutrophil–lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma undergoing TAE combined with Sorafenib. Med Oncol. 2014;31:969.
Peng W, Li C, Wen TF, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res. 2014;192:402–8.
Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS ONE. 2013;8:e58184.
Bo S, Gambino R, Durazzo M, et al. Associations between gamma-glutamyl transferase, metabolic abnormalities and inflammation in healthy subjects from a population-based cohort: a possible implication for oxidative stress. World J Gastroenterol. 2005;11:7109–17.
Song P, Feng X, Zhang K, et al. Screening for and surveillance of high-risk patients with HBV-related chronic liver disease: promoting the early detection of hepatocellular carcinoma in China. Biosci Trends. 2014;7:1–6.
Lee YJ, Kim JK, Lee JH, Lee HR, Kang DR, Shim JY. Association of serum gamma-glutamyltransferase with C-reactive protein levels and white blood cell count in Korean adults. Clin Chem Lab Med. 2008;46:1410–5.
Saijo Y, Utsugi M, Yoshioka E, et al. The relationship of gamma-glutamyltransferase to C-reactive protein and arterial stiffness. Nutr Metab Cardiovasc Dis. 2008;18:211–9.
Lee DH, Jacobs DR Jr. Association between serum gamma-glutamyltransferase and C-reactive protein. Atherosclerosis. 2005;178:327–30.
Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553–61.
Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187:411–7.
Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48:202–8.
Kobayashi M, Kubo T, Komatsu K, et al. Changes in peripheral blood immune cells: their prognostic significance in metastatic renal cell carcinoma patients treated with molecular targeted therapy. Med Oncol. 2013;30:556.
Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–16.
Sun Y, Campisi J, Higano C, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–68.
Seton-Rogers S. Tumour microenvironment: means of resistance. Nat Rev Cancer. 2013;13:607.
Masuda S, Izpisua Belmonte JC. The microenvironment and resistance to personalized cancer therapy. Nat Rev Clin Oncol. 2013;10:64.
Motomura T, Shirabe K, Mano Y, et al. Neutrophil–lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64.
Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–30.
Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–27.
Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94.
Acknowledgments
This project was supported by a Grant from the National Natural Science Foundation of China (No. 81101871 & No.81372635), a Grant from the Tianjin Municipal Science and Technology Commission (TSTC, No.14JCYBJC25200), and a Grant from the Major Programs of the National Natural Science Foundation of Tianjin (No. 11JCZDJC18800). Grants were also received from the Program for a New Generation of Exceptional Personnel of Tianjin Medical University Cancer Hospital and the Tianjin Medical University Cancer Institute and Hospital of the National Clinical Research Center for Cancer.
Conflict of interest
All authors declare that they do not have a commercial or other association that might pose a conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, W., Zhao, G., Wei, K. et al. Adjuvant sorafenib therapy in patients with resected hepatocellular carcinoma: evaluation of predictive factors. Med Oncol 32, 107 (2015). https://doi.org/10.1007/s12032-015-0549-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0549-3