Abstract
PinX1 induces apoptosis and suppresses cell proliferation in some cancer cells, and the expression of PinX1 is frequently decreased in some cancer and negatively associated with metastasis and prognosis. However, the precise roles of PinX1 in gliomas have not been studied. In this study, we found that PinX1 obviously reduced the gliomas cell proliferation through regulating the expressions of cell cycle-relative molecules to arrest cell at G1 phase and down-regulating the expression of component telomerase reverse transcriptase (hTERT in human), which is the hardcore of telomerase. Moreover, PinX1 could suppress the abilities of gliomas cell wound healing, migration and invasion via suppressing MMP-2 expression and increasing TIMP-2 expression. In conclusion, our results suggested that PinX1 may be a potential suppressive gene in the progression of gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most commonly diagnosed malignancies and contribute to most of the deaths among all the brain and central nervous system cancers, and in 2014, nearly 22,810 new cases were diagnosed worldwide [1, 2]. As the most aggressive type of gliomas, glioblastoma multiform (GBM) is the grade IV histological malignancy according to the WHO classification [3]. A series of treatments for GBM, including the tumor resection, radiotherapy, chemotherapy, as well as inhibiting signaling pathways and immunotherapy have been employed; however, the survival of GBM is still less than 12 months [4–6]. The main cause of death for GBM patients is its rapid proliferation and distinctly infiltrating to the adjacent normal brain tissues [7], and increased studies have demonstrated that the progression of gliomas is correlated with the abnormal gene expressions [3]; therefore, it is urgent for us to search and discover the effective biomarkers, which are associated with the disordered cell cycle and invasion of gliomas cells.
PinX1, a conserved nuclear protein, can directly interact with the telomerase catalytic component hTERT and inhibit telomerase activity [8–10], and the spontaneous incidence of malignant tumors in the PinX1 knockout heterozygous mice (PinX1 ±) is significantly increased [11]. These evidences suggest that PinX1 is an endogenous telomerase inhibitor and a potential tumor suppressor. Moreover, series of studies about PinX1 in cancers have demonstrated that the expression of PinX1 in cancer tissues is frequently down-regulated when compared to the normal tissues, and reduced PinX1 expression is associated with increased tumor cell metastasis and poor prognosis in some cancers, including gastric cancer [12, 13], prostate cancer [14] and ovarian cancer [15]. PinX1 over-expression in some cancer cells induces apoptosis and suppresses cell proliferation [16, 17]. However, the precise role of PinX1 in gliomas cell is still unclear so far.
In this study, we investigated the association of PinX1 expression with gliomas cell proliferation, migration and invasion. Simultaneously, we also further studied the probable mechanisms of PinX1 regulating gliomas cell proliferation, migration and invasion.
Methods
Cell lines, plasmids and the transient transfections
Human glioblastoma cell lines U87 and U251 were purchased from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. Cells were cultured in DMEM medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 10 % fetal bovine serum. The cells were grown at 37 °C in the presence of 5 % CO2 in a humidified incubator.
The pEGFP-C3 and pEGFP-C3-PinX1 plasmids were obtained from Dr. Xiao-Fen Lai (Southern Medical University, Guangzhou, China), and all the constructs were confirmed before using by DNA sequencing. Then, the pEGFP-C3-PinX1 and the pEGFP-C3 were transiently transfected into the U87 and U251 cells using Lipofectamine 2000 transfection reagent (Invitrogen) following the manufacturer’s protocol.
Western blot and antibodies
Western blot was performed as described before [18]. And PinX1 (1:200, Novus Biologicals); MMP-2 (1:1000; Cell Signaling Technology); TIMP-2 (1:200, Santa Cruz); β-actin (1:1000; Cell Signaling Technology); cyclin D1 (1:1000, Cell Signaling Technology); cyclin E (1:1000, Cell Signaling Technology); p27 (1:1000, Cell Signaling Technology); and hTERT (1:1000, Cell Signaling Technology) were applied as the primary antibodies to the blot incubation at 4 °C overnight. The signals were detected with Odyssey Infrared Imaging system (LI-COR).
Cell growth assay
Cell growth was analyzed using a WST-8 Cell Counting Kit-8 (Beyotime, Nantong, China). The 100-microliter complete DMEM medium including 5 × 103 U251 and U87 cells was seeded in 96-well plate and cultured for 1, 2, 3 and 4 days. At the exact time, ten-microliter cell counting CCK-8 solution was added to each well and incubated at 37 °C for 1 h. Then, the absorbance at 450 nm was measured on an ELX-800 spectrometer reader (Bio-Tek Instruments, Winooski, USA).
Cell cycle analysis
The U87 and U251 cells transfected with pEGFP-C3 and pEGFP-C3-PinX1 for 36 h were treated with 1 μg/ml aphidicolin for 12 h, and then the medium was removed. The cell was rinsed with PBS and then incubated in the fresh medium containing 50 ng/ml nocodazole for 0, 3 and 6 h. Then, the cells were fixed with 70 % ethanol at 4 °C overnight and stained with 40 μg/ml propidium iodide in hypotonic fluorochrome buffer (0.1 % Triton X-100, 0.1 % sodium citrate and 25 μg/ml RNase A) for 30 min. Samples were then analyzed using a FACS Canto flow cytometer (BD Biosciences). Cell distribution in the different phases of the cell cycle was analyzed using ModFit LT3.0 software.
Cell migration and invasion assays
The migration and invasion assays were carried out using a modified two-chamber plate with a pore size of 8 μm. The transwell filter inserts were coated with or without Matrigel for the cell migration and invasion assays, respectively. 1 × 105 cells were seeded in serum-free medium in the upper chamber. After incubation for 24 h at 37 °C, cells in the upper chamber were carefully removed with a cotton swab and the cells that had traversed the membrane were fixed in methanol, stained with crystal violet (0.04 % in water; 100 μl), and the permeating cells were counted under the inverted microscope and photographed.
Statistical analysis
All the statistical analyses were performed by STATA statistical software (version 10.1; StataCorp, College Station, TX). The Student’s t test was used to evaluate the significance, a P value of < 0.05 was deemed statistically significant, and all tests were two-sided.
Results
Over-expression of PinX1 reduced cell proliferation in gliomas cells
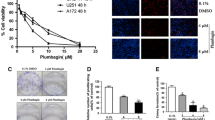
Accumulated studies have showed that PinX1 over-expression in some types of cancer cells can suppress proliferation [16, 17]; however, the precise roles of PinX1 in gliomas cell proliferation are still not explored. In order to test the role of PinX1 expression in gliomas cell growth, we over-expressed PinX1 in U87 and U251 cells (Fig. 1a), respectively, and then, the CCK-8 assays were used to detect the gliomas cell proliferation. Our data indicated that the abilities of cell growth were drastically decreased after PinX1 over-expression when compared to the respective controls in these two cell lines (Fig. 1b, c, P < 0.05 in these two cell lines).
PinX1 reduced cell proliferation in gliomas cells. a Western blot was used to test the PinX1 expression levels after gliomas cells were transfected by GFP-CON and GFP-PinX1 plasmids. b, c The CCK-8 assays were performed after PinX1 over-expression in U87 and U251 cells. Data were presented as mean ± SD, *P < 0.05, Student’s t test
PinX1 suppressed gliomas cell growth by regulating cell cycle-relative biomarkers and hTERT in gliomas cells
Since cell proliferation is tightly controlled by the cell cycle, we next investigated whether PinX1-reduced cell proliferation was due to the change of cell cycle. After cells were incubated in the fresh medium containing 50 ng/ml nocodazole for 3 and 6 h, the data of flow cytometer showed that the G1 population was significantly increased in these two cell lines with PinX1 over-expression when compared to the corresponding control cells (Fig. 2a−d, P < 0.05 in these two cell lines). Studies have indicated that PinX1 controls the expression of hTERT and cyclin-CDK complexes in the process of suppressing cell growth [17, 19, 20]. Therefore, Western blot was used to explore the expressions of hTERT and the cell cycle-relative biomarkers. Our results showed that PinX1 negatively regulated hTERT, Cyclin D and Cyclin E expression, whereas positively regulated p27 expression (Fig. 3).
PinX1 arrested cell cycle in gliomas cells. a, c The representative pictures of cell cycle in U87 and U251 cells with PinX1 over-expression and controls. b, d The percentages of G1 population cells were calculated in U87 and U251 cells with the different PinX1 expression levels. Data were presented as mean ± SD, *P < 0.05, Student’s t test
PinX1 decreased cell migration and invasion in gliomas cells
Previous evidences about cancers have indicated that low expression of PinX1 is associated with metastasis and poor prognosis in some cancers [12–15], and the diffusion of gliomas cells to adjacent tissues is another aggressive phenotype of gliomas [7]. In this study, we further investigated the role of PinX1 in gliomas migration and invasion. The results of wound-healing assays showed that when compared to the corresponding controls, PinX1 over-expression significantly lowered the speeds of gliomas cell migration by 19 and 22 % in U87 and U251 cells, respectively, after 24 h of wound healing (Fig. 4a, b, P < 0.05 in these two cell lines). The cell transwell assays were used to go on validating the finding in wound-healing assay. Our data went on revealing that the average numbers of migration were decreased by 51 % and 45 % in U87 and U251 cells with PinX1 over-expression in comparison with the relative controls, respectively (Fig. 5a, b, P < 0.05 in these two cell lines), which was accordant with the former results. Moreover, the results of the cell invasion assays also showed that the abilities of invasion were reduced by 45 % and 55 % in these two cell lines with PinX1 over-expression in comparison with their controls (Fig. 5c, d, P < 0.05 in these two cell lines).
PinX1 inhibited the speeds of gliomas cell wound healing. a, c The representative pictures of wound healing in U87 and U251 cells with PinX1 over-expression and controls. b, d The percentages of wound healing after 24 h were calculated in U87 and U251 cells with the different PinX1 expression levels. Data were presented as mean ± SD, *P < 0.05, Student’s t test
PinX1 suppressed the gliomas cell migration and invasion. a, c The representative pictures of migration and invasion in U87 and U251 cells with PinX1 over-expression and controls. b, d The number of cell migration and invasion per field were counted in five random fields for PinX1 over-expressing and control groups (n = 3/group) (B, D). Data were presented as mean ± SD, *P < 0.05, Student’s t test
PinX1 regulated the expression of MMP-2 and TIMP-2 to control the gliomas cell migration and invasion
Because MMP-2 can degrade ECM components to make the cancer cell expanding without resistance, whereas TIMP-2 is a specific inhibitor against MMP-2 [21, 22], MMP-2 and TIMP-2 play important roles in mediating the cell metastasis. Here, we validated that PinX1 could regulate the expressions of MMP-2 and TIMP-2 in these two gliomas cells. Our results demonstrated that when compared to the respective corresponding controls, expression of MMP-2 was vastly down-regulated in U87 and U251 cells after PinX1 over-expression; in contrast, the expression of TIMP-2 was dramatically up-regulated in both gliomas cells after PinX1 over-expression, (Fig. 6).
Discussion
Studies have revealed that the changed expressions of some oncogenes and tumor suppressor genes are associated with the aggressive phenotypes and progression of gliomas [3]. The expression of PinX1 is frequently reduced in some human cancers and negatively associated with the poor outcome of the cancer patients [13, 15, 16, 23]. Moreover, the suppressive role of PinX1 in regulating some cancer cell proliferation via the inhibition of telomerase activity and cell cycle-relative molecules has been confirmed [17, 20], and one typical malignant feature of gliomas is the infinite growth [7]. Therefore, in this study, firstly, we investigated the exact role of PinX1 in gliomas proliferation and the relevant mechanisms. Our data demonstrated that over-expression of PinX1 in gliomas cells significantly decreased the cell proliferation through arresting cell cycle at G1 phase. Many researchers have reported that cyclin-CDK complexes, such as cyclin D1 and cyclin E, drive the progression of the cell cycle, whereas Cip/Kip proteins, such as p27, by binding to cyclin-CDKs, suppress the process of the cell cycle [19]. Our results indicated that PinX1 negatively regulated the expression of cyclin D1 and cyclin E, whereas positively associated with p27 expression, which was in accordance with the results in bladder urothelial carcinoma cell [17]. In addition, studies have showed that PinX1 suppresses cell immortalization by inhibiting the telomerase activity, which is controlled by hTERT expression [17, 20, 24]. Our data also validated that PinX1 restrained hTERT expression and therefore was responsible for gliomas proliferation [25].
Another aggressive characteristic of gliomas is their extensive invasion [7, 26, 27], and some data of retrospective cancer patient cohorts have suggested that PinX1 expression is negatively correlated with the lymph node metastasis [13, 15, 16, 23]. Here, we investigated the effect of PinX1 in gliomas invasion. Our results showed that PinX1 over-expression significantly inhibited the wound-healing abilities of gliomas cells. Simultaneously, in the migration and invasion assays of gliomas cells, we also found that PinX1 remarkably reduced the capabilities of gliomas cell migration and invasion. As we all know, the process that glioma cells escape from the original site and expand into the surrounding normal brain parenchyma is involved in a diverse array of biological incidents [28]. Number of studies have shown that MMP-2 degrades ECM components and plays a key role in tumor cells migrating and infiltrating smoothly to the neighbor tissues, whereas TIMP-2 has the inhibitory role against MMP-2 to suppress the cancer cell migration and invasion [21, 22]. Therefore, we checked the expressions of MMP-2 and TIMP-2 in gliomas cells with PinX1 over-expression. Our results displayed that PinX1 could negatively regulate MMP-2 expression, whereas it was positively related to the expression of TIMP-2, which also validated the findings that MMP-2 and TIMP-2 have been proved to perform an important role in gliomas aggression [29, 30].
In summary, PinX1 over-expression could obviously reduce the gliomas cell proliferation through the retardation of cell cycle at G1 phase and suppress the abilities of gliomas cell migration and invasion via down-regulating MMP-2 expression and up-regulating TIMP-2 expression. Combined with the previous reports of PinX1 about cancer, these results suggested that PinX1 may play a suppressive role in the progression of gliomas. However, the exact molecular mechanism that PinX1 regulates MMP-2, TIMP-2 and cell cycle protein expression still needs to be studied.
References
Ostrom QT, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):1–56.
Goodenberger ML. RB Jenkins. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–21.
Urbanska K, et al. Glioblastoma multiforme - an overview. Contemp Oncol (Pozn). 2014;18(5):307–12.
Mrugala MM. Advances and challenges in the treatment of glioblastoma: a clinician’s perspective. Discov Med. 2013;15(83):221–30.
Sorensen AG, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–7.
Specenier P. Bevacizumab in glioblastoma multiforme. Expert Rev Anticancer Ther. 2012;12(1):9–18.
Karcher S, et al. Different angiogenic phenotypes in primary and secondary glioblastomas. Int J Cancer. 2006;118(9):2182–9.
Zhou XZ, Lu KP. The Pin2/TRF1-interacting protein PinX1 is a potent telomerase inhibitor. Cell. 2001;107(3):347–59.
Yoo JE, Park YN, Oh BK. PinX1, a telomere repeat-binding factor 1 (TRF1)-interacting protein, maintains telomere integrity by modulating TRF1 homeostasis, the process in which human telomerase reverse Transcriptase (hTERT) plays dual roles. J Biol Chem. 2014;289(10):6886–98.
Yonekawa T, Yang S, Counter CM. PinX1 localizes to telomeres and stabilizes TRF1 at mitosis. Mol Cell Biol. 2012;32(8):1387–95.
Zhou XZ, et al. The telomerase inhibitor PinX1 is a major haploinsufficient tumor suppressor essential for chromosome stability in mice. J Clin Invest. 2011;121(4):1266–82.
Kondo T, et al. Loss of heterozygosity and histone hypoacetylation of the PINX1 gene are associated with reduced expression in gastric carcinoma. Oncogene. 2005;24(1):157–64.
Ma Y, et al. The correlation of genetic instability of PINX1 gene to clinico-pathological features of gastric cancer in the Chinese population. J Cancer Res Clin Oncol. 2009;135(3):431–7.
Shi R, et al. Reduced expression of PinX1 correlates to progressive features in patients with prostate cancer. Cancer Cell Int. 2014;14:46.
Cai MY, et al. Decreased expression of PinX1 protein is correlated with tumor development and is a new independent poor prognostic factor in ovarian carcinoma. Cancer Sci. 2010;101(6):1543–9.
Zhang R, et al. PinX1 without the G-patch motif suppresses proliferation, induces senescence, but does not inhibit telomerase activity in colorectal cancer SW480 cells. Oncol Rep. 2014;32(1):286–92.
Liu JY, et al. PinX1 suppresses bladder urothelial carcinoma cell proliferation via the inhibition of telomerase activity and p16/cyclin D1 pathway. Mol Cancer. 2013;12(1):148.
Bai J, et al. JWA regulates melanoma metastasis by integrin alphaVbeta3 signaling. Oncogene. 2010;29(8):1227–37.
Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18(8):851–5.
Zuo J, et al. Expression and mechanism of PinX1 and telomerase activity in the carcinogenesis of esophageal epithelial cells. Oncol Rep. 2013;30(4):1823–31.
Chaudhary AK, et al. Matrix metalloproteinase and its drug targets therapy in solid and hematological malignancies: an overview. Mutat Res. 2013;753(1):7–23.
Libra M, et al. Uterine cervical carcinoma: role of matrix metalloproteinases (review). Int J Oncol. 2009;34(4):897–903.
Wan SM, et al. Silencing of the hPOT1 gene by RNA inference promotes apoptosis and inhibits proliferation and aggressive phenotype of gastric cancer cells, likely through up-regulating PinX1 expression. J Clin Pathol. 2011;64(12):1051–7.
Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat Rev Mol Cell Biol. 2006;7(7):484–94.
Zhang B, et al. Anthracyclines disrupt telomere maintenance by telomerase through inducing PinX1 ubiquitination and degradation. Oncogene. 2012;31(1):1–12.
Nakada M, et al. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64(4):458–78.
Cuddapah VA, et al. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15(7):455–65.
Nie E, et al. beta-Catenin is involved in Bex2 down-regulation induced glioma cell invasion/migration inhibition. Biochem Biophys Res Commun. 2014.
Roomi MW, et al. Modulation of uPA, MMPs and their inhibitors by a novel nutrient mixture in human glioblastoma cell lines. Int J Oncol. 2014;45(2):887–94.
Singh MK, et al. T11TS inhibits glioma angiogenesis by modulation of MMPs, TIMPs, with related integrin alphav and TGF-beta1 expressions. Tumour Biol. 2014;35(3):2231–46.
Acknowledgments
This project is supported by grants from the National Natural Science Foundation of China (Nos. 81472663, 81201636), the Science and Technology Department of Jiangsu Province (No. BK2012139) and China Postdoctoral Science Foundation (No. 2014T70549, 2012M511323).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Peng-Jin Mei and Yan-Su Chen contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Mei, PJ., Chen, YS., Du, Y. et al. PinX1 inhibits cell proliferation, migration and invasion in glioma cells. Med Oncol 32, 73 (2015). https://doi.org/10.1007/s12032-015-0545-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0545-7