Abstract
The aim of this study was to evaluate the efficiency and safety of single-agent bevacizumab therapy for recurrent glioblastoma multiforme (GBM). We identified patients with histologically confirmed glioblastoma and World Health Organization Grade III glioma who were previously treated with temozolomide plus radiotherapy and received 10 mg/kg bevacizumab intravenous infusion every 2 weeks until disease progression for recurrent disease. A total 24 patients included to this study. Twenty-two patients had GBM, and two patients had WHO grade III glioma. No complete response was observed, five patients (20.8 %) had partial response, nine patients (37.5 %) had stable diseases, and ten patients (41.7 %) had progressive diseases. The overall response rate was 20.8 %. The 6-month PFS rate (PFS6) and median PFS were determined as 37.5 % and 4.1 months, respectively. Median OS was 6.4 months. Performance status of 17 (70.8 %) patients was improved following bevacizumab regimen. Univariate analysis showed that improvement in performance status (IPS) following bevacizumab therapy was a significant predictor of both PFS (p < 0.001) and OS (p < 0.020). Bevacizumab-related adverse effects were observed in 13 (54.1 %) patients. Grade 3–4 toxicity was observed in 4 (16.6 %) patients. Therapy interruptions were experienced in two patients due to adverse effects. Single-agent bevacizumab is an effective and safe treatment alternative in recurrent GBM. IPS following bevacizumab therapy was a significant predictor of both PFS and OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most common and the most aggressive primary brain tumor in adults. The average survival in new diagnosed GBM is around 15–19 months, and the 5-year survival rate is less than 10 % [1, 2]. The standard treatment in GBM is maximum safe surgical resection followed by radiotherapy and temozolomide (TMZ) with additional maintenance TMZ [3]. The prognosis is poor, after recurrence the median overall survival (OS) has been reported between 3 and 9 months in various publications [4]. There is no standard treatment after recurrence and the currently available treatments have limited efficiency [5].
Malignant gliomas are highly vascular tumors. Vascular endothelial growth factor (VEGF), an important regulator of angiogenesis, is associated with the pathologic vascularization in tumor development [6]. The high expression of VEGF in GBM has been shown to be correlated with poor prognosis and high-grade malignancy [7, 8]. Bevacizumab humanized monoclonal antibody developed against VEGF is being widely used in patients with various types of cancers including colorectal cancers, lung cancers [9, 10]. The limited number of phase 2 studies conducted in recent years report that bevacizumab increases overall response rate (ORR) and progression-free survival (PFS) when used alone or in combination with chemotherapy in GBM treatment [11–13]. However, the treatment decision is still difficult because these findings are not supported with phase 3 randomized trials and a few phase 2 studies with single-agent bevacizumab resulted similar to bevacizumab and irinotecan combination.
Given the current evidence for bevacizumab in recurrent GBM, we reviewed our single center experience regarding the efficacy and safety of single-agent bevacizumab in unselected Turkish patients with previously untreated recurrent GBM.
Materials and methods
We retrospectively reviewed our records at Trakya University Medical Oncology Department from between June 2009 and May 2014. This retrospective study was proved by the ethics committee of our center.
We included only the cases that met the following criteria: pathologically proven GBM (WHO Grade IV) or WHO grad III gliomas in first diagnosis or reoperation, first progression after standard temozolomide and radiotherapy treatment and a measurable lesion larger than 1 cm in pre-treatment imaging, received 10 mg/kg bevacizumab every 2 (q2) weeks as first-line treatment until disease progression, unacceptable toxicity, the patient refusal or physician decision. Review was conducted on the 24 patients those met the above criteria. Patient’s treatment, pathological characteristics, and outcomes were reviewed. Magnetic resonance images (MRI) with contrast were obtained every 8–12 weeks. Laboratory tests, including complete blood count, urine analysis, renal and liver function were assessed before every treatment cycle. Treatment was administered until progression or unacceptable toxicity. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for (CTCAE), version 4.0 [14].
Treatment response and progression assessment
Previously published response assessment in neuro-oncology (RANO) criterias, including parameters for changes in the T1-weighted gadolinium-enhancing lesion and non-enhancing T2/FLAIR progression, were used to assess treatment response [15].
Imaging was reviewed independently of clinical data to determine radiographic response and progression. Clinical progression, according to provider records, was also as criteria of progression. If a patient died due to presumed progressive disease in the absence of radiographic evidence of progression, the date of death was used as the date of progression. Improvement in performance status (IPS) was evaluated by using the Karnofsky performance score (KPS) based on the corresponding physician’s subjective evaluation of findings or the recorded patient disclosure. ORR was defined as sum of percentage of patients with partial and complete response rate according to response evaluation criteria that we used.
Statistical analysis
PFS was calculated from start of bevacizumab therapy to progression. PFS6 was defined as the percentage of patients alive and progression free at 6 months. OS was calculated from start of bevacizumab therapy to death or last follow-up. Progression-free survival and OS estimates were analyzed using Kaplan–Meier plots, factors related with PFS and OS compared with the log-rank test, and related 95 % confidence intervals (CIs) was also calculated. Survival curves were created using the software 20.0 (SPSS Inc, Chicago, IL). The safety analyses were performed by descriptive statistics. A p value <0.05 was considered statistically significant.
Results
We analyzed 24 patients (14 males, 10 females). Baseline characteristics of patients are summarized in Table 1. The median age at the diagnosis was 51 years (range 19–76 years). The median KPS prior to bevacizumab therapy was 70 (60–100).
The majority of patients (22; 91.6 %) had GBM, and other two patients had grade III glioma. The diagnoses of the other two patients were anaplastic oligodendroglioma and anaplastic astrocytoma. At time of GBM diagnosis, three patients (12.5 %) underwent biopsy and 21 patients (87.5 %) underwent surgery.
Median follow-up time was 21 (6–53) months. At the time of analysis, 21 (87.5 %) patients had died. The median PFS and OS from the first diagnosis were 13.9 (95 % CI 9.7–17.8) months and 22.9 (95 % CI 16.0–29.7) months, respectively.
No complete response was observed. Five patients (20.8 %) had partial response, nine patients (37.5 %) had stable diseases, and ten patients (41.7 %) had progressive diseases. One partial response and one progressive disease was observed in patients had grade III glioma. Deaths (four patients) during treatment before radiological evaluation accepted as death due to disease progression. The ORR with bevacizumab therapy was 20.8 %.
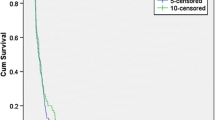
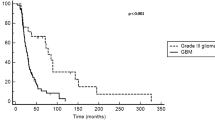
PFS6 was found 37.5 %, and median PFS was 4.1 months (95 % CI 2.8–5.5) (Fig. 1). The median OS was 6.4 months (95 % CI 5.0–7.8) (Fig. 2). Median survival after failure of bevacizumab was 1.7 months. IPS following bevacizumab therapy was observed in 17 (70.8 %) patients.
Univariate analysis showed that IPS following bevacizumab therapy was a significant predictor of both PFS (p < 0.001) and OS (p < 0.020). Age, gender, tumor localization, pre-bevacizumab-treatment KPS and the time from original diagnosis to recurrence were not related with PFS and OS (Table 2).
In general, bevacizumab therapy was well tolerated. Bevacizumab-related adverse effects (AEs) were observed in 13 (54.1 %) patients. Toxicities observed during the treatment are summarized in Table 3. The most common AE was hypertension, observed in five patients (20.8 %). While grade 4–5 toxicity was not observed in any patient, six (25.0 %) patients had grade 3 AEs of special interest to bevacizumab, comprising deep venous thrombosis in 2 (8.3 %) patients, hypertension in 2 (8.3 %) patients, leukopenia in 1 (4.1 %) patient and fatigue in 1 (4.1 %) patient. Bevacizumab treatment was discontinued in two patients because of bevacizumab-related AEs which was deep venous thrombosis.
Discussion
Angiogenesis is closely associated with tumor aggressiveness and prognosis as much as tumor development and survival [16]. Malignant gliomas are highly vascular tumors that produce VEGF [17]. Inhibition of angiogenesis by bevacizumab improves delivery of chemotherapy to tumor secondary to vascular normalization [18]. The promising results obtained in preliminary studies conducted with bevacizumab and irinotecan combination have led to wide use of this regimen in recurrent GBM [19–21]. Recently, three phase 2 studies have shown effectiveness of single-agent bevacizumab on ORR and PFS in recurrent GBM [11, 13, 22]. However, lack of subsequent phase 3 studies, different characteristics and low number of patients enrolled in phase 2 studies, and the clinical benefits that do not affect OS make it difficult to evaluate the effectiveness of the chemotherapy agents combined with bevacizumab in treatment of recurrent GBM. Currently, there are no widely accepted, effective chemotherapy alternatives for recurrent GBM.
This is the first retrospective analysis investigating the efficiency and safety of single-agent bevacizumab in recurrent GBM with an unselected Turkish patient population. The results of current study demonstrated that single-agent bevacizumab is an effective and safe treatment alternative in recurrent GBM. Moreover, performance status of the majority (70.8 %) of the patients improved during treatment. IPS was determined as a predictive factor for both PFS and OS. While PFS was higher among females than in males, there was no statistically significant difference between genders (mPFS: 6.2 vs. 3.8 months; p: 0.070).
The effectiveness of combinations of bevacizumab with various chemotherapy agents in recurrent GBM treatment has been evaluated in retrospective and prospective studies. The results were not found to be better than bevacizumab monotherapy. While in the combination chemotherapy studies ORR, PFS6 and OS had been reported as 20–57 %, 19–46.5 %, and 5.0–10.2 months [20, 23–29], respectively, in single-agent bevacizumab studies ORR, PFS6 and OS reported as 29–42 %, 25.0–42.6 %, and 6.5–10.5 months, respectively [12, 13, 22, 30]. In our study, ORR was 20.8 %, PFS6 was 37.5 % and OS was 6.7 months. Our results were in same direction with other published studies including single-agent bevacizumab or combination regimens. While ORR was lower than previous studies, our study had higher rates of stable disease with lower disease progression.
Bevacizumab was well tolerated. The incidence of adverse events associated with bevacizumab was 54.1 %. This rate was similar to those reported in previously published studies on single-agent bevacizumab use [11, 13, 22]. Friedman et al. [13] reported that incidence of grade 3 and higher adverse events were 46.4 % in single-agent bevacizumab arm, this rate was 65.8 % in the bevacizumab and irinotecan combination arm. In the same study, the rate of therapy interruption due to adverse events was 4.8 % in the single-agent bevacizumab arm and 17.7 % in the bevacizumab and irinotecan arm. In a meta-analysis conducted by Zhang et al. [31], therapy interruption rate was significantly higher in the bevacizumab and irinotecan arm compared to the single-agent bevacizumab arm. In other words, a comparison of combination to single-agent therapy showed that it was associated with disputable clinical benefits that are not reflected in survival, along with additional treatment toxicity and lower therapy continuation.
The study had certain limitation related to retrospective design. Firstly, IPS is a subjective criteria and performance status is not a precise criterion for evaluating the general status of patients. The other limitations of our study were that no generalizations can be made for grade 3 glioma patients due to low sample size and the heterogeneous distribution of patient groups.
In conclusion, as long as no phase 3 studies are conducted that including evaluation of predictive biomarkers directing treatments and quality of life assessments, use of bevacizumab combination regimens is still controversial, especially in countries with limited budgets assigned to oncologic treatments.
Single-agent bevacizumab provides significant clinical benefits in treatment of recurrent GBM. Single-agent bevacizumab use can allow for prevention of chemotherapy toxicities and additional costs while obtaining similar PFS, OS and ORR results. In our study, improved performance status with bevacizumab therapy was determined to be significant a predictor of PFS and OS. We believe that results of our study provide a considerable reference despite the small patient number included in this retrospective analysis.
References
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–9.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–93.
Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro-Oncology. 2013;15(1):4–27.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76.
Godard S, Getz G, Delorenzi M, Farmer P, Kobayashi H, Desbaillets I, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63(20):6613–25.
Jain HV, Nor JE, Jackson TL. Modeling the VEGF-Bcl-2-CXCL8 pathway in intratumoral angiogenesis. Bull Math Biol. 2008;70(1):89–117.
Fang Y, Qu X, Cheng B, Chen Y, Wang Z, Chen F, et al. The efficacy and safety of bevacizumab combined with chemotherapy in treatment of HER2-negative metastatic breast cancer: a meta-analysis based on published phase III trials. Tumour Biol. 2014. doi:10.1007/s13277-014-2799-7.
Lange A, Prenzler A, Frank M, Golpon H, Welte T, von der Schulenburg JM. A systematic review of the cost-effectiveness of targeted therapies for metastatic non-small cell lung cancer (NSCLC). BMC Pulm Med. 2014;14(1):192.
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5.
Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96(2):259–69.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40.
Cirillo M, Venturini M, Ciccarelli L, Coati F, Bortolami O, Verlato G. Clinician versus nurse symptom reporting using the National Cancer Institute—common terminology criteria for adverse events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol. 2009;20(12):1929–35.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72.
Jansen M, de Witt Hamer PC, Witmer AN, Troost D, van Noorden CJ. Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res Brain Res Rev. 2004;45(3):143–63.
Huang H, Held-Feindt J, Buhl R, Mehdorn HM, Mentlein R. Expression of VEGF and its receptors in different brain tumors. Neurol Res. 2005;27(4):371–7.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Cloughesy T, Prados M, Wen PY. A phase II, randomized, noncomparative clinical trial of bevacizumab alone or in combination with irinotecan prolongs 6-month PFS in recurrent, treatment-refractory glioblastoma [abstract]. J Clin Oncol. 2008;26:2010b.
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9.
Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–60.
Nagane M, Nishikawa R, Narita Y, Kobayashi H, Takano S, Shinoura N, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;42(10):887–95.
Nghiemphu PL, Liu W, Lee Y, Than T, Graham C, Lai A, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72(14):1217–22.
Francesconi AB, Dupre S, Matos M, Martin D, Hughes BG, Wyld DK, et al. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci. 2010;17(8):970–4.
Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sorensen M, Kosteljanetz M, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro-Oncology. 2010;12(5):508–16.
Sathornsumetee S, Desjardins A, Vredenburgh JJ, McLendon RE, Marcello J, Herndon JE, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro-Oncology. 2010;12(12):1300–10.
Verhoeff JJ, Lavini C, van Linde ME, Stalpers LJ, Majoie CB, Reijneveld JC, et al. Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol. 2010;21(8):1723–7.
Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE 2nd, Bailey L, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–12.
Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol. 2012;107(1):155–64.
Raizer JJ, Grimm S, Chamberlain MC, Nicholas MK, Chandler JP, Muro K, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–305.
Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012;19(12):1636–40.
Conflict and interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hacibekiroglu, I., Kodaz, H., Erdogan, B. et al. Single-agent bevacizumab is an effective treatment in recurrent glioblastoma. Med Oncol 32, 12 (2015). https://doi.org/10.1007/s12032-014-0460-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0460-3