Abstract
Disruption of cancer lymphatic vessel barrier function occurs has been reported to involve in cancer lymphatic metastasis. Hyaluronan (HA), a major glycosaminoglycan component of the extracellular matrix, is associated with cancer metastasis. We investigated the effect of high/low molecular weight hyaluronan (HMW-HA/LMW-HA) on regulation of barrier function and tight junctions in cancer lymphatic endothelial cell (LEC) monolayer. Results showed that LMW-HA increased the permeability of cancer LEC monolayers and induced disruption of Zonula Occludens-1 (ZO-1)-mediated intercellular tight junction and actin stress fiber formation. HMW-HA treatment decreased permeability in cancer LEC monolayers and cortical actin ring formation. As reported, sphingosine 1-phosphate (S1P) receptors are involved in vascular integrity. After silencing of lymphatic vessel endothelial hyaluronan receptor (LYVE-1), upregulation of S1P receptors (S1P1 and S1P3) induced by HMW-HA/LMW-HA were inhibited, respectively. With S1P3 silenced, the disruption of ZO-1 as well as stress fiber formation and the ROCK1/RhoA signaling pathway induced by LMW-HA was not observed in cancer LEC. These results suggested that S1P receptors may play an important role in HMW-HA-/LMW-HA-mediated regulation of cancer lymphatic vessel integrity, which might be the initial step of cancer lymphatic metastasis and a useful intervention of cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The invasion of cancer cells into lymphatic vessels is an important step in the metastatic cascade, wherein cancer cell migration and adhesion to lymphatic vessels are followed by invasion through the vessel wall and subsequent systemic spread [1]. Disruption of cancer lymphatic vessel barrier function results in promoting cancer lymphatic vessel invasion and metastasis [2]. A continuous layer of cancer lymphatic endothelial cells is attached to each other by tight junctions, and the early changes of cancer lymphatic vessel barrier function are attributed to alterations in cell shape and cell junctions, described as contraction or intercell gap formation [3]. Regrettably, the mechanism of cancer lymphatic vessel barrier dysfunction is less understood. Previous studies showed that cancer barrier function is primarily mediated by intercellular tight junctions. Zonula Occludens-1 (ZO-1) is an essential component of tight junction that is functionally critical in regulating endothelial permeability barrier and is linked to the underlying actin cytoskeleton [4]. ZO-1 binds to the adhering junction protein and connexins in gap junctions, indicating an important scaffolding function of ZO-1 in cell–cell contact [5]. However, the exact molecular mechanism underlying ZO-1-mediated cancer lymphatic dysfunction is unclear.
Hyaluronan (HA) is composed of repeating disaccharide units of D-glucuronic acid and N-acetylglucosamine that exist as a ~1 × 106 Da random coil structure in vivo (referred to as HMW-HA) [6, 7]. LMW-HA is generated by either hyaluronidase-mediated degradation or oxidative hydrolysis of native HA under tumor microenvironment, which is associated with cancer cell metastasis and poor prognosis in certain cancer [8]. The size-dependent effects are widely documented, with HMW-HA exerting anti-angiogenesis and immunosuppressive effects and LMW-HA stimulating cancer LEC proliferation and motility, exhibiting pro-inflammatory and pro-angiogenic effects under cancer conditions [9, 10]. In addition, HA with distinct molecular weight play an important role in regulation of the vascular integrity [11]. Nevertheless, little is known regarding the function of HMW-HA and LMW-HA on regulating lymphatic vessel barrier function under tumor microenvironment.
Sphingosine 1-phosphate (S1P) receptors are expressed on various cell types including neurons, cardiomyocytes, and endothelial cells [12]. Of the five members of S1P receptors, cancer LEC principally expresses S1P1 and S1P3 that participate in protecting and disrupting barrier integrity, respectively [13]. S1P1 signaling couples with the Gi pathway and activates Rac to regulate vascular stability, whereas S1P3 signaling couples with the Gi, Gq/11, and G12/13 pathways and activates RhoA to disrupt vascular endothelial cell interjunction integrity [14]. Despite development in understanding the involvement of S1P receptors in vascular integrity, the effect of S1P receptors on LEC to regulate cancer lymphatic vessel integrity has not been investigated.
Our study verified the effects of LMW-HA on the barrier function and tight junctions in of LEC monolayer, which simulated the tumor microenvironment. The results approved that LMW-HA could increase permeability of cancer LEC monolayers and disrupted ZO-1-mediated LEC junction. HMW-HA strengthened tight junction. To further explore the molecular mechanism, our results showed that HMW-HA/LMW-HA increased S1P1/S1P3 expression and induced downstream signal pathways, respectively. In addition, we further examined the role of S1P receptors in HMW-HA- and LMW-HA-mediated regulation of tight junction and cytoskeleton.
Materials and methods
Reagents
Native HMW-HA was obtained from Sigma-Aldrich (St. Louis, MO). LMW-HA was prepared as described previously [15]. LMW-HA was a mixed fraction of average molecular weight of 2.5 × 106 composed of 3–10 disaccharide units that were fractionated from testicular hyaluronidase type 1-S (Sigma-Aldrich) digests of hyaluronan sodium salt (Sigma-Aldrich). Polyclonal antibody against ZO-1 was obtained from Invitrogen (Carlsbad, MA). Mouse anti-GAPDH, rabbit anti-myosin IIa, anti-AKT, anti-Rac1, anti-ROCK1, and anti-RhoA were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-LYVE-1 receptor was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-S1P1 receptor and anti-S1P3 receptor were purchased from Abcam (Cambridge, MA, USA). Alexa Fluxo 488-conjugated goat anti-rabbit IgG, HRP-conjugated rabbit anti-mouse, and HRP-conjugated goat anti-rabbit IgG were all from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
Cell cultures
SVEC4-10 cells (mouse endothelial cell line, an LEC-like cell line) were purchased from ATCC. SVEC4-10 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Invitrogen) supplemented with 10 % fetal bovine serum in a humid atmosphere with 5 % CO2 at 37 °C.
Permeability of the monolayer barrier
Confluent SVEC4-10 cells were seeded on the insides of inserts (Transwell™ inserts, diameter 6.5 mm, 3.0-µm pore size; Corning, Mid-land, MI, USA) then placed into 24-well plates for 24 h. Inserts were washed once with serum-free DMEM, and the lower chamber was filled with 0.6 ml of DMEM. Then, 0.1 ml of vehicle, HMW-HA/LMW-HA (10 µg/ml) in serum-free DMEM along with sodium fluorescein (Na–F) 100 µg/ml (average MW ~376) was added to the top chamber. The flux of Na–F across the endothelial cell layers was determined as previously described [16].
Immunofluorescence
Cells grown on coverslips for 2 days (90–100 % confluence) were treated with 10 µg/ml HMW-HA and LMW-HA or vehicle only for 30 min in serum-free DMEM. After treatment, cells were washed with PBS twice and then were fixed with methanol at −20 °C for 10 min. Cells were washed with PBS and blocked with 1 % bovine serum albumin in PBS at room temperature for 1 h. Cells were incubated overnight with anti-Z0-1 antibody and anti-myosin IIa antibody at 1:100 and 1:25 dilution. After extensive washing, cells were incubated with Alexa Fluxo 488-conjugated goat anti-rabbit IgG antibody at 1:300 dilution for 1 h at room temperature. Images were acquired using a confocal microscope (Nikon, Tokyo, Japan).
Western blotting
Cells were scraped and lysed in 1 % Triton X-100 lysis buffer. The total protein concentration in the cell lysate was determined using a BCA protein assay kit (Pierce, Rockford, IL). Proteins from each sample were electrophoretically separated on 10 % SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF membranes were blocked with Tris-buffered saline (TBS) containing 5 % non-fat milk powder at room temperature for 1 h, and immunoblot analysis was performed with mouse anti-GAPDH, rabbit anti-S1P1 receptor, anti-S1P3 receptor, anti-AKT, anti-Rac1, anti-ROCK1, and anti-RhoA at 4 °C overnight. The membranes were washed with Tris-buffered saline/Tween-20 (TBS/T) buffer for three times (5 min each time) and then incubated with HRP-conjugated polyclonal secondary antibody for 1 h at room temperature. The membranes were developed with the enhanced plus chemiluminescence assay (Pierce) according to the manufacturer’s instructions. Images were analyzed using Image-Pro Plus 6.0 software.
siRNA transfection
Transfection of cells with S1P1, S1P3, LYVE-1, and scramble siRNA was performed according to the manufacturer’s protocol (Santa Cruz Biotechnology). Briefly, SVEC4-10 cells were grown on the 6-well tissue culture plate to 60–80 % confluence. Cells were then washed twice with transfection medium (sc-36868). A 0.2-ml aliquot of transfection medium containing S1P1 siRNA (sc-37087), S1P3 siRNA (sc-35262), LYVE-1 siRNA (sc-42902), scramble siRNA (sc-37007), and transfection reagent (sc-29528) was incubated at room temperature for 30 min before adding to the cells. Cells were used for experiments for 48 h after transfection.
Statistical analysis
The analyses were performed using the statistical software SPSS 13.0. Differences were considered statistically significant at P < 0.05. All experiments were performed at least three independent experiments.
Results
Effect of HMW-HA and LMW-HA on barrier function and tight junction of LEC monolayer
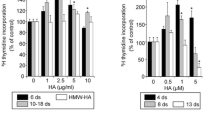
Using a transwell system, we determined the effect of HMW-HA and LMW-HA on the permeability of SVEC4-10 monolayer by measuring the passage of Na–F from the upper chamber to the lower chamber. Stimulation of cells with LMW-HA significantly increased transit of Na–F across the cell monolayer when compared to control at 30, 60, and 90 min (*P < 0.05). HMW-HA just decreased the permeability of Na–F at 30 min (Fig. 1A). The permeability of Na–F was used to detect the barrier integrity of tight junction, and tight junction and cytoskeleton are critical determinants of lymphatic vessel integrity and barrier regulation [17]. Thus, we analyzed the effect of HMW-HA and LMW-HA on the distribution of ZO-1 and myosin in SVEC4-10 monolayer for 30 min. Our data showed that HMW-HA enhanced ZO-1 distribution in the cell borders to form a linear pattern and enhanced myosin polymerization, which was confined to the cortical cytoskeletal ring (Fig. 1B, b e). Conversely, ZO-1 was rapidly redistributed and “lost,” disorganizing into a zigzag pattern by LMW-HA (Fig. 1B, c). In addition, the appearance of increased stress fibers or paracellular gaps was also caused by LMW-HA (Fig. 1B, f).
HMW-HA and LMW-HA regulated barrier function and tight junction in LEC monolayer. A SVEC4-10 cells (1.3 × 105) were plated into 6.5-mm transwell membrane inserts and grown overnight. Cell monolayers were treated with medium only, 10 µg/ml HMW-HA and LMW-HA. Na–F (100 µg/ml) was then added to the upper chamber. At each time period (30, 60, 90, 120 min), an aliquot was taken from the lower chamber to measure its fluorescence intensity (A.U.). *P < 0.05 was compared with control. B SVEC4-10 cells were seeded on coverslips and treated with either vehicle (a, d), HMW-HA (10 µg/ml) (b, e) or LMW-HA (10 µg/ml) (c, f) for 30 min. Cells were stained with anti-ZO-1 antibody (a–c), and myosin staining was evaluated with anti-myosin IIa anti-body (d–f) and analyzed using confocal microscope
HMW-HA and LMW-HA regulate the expression of S1P receptors in LECs through LYVE-1
The S1P receptors were originally identified and isolated as an immediate early gene product that is abundantly induced after activation of vascular endothelial cells, S1P1 receptor up-regulation by VEGF was seen within 30 min in endothelial cells [18]. As reported previously, S1P1/S1P3 participated in vessel barrier integrity. To determine the involvement of S1P1/S1P3 in HA-induced lymphatic vessel integrity, their expression was assessed by Western blotting. Our results showed that the protein expression of S1P1 receptor and S1P3 receptor increased significantly within 30 min of HMW-HA and LMW-HA treatment, respectively (*P < 0.05). To assess the role of LYVE-1 in S1P receptor expression evoked by HA, siRNA was used to silence LYVE-1 in SVEC4-10 cells (Fig. 2A), which resulted in reduced the expression of S1P1 and S1P3 induced by HMW-HA and LMW-HA, respectively (Fig. 2B).
LYVE-1 is required for HMW-HA- and LMW-HA-induced expression of S1P receptors on LECs. A knockdown LYVE-1 with siRNA was confirmed by Western blotting, scramble siRNA used as a loading control. B SVEC4-10 cells were treated with scramble siRNA, LYVE-1 siRNA for 48 h. After reaching post-confluence, the cells were induced to 10 µg/ml HMW-HA and LMW-HA or medium only for 30 min, and the lysates were analyzed by Western blotting. The blots are representative of at least three independent experiments
HMW-HA and LMW-HA induce ROCK1/RhoA, AKT/Rac signaling pathways through S1P receptors in LECs
The ROCK1/RhoA signaling pathway is known to regulate myosin contractility and negative regulation of cell integrity [19, 20]. Another member of the Rho family GTPase, Rac, directs actin assembly that results in the formation of lamellipodia and filopodia [21]. S1P1/S1P3 is involved in HA-mediated lung vascular barrier regulation [22]. Therefore, to determine whether HMW-HA and LMW-HA stimulation required S1P receptor signaling to exert their effect on ROCK1/RhoA and AKT/Rac expression, S1P1 and S1P3 were silenced in SVEC4-10 cells with RNAi (Fig. 3A, B). The expression of AKT/Rac increased by HMW-HA was blocked by silencing of S1P1 (*P < 0.05) (Fig. 3C). In contrast, LMW-HA-induced activation of ROCK1/RhoA signal pathway was significantly inhibited by S1P3 knockdown (*P < 0.05) (Fig. 3D).
HMW-HA and LMW-HA induce ROCK1/RhoA and AKT/Rac1 signaling pathway in LECs through S1P receptors. SVEC4-10 cells were grown on plate to 60–80 % confluence and transfected with S1P1 receptor and S1P3 receptor siRNA, which were confirmed by Western blotting (A, B). Western blot analysis of the cells was performed using the antibodies indicated, with GAPDH as a loading control, after silencing of S1P1 and S1P3 receptor weakened the expression of AKT/Rac and ROCK/RhoA signal pathways induced by HMW-HA and LMW-HA, respectively (C, D) *P < 0.05. The blots are representative of at least three independent experiments
HMW-HA and LMW-HA influence the tight junction and myosin rearrangement of LECs through S1P receptors
Investigating the involvement of S1P1/S1P3 in the ZO-1 and myosin rearrangement of SVEC4-10, our results showed that reduction of S1P1 expression significantly attenuated HMW-HA-induced ZO-1 to form a linear pattern in the cell borders and eliminated cortical actin ring formation (Fig. 4). Reduction of S1P3 expression also blocked LMW-HA-induced ZO-1 “lost” and paracellular gaps and stress fiber formation (Fig. 4), suggesting that S1P receptors are required for HA-induced tight junction and myosin rearrangement in cancer LECs.
HMW-HA and LMW-HA influence the ZO-1 and myosin of LECs through S1P receptors. SVEC4-10 cells treated with scramble, S1P1, S1P3 siRNA for 48 h. Cells were stimulated with either medium only, HMW-HA (10 µg/ml) or LMW-HA (10 µg/ml) for 30 min. Cells were then fixed and stained with anti-ZO-1 antibody (A) and anti-myosin IIa anti-body (B) and analyzed using a confocal microscope. RNAi S1P1 significantly attenuated HMW-HA-induced ZO-1 to form a linear pattern in the cell borders and eliminated cortical actin ring formations indicated by arrows, RNAi S1P3 receptor blocked LMW-HA-induced ZO-1 “lost” indicated by arrows
Discussion
Invasion into lymphatic vessels is a key step in the metastasis of primary tumors to draining lymph nodes [23]. Breakdown in the barrier function of cancer lymphatic vessel plays an important role in cancer metastasis [24]. Previous studies showed that the cancer lymphatic endothelial cell barrier function is tightened by enhancing the organization of junctional component ZO-1 [25]. Moreover, ZO-1 is linked to the actin cytoskeleton myosin, which remodel in a temporal fashion consistent with barrier enhancement [26]. Despite the growing interest in alterations to cancer lymphatic vessel barrier function, the associated molecular mechanism is unclear.
Studies have shown that HA regulates malignant tumor growth, invasion, and metastasis [27, 28]. It is fascinating that HMW-HA contributes to space filling and is a novel inhibitor of vascular leakiness, suggesting that HMW-HA plays a significant role in providing a protective barrier between vascular endothelial cells. On the other hand, elevated LMW-HA exerts a disruptive effect on vascular integrity under cancer condition [29, 30]. However, the roles of HMW-HA and LMW-HA in cancer LEC barrier function are not well defined. Recent studies have demonstrated that HMW-HA/LMW-HA induces CD44 association with S1P1 and S1P3 leading to barrier enhancement and barrier disruption in human pulmonary endothelial cell, respectively [22]. However, the role of S1P receptors in cancer lymphatic vessel barrier function has not been studied.
In this study, we presented evidence that LMW-HA increased permeability in LEC monolayers and produced a significant increase in Na–F permeability in LEC monolayer, and this finding suggested LMW-HA disrupted cancer lymphatic vessel barrier. HMW-HA decreased the permeability of LEC monolayer just for a short time (Fig. 1A). Consistent with the previously reported, LMW-HA induced endothelial cell barrier disruption [11]. The permeability of Na–F was used to detect the barrier integrity of tight junction [31], and thus, we further explored the effect of HMW-HA and LMW-HA on ZO-1 and myosin distribution in LEC. Our results showed that HMW-HA enhanced ZO-1 distribution in cancer LEC borders and formed cortical actin rings (Fig. 1B, b e). On the contrary, LMW-HA opened ZO-1 intercellular junction and promoted actin stress fiber or paracellular gap formation (Fig. 1B, c f). HMW-HA/LMW-HA treatment for 15 or 30 min induced cortical actin ring and actin stress fiber formation in endothelial cell, respectively [22]. It is, therefore, conceivable that LMW-HA may disrupt cancer lymphatic vessel barrier function.
Next, we determined the mechanisms by which HMW-HA/LMW-HA regulated barrier function of LEC monolayer. S1P1 receptor is more critical for Rac-dependent cortical actin assembly and barrier protection, whereas S1P3 receptor controls Rho and stress fiber/focal contact pathway [32]. We found that silencing of LYVE-1 abrogated the increased expression of S1P1 and S1P3 triggered by HMW-HA and LMW-HA in cancer LEC, respectively (Fig. 2B), suggesting that S1P receptors may be involved in HMW-HA- and LMW-HA-mediated cancer lymphatic vessel barrier function. RhoA and Rac, the two best-characterized members of the Rho GTPases family, are key regulators of the actin cytoskeleton and intercellular junctions [33, 34]. Previous studies have shown that Rac is required for the assembly and maturation of endothelial cell junctions, RhoA-mediated myosin contractility and broken endothelial barrier function [35, 36]. Hence, we examined whether they were involved in HA-regulated cancer lymphatic vessel barrier function, and the results showed that HMW-HA increased AKT and Rac expression in LECs, which were weakened by silencing of S1P1 (Fig. 3C), On the contrary, LMW-HA significantly enhanced ROCK1 and RhoA expression in LECs that were inhibited with S1P3 silenced (Fig. 3D). Last, we tried to determine the role of S1P receptor in LEC barrier integrity. Our data showed that LMW-HA disturbed the distribution of ZO-1 and formed stress fiber in LECs could be influenced by S1P3 siRNA (Fig. 4A, B). HMW-HA-induced ZO-1 distribution in LEC borders and cortical actin ring formation was ameliorated by S1P1 siRNA (Fig. 4A, B). The results indicated that S1P receptors may participate in HMW-HA-/LMW-HA-regulated lymphatic integrity in cancer LECs.
In conclusion, we demonstrated that LMW-HA disrupted barrier properties of cancer LEC monolayer by increasing S1P3 expression. On the other hand, HMW-HA enhanced cancer lymphatic vessel barrier function through elevating S1P1 expression. This is the first time demonstrating that HMW-HA and LMW-HA regulate cancer lymphatic vessel barrier function through S1P receptors. The results suggest a new means for understanding the molecular mechanism of HMW-HA and LMW-HA in tumor lymphatic metastasis, which might be a useful intervention for lymphatic integrity-associated tumor progression.
References
Safuan S, Storr SJ, Patel PM, Martin SG. A comparative study of adhesion of melanoma and breast cancer cells to blood and lymphatic endothelium. Lymphat Res Biol. 2012;10:173–81.
Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 2014;17:395–406.
Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol. 2007;127:762–74.
Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, Wang E, Lee MJ. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281:29190–200.
Müller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G. The tight junction protein occludin and the adherens junction protein-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–56.
Auvinen P, Tammi R, Kosma VM, Sironen R, Soini Y, Mannermaa A, Uljas E, Tammi M, Tammi R. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int J Cancer. 2013;132:531–9.
Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39.
Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61.
Wu M, Du Y, Liu Y, He Y, Yang C, Wang W, Gao F. Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS ONE. 2014;9:e92857.
Wang YZ, Cao ML, Liu YW, He YQ, Yang CX, Gao F. CD44 mediates oligosaccharides of hyaluronan-induced proliferation, tube formation and signal transduction in endothelial cells. Exp Biol Med. 2011;236:84–90.
Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol. 2010;299:L639–51.
O’Sullivan C, Dev KK. The structure and function of the S1P1 receptor. Trends Pharmacol Sci. 2013;34:401–12.
English D, Brindley DN, Spiegel S, Garcia JG. Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim Biophys Acta. 2002;1582:228–39.
Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F, Garcia JGN. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–67.
Gao F, Cao M, Yang C, He Y, Liu Y. Preparation and characterization of hyaluronan oligosaccharides for angiogenesis study. J Biomed Mater Res B Appl Biomater. 2006;78:385–92.
Dohgu S, Sumi N, Nishioku T, Takata F, Watanabe T, Naito M, Shuto H, Yamauchi A, Kataoka Y. Cyclosporin A induces hyperpermeability of the blood-brain barrier by inhibiting autocrine adrenomedullin-mediated up-regulation of endothelial barrier function. Eur J Pharmacol. 2010;644:5–9.
Downes M, François M, Ferguson C, Parton RG, Koopman P. Vascular defects in a mouse model of hypotrichosis–lymphedema–telangiectasia syndrome indicate a role for SOX18 in blood vessel maturation. Hum Mol Genet. 2009;18:2839–50.
Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA. 2009;16:10664–9.
Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261:1–12.
Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–60.
Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14.
Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–93.
Witte MH, Jones K, Wilting J, Dictor M, Selg M, McHale N, Gershenwald JE, Jackson DG. Structure function relationships in the lymphatic system and implications for cancer biology. Cancer Metastasis Rev. 2006;25:159–84.
Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: new insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006;25:677–94.
Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–41.
Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701.
Ikuta K, Urakawa H, Kozawa E, Arai E, Zhuo L, Futamura N, Hamada S, Kimata K, Ishiguro N, Nishida Y. Hyaluronan expression as a significant prognostic factor in patients with malignant peripheral nerve sheath tumors. Clin Exp Metastasis 2014;31:715–25.
Dang S, Peng Y, Ye L, Wang Y, Qian Z, Chen Y, Wang X, Lin Y, Zhang X, Sun X, Wu Q, Cheng Y, Nie H, Jin M, Xu H. Stimulation of TLR4 by LMW-HA induces metastasis in human papillary thyroid carcinoma through CXCR7. Clin Dev Immunol. 2013;10:712561.
Genasetti A, Vigetti D, Viola M, Karousou E, Moretto P, Rizzi M, Bartolini B, Clerici M, Pallotti F, De Luca G, Passi A. Hyaluronan and human endothelial cell behavior. Connect Tissue Res. 2008;49:120–3.
Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu YY, Garg H, Quinn DA, Hales CA. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology. 2010;15:1131–9.
Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood–brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127.
Hla Timothy. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol Res. 2003;47:401–7.
Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701.
Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vasc Pharmacol. 2002;39:187–99.
Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanism. J Cell Physiol. 2004;201:55–70.
Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from G12/13and G11/q. J Biol Chem. 2008;283:29888–96.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81071814, 81172027, 81272479, 81402419), and the Natural Science Foundation of Shanghai Municipality (12ZR1447400, 14YF1412200) and the Doctorial Innovation Foundation (BXJ201238), and the program of Shanghai Shen-Kang Hospital Development Center (SHDC22014004).
Conflict of interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mengsi Yu and Pingqing He have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, M., He, P., Liu, Y. et al. Hyaluroan-regulated lymphatic permeability through S1P receptors is crucial for cancer metastasis. Med Oncol 32, 381 (2015). https://doi.org/10.1007/s12032-014-0381-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0381-1