Abstract
Parkinson’s disease (PD) is characterized by astrocyte activation and disruptions in circadian rhythm. Within the astrocyte population, two distinct reactive states exist: A1 and A2. A1 astrocytes are associated with neurotoxicity and inflammation, while A2 astrocytes exhibit neuroprotective functions. Our investigation focused on the role of REV-ERBα, a member of the nuclear receptor superfamily and a key regulator of the circadian clock, in astrocyte activation. We observed that REV-ERBα expression in A1 astrocytes was reduced to one-third of its normal level. Notably, activation of REV-ERBα prompted a transformation of astrocytes from A1 to A2. Mechanistically, REV-ERBα inhibition was linked to the classical NF-κB pathway, while it concurrently suppressed the STAT3 pathway. Furthermore, astrocytes with low REV-ERBα expression were associated with dopaminergic neurons apoptosis. Intriguingly, the opposite effect was observed when using a REV-ERBα agonist, which mitigated astrocyte activation and reduced dopaminergic neuron damage by 50%. In summary, our study elucidates the pivotal role of REV-ERBα in modulating astrocyte function and its potential implications in PD pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytes, the most abundant type of glial cells in the central nervous system (CNS), play a crucial role in maintaining CNS homeostasis. In normal physiological conditions, astrocytes fulfill protective functions by secreting neurotrophins and antioxidants and facilitating neuronal waste disposal (Lee et al. 2022). When exposed to stimuli such as injury or disease, reactive astrocytes undergo dynamic changes, including hypertrophy, increased glial fibrillary acidic protein (GFAP) expression, and gene expression alterations, highlighting their dynamic nature. Reactive astrocytes play diverse roles, contributing to both neurotoxic and neuroprotective functions in the complex landscape of neuroinflammatory responses (Escartin et al. 2021). Similar to microglia, reactive astrocytes exhibit two distinct phenotypes: A1 (pro-inflammatory) and A2 (anti-inflammatory), which can be induced by neuroinflammation and ischemia, respectively (Escartin et al. 2021; Liddelow et al. 2017).
A1 astrocytes are typically induced by neuroinflammatory factors such as complement component C1q, tumor necrosis factor TNF-α, and interleukin IL-1α. They exhibit neurotoxic and pro-inflammatory properties. A1 cells lose many normal functions, such as maintaining synapses, and upregulate many genes that are harmful to synapses. A1 astrocytes produce and release pro-inflammatory factors, chemokines, and neurotoxic mediators, leading to dysfunctions in cell survival, proliferation, and differentiation (Liddelow et al. 2017). On the other hand, A2 astrocytes are usually induced by conditions such as hypoxia and exhibit neuroprotective and anti-inflammatory properties. A2 cells upregulate neurotrophic factors or anti-inflammatory genes, promoting the survival and growth of neurons. A2 astrocytes exert neuroprotective effects through their phagocytic functions and the release of neurotrophic factors and neurogenic transcription factors (Lee et al. 2022; Colombo and Farina 2016).
A1 astrocytes, when activated by neuroinflammatory factors such as complement component C1q, tumor necrosis factor TNF-α, and interleukin IL-1α, secrete inflammatory cytokines and chemokines, ultimately leading to neuronal death and neurodegenerative diseases. A2 astrocytes, on the other hand, secrete neurotrophic factors, including glial cell line-derived neurotrophic factor (GDNF), which play a protective role in preserving neurons.
Parkinson’s disease (PD) is a degenerative brain disorder characterized by the loss of dopamine (DA)-producing neurons in the brain. Neuroinflammation significantly contributes to the disease’s etiology (Colwell 2021; Roth and Ding 2020). During the progression of PD, activated microglia stimulate the activation of A1 astrocytes, resulting in neurotoxicity release and subsequent death of DA neurons (Escartin et al. 2021; Araujo et al. 2022). Conversely, the neuroprotective properties of A2 astrocytes may help slow the progression of PD. A2 cells, by releasing neurotrophic factors and anti-inflammatory mediators, may promote the survival and functional recovery of dopaminergic neurons. Regulating the activation states of A1/A2 reactive astrocytes to reduce toxic effects and enhance protective effects provides new insights and potential therapeutic targets for the treatment of PD.
Glial cells harbor functional circadian clocks that govern their responses to daily oscillations in brain activity, cellular stress, and metabolism (Koronowski and Sassone-Corsi 2021). Circadian dysfunction commonly occurs in aging and neurodegenerative disease (McKee et al. 2020). Astrocyte reactivity and activity exhibit circadian rhythm, with GFAP, serving as a specific marker for these cellular oscillations (Leone et al. 2006). Mammalian circadian rhythm, at the molecular level, is regulated by a transcription-translation feedback loop, including Basic Helix-Loop-Helix ARNT Like 1 (BMAL1), Circadian Locomoter Output Cycles Protein Kaput (CLOCK), and REV-ERBα (Ikegami et al. 2019). The core circadian clock protein BMAL1 modulates astrogliosis through a cell-autonomous mechanism (Lananna et al. 2018).
REV-ERBα, a transcriptional repressor, participates in regulating circadian rhythm, immune function and metabolism and other physiological processes (Guo et al. 2019). REV-ERBα was named because it resides on the opposite strand of ERBA (THRA) oncogene (Everett and Lazar 2014). In animal models of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine(6-OHDA), or rotenone, the expressions of major clock genes BMAL1, REV-ERBα, Cryptochrome 1 (CRY1), and Period 2 (PER2) were altered (Shkodina et al. 2022; Hayashi et al. 2013). The expression of REV-ERBα is abnormal in PD patients and animal models (Breen et al. 2014; Kou et al. 2022). Elevated GFAP expression was observed in the cortex and hippocampus of Rev-erbα KO mice (Griffin et al. 2019). However, it is not clear whether Rev-erbα deletion directly leads to astrocyte activation. In this study, we propose that circadian clock protein REV-ERBα may regulate the polarization of astrocytes.
Materials and Methods
Animal Study
C57BL/6 mice (8 weeks, Male) weighing 25–30 g were purchased from SLAC Lab Animal Ltd (Shanghai, China) and were housed with a 12:12-h light/dark cycle. The mice were used to detect circadian rhythm of REV-ERBα in mouse brains. The mice were killed by injection of pentobarbital sodium (50 mg/kg i.p.) at indicated time points. Fresh mice brain tissues were crushed by a homogenizer. All animal experiments were approved by the Animal Committee of Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences. The ethics commission number is IRB202005003RI.
Primary Astrocyte Cultures
Newborn mice were only used to extract primary astrocyte. Primary mouse astrocytes were extracted from 2-day-old mouse cerebral cortices and prepared as previously described (Chen et al. 2018). Briefly, newborn mice were killed at 1 pm. Cerebral cortices were digested using 0.25% trypsin and DNase (100 μg/mL). Mixed glia cells were cultured in poly-D-lysine-coated bottles. After 2 weeks, non-astrocytic cells were detached from the flasks by shaking at 250 rpm for 18 h and changed the medium after washing 3 times by PBS.
Cell Culture and Drugs
The BV2, C8D1A, and SH-SY5Y cell lines were presented from Wang lab research group, School of Pharmacy, Soochow University. Primary astrocyte and C8D1A (a murine astrocyte cell line) were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS, Gibco) and penicillin/streptomycin (100 μg/mL), with a seeding density of 1 × 105 cells per well in 6-well plates. The confluency at the time of treatment was approximately 50%. BV2 (a murine microglia cell line) was cultured in high-glucose DMEM with 10% heat-inactivated FBS, with a seeding density of 1 × 105 cells per well in 6-well plates and a confluency of approximately 50% at the time of treatment. SH-SY5Y (a human-derived neuronal cell line, expressing dopaminergic markers) was cultured in DF12 with 10% FBS, with a seeding density of 1 × 105 cells per well in 6-well plates and a confluency of approximately 50% at the time of treatment. All cells were cultured at 37 ℃ in a mixture of 5% CO2 and 95% O2. SR9009 and SR8278 were purchased from Selleck company, which are agonist and inhibitor of REV-ERBα, respectively (Selleck Chemicals, Houston, TX, USA).
Conditioned Media (CM)
BV2 cells were stimulated by PBS or LPS (100 ng/mL) for 24 h. The supernatants were filtered through 0.45-μm filters and used to culture astrocyte cells for 24 h. Similarly, astrocytes were pretreated by SR9009 or SR8278, and then astrocyte supernatant were filtered and used to culture SH-SY5Y cell for 48 h. After culture, SH-SY5Y cells were used for further experiments.
Genetic and Pharmacological Intervention
For plasmid transfection, cells were transfected with HA-Rev-erbα using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. siRNAs against the mouse Rev-erbα gene were synthesized with the following sequences: Rev-erbα: 5′-GCAUCGUUGUUCAACGUGATT-3′ and 5′-UCACGUUGAACAACGAUGCAA-3′. For small interfering RNA (siRNA) knockdown, cells were transfected with siRNAs using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. For pharmacological intervention, astrocytes were treated by DMSO or SR9009/SR8278 (20 μM) for 24 h.
Real-Time Quantitative RT-PCR
The qPCR machine (Roche, cobas z 480) was used. Total RNA was isolated with TRIzol reagent (Vazyme, Jiangsu, China). cDNA was reverse-transcribed from total RNA (500 ng) using PrimeScript RT Master Mix (Vazyme, Jiangsu, China). RNA concentration was measured using a nanodrop. Housekeeping gene Actin was used for normalization. qRT-PCR analysis was calculated using the 2−ΔΔCT formula for the relative quantification. Quantitative PCR primers were designed as following:
iNos, forward primer 5′-TCCCAGCCTGCCCCTTCAAT-3′, reverse primer 5′-CGGATCTCTCTCCTCCTGGG-3′; Rev-erbα, forward primer 5′- GGGCACAAGCAACATTACCA-3′, reverse primer 5′- CACGTCCCCACACACCTTAC-3′; C3, forward primer 5′- AAGCATCAACACACCCAACA-3′, reverse primer 5′- CTTGAGCTCCATTCGTGACA-3′; S100a10, forward primer 5′- CCTCTGGCTGTGGACAAAAT-3′, reverse primer 5′- CTGCTCACAAGAAGCAGTGG-3′; Gdnf, forward primer 5′-TTGCAGCGGTTCCTGTGAAT-3′, reverse primer 5′- TCTTAGAATATGGTAAACCAGGTTGTCA-3′; Cxcl10, forward primer 5′- CCAAGTGCTGCCGTCATTTTC-3′, reverse primer 5′- GGCTCGCAGGGATGATTTCAA-3′; Gfap, forward primer 5′- AGAAAGGTTGAATCGCTGGA-3′, reverse primer 5′- GAACCCGTCTTCCATCGTTA-3′; β-Actin, forward primer 5′-GACCTGACTGACTACCTC-3′, reverse primer 5’-GACAGCGAGGCCAGGATG-3′.
Immunoblot Analysis
Immunoblot analysis was carried out as previously described (Xu et al. 2023). Protein expression was quantified by Western blot assay. Blots were incubated with the following primary antibodies: REV-ERBα (Santa Cruz, CA, USA), GAPDH (Santa Cruz Biotechnology, United States), iNOS (Abcam, United Kingdom), p-p65 (CST, United States), p65 (Santa Cruz, CA, USA), p-STAT3 (Abcam, United Kingdom), STAT3 (Abcam, United Kingdom), Cxcl10 (Abcam, United Kingdom), Caspase-3 (CST, United States), and GDNF (Abcam, United Kingdom). The secondary antibodies, horseradish peroxidase (HRP)-conjugated sheep anti-mouse, or anti-rabbit antibodies (BBI, Canada) were used. The gels were imaged with chemiluminescence imaging analysis system (Clinx, China) and analyzed by ImageJ software (National Institutes of Health, USA).
Statistical Analysis
Data were analyzed with GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA). One-way analysis of variance was used for comparison between groups and t-tests for two groups. The criterion of significance was set as P < 0.05. All results are presented as means ± SD.
Results
REV-ERBα Expression Declined in A1 Astrocytes
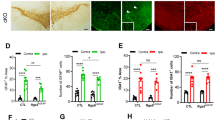
Discovered in 1989, REV-ERBα derives its name from its position on the antisense chain of the nuclear receptor, the thyroid hormone receptor (erbA alpha) gene (Adlanmerini et al. 2021). REV-ERBα, a core CLOCK protein, belongs to the nuclear receptor transcription factor superfamily. In mouse brains, REV-ERBα exhibits a circadian rhythm in both protein and mRNA levels (Fig. 1A–B), which typically do not involve statistical significance comparisons (Kou et al. 2022). LPS-activated microglia induced A1 reactive astrocytes, characterized by elevated levels of inflammatory proteins(Liddelow et al. 2017; Liddelow and Barres 2017). We employed BV2 microglia supernatant treated by LPS to stimulate astrocyte activation. REV-ERBα expression decreased in both C8D1A astrocyte cells line (Fig. 1C–D) and primary astrocytes (Fig. 1C–F) cultured with BV2 supernatant following LPS stimulation.
The expression of REV-ERBα in astrocytes. A REV-ERBα protein levels in mouse brains show circadian rhythm, indicating its fluctuation over a 24-h period. B Rev-erbα mRNA levels in mouse brains exhibit circadian rhythm, with n = 8 mice used for the analysis. C REV-ERBα protein levels in C8D1A astrocytes cultured with supernatant from BV2 microglia cell line. D Relative expression levels of indicated proteins in (C) were determined from n = 3 independent experiments. E REV-ERBα protein levels in primary astrocytes cultured with supernatant from BV2 microglia cell line. F Relative expression levels of indicated proteins in (E) were determined from n = 3 independent experiments. ***P < 0.001
REV-ERBα Regulated Astrocyte Phenotype
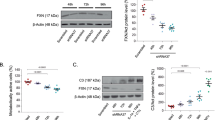
Although REV-ERBα decreased in A1 astrocytes, it remains unclear whether REV-ERBα directly regulates astrocyte phenotype. We modulated REV-ERBα expression by transfecting HA-Rev-erbα plasmid or siRNA in C8D1A astrocytes and subsequently assessed A1/A2 biomarker mRNA levels. In Rev-erbα knockdown astrocytes, A1 biomarker mRNA levels of iNos and C3 increased, while A2 biomarker mRNA level of S100a10 decreased (Fig. 2A). In REV-ERBα overexpressed astrocytes, A1 biomarker mRNA levels of iNos and C3 decreased (Fig. 2B). We also detected A1/A2 biomarker protein levels in REV-ERBα overexpressed astrocytes. iNOS increased and GDNF decreased in Rev-erbα knockdown astrocytes (Fig. 2C–D).
REV-ERBα regulated astrocyte phenotype. A A1/A2 biomarker mRNA levels in REV-ERBα knockdown astrocyte, determined from n = 3 independent experiments. B A1/A2 biomarker protein levels in REV-ERBα overexpression astrocyte, with ns indicating no statistical significance, from n = 3 independent experiments. C A1/A2 biomarker protein levels in REV-ERBα knockdown astrocyte. D Relative expression levels of indicated proteins in (C) were determined from n = 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
REV-ERB Regulated Signaling Pathways of the Astrocyte Phenotype
A1 reactive astrocytes exhibit NF-κB activation, while A2 reactive astrocytes show STAT3 activation (Colombo and Farina 2016; Liddelow and Barres 2017). Phosphorylation of p65, a critical indicator of NF-κB pathway activation, increased in Rev-erbα knockdown astrocytes (Fig. 3A–C). Phosphorylation levels of STAT3 serve as an indicator of STAT3 pathway activation, and these levels increased in Rev-erbα knockdown astrocytes (Fig. 3D–F).
REV-ERBα regulated signaling pathways of the astrocyte phenotype. A NF-κB pathway involvement in REV-ERBα knockdown astrocytes. B–C Relative expression levels of indicated proteins in (A) were determined. ns, no statistical significance, from n = 3 independent experiments. D STAT3 pathway involvement in REV-ERBα knockdown astrocytes. E–F Relative expression levels of indicated proteins in (D) were determined from n = 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
Astrocyte Activation Mediated by REV-ERBα Induced the Death of Dopaminergic Neurons
Pro-inflammatory mediators, secreted by A1 reactive astrocytes, contribute to neuron death (Liang et al. 2023; Park et al. 2021). Cxcl10, a cytokine within the CXC chemokine family, plays a crucial role in mediating the inflammatory response in astrocytes during various CNS diseases (Liang et al. 2023). In Rev-erbα knockdown astrocytes, mRNA levels of Cxcl10 and GFAP increased (Fig. 4A). Additionally, the protein level of Cxcl10 increased in Rev-erbα knockdown astrocytes (Fig. 4B–C). Cleaved caspase-3, an activated form of caspase 3, serves as an indicator of apoptosis. Treatment of SH-SY5Y neurons with C8D1A astrocyte supernatant revealed that Rev-erbα knockdown astrocytes promotes neuronal apoptosis (Fig. 4D–E).
Astrocyte activation mediated by REV-ERBα induced the death of dopaminergic neurons. A Chemokine mRNA levels in REV-ERBα knockdown astrocyte, determined from n = 3 independent experiments. B Cxcl10 protein levels in REV-ERBα knockdown astrocyte. C Relative expression levels of indicated proteins in (B) were determined from n = 3 independent experiments. D Cleaved Caspase-3 levels in dopaminergic neuron cells treated with astrocyte medium knockdown with Rev-erbα. E Relative expression levels of indicated proteins in (D) were determined from n = 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
Pharmacological Activation of REV-ERBα in Astrocyte Rescued the Death of Dopaminergic Neurons
Currently, a variety of REV-ERBα agonists and inhibitors have been utilized in pharmacological studies (Uriz-Huarte et al. 2020). Specifically, SR9009 functions as an agonist, while SR8278 acts as inhibitor of REV-ERBα. We investigated whether pharmacological activation or inhibition of REV-ERBα in astrocyte impacts the survival of dopaminergic neurons. We pre-treated C8D1A astrocytes with SR9009 or SR8278, followed by culturing them with BV2 supernatant treated with LPS. Subsequently, astrocytes supernatant was used to culture SH-SY5Y cell (Fig. 5A). Caspase-3, a key factor, renders dopaminergic neurons susceptible to apoptotic death in PD. In PD patients, the percentage of dopaminergic neurons testing positive for active caspase-3 was significantly higher than in the control group (Hartmann et al. 2000). Supernatant from activated astrocytes induced apoptosis in dopaminergic neurons. Furthermore, the REV-ERBα inhibitor SR8278 exacerbated damage to dopaminergic neurons (Fig. 5B–C). Conversely, the REV-ERBα agonist SR9009 mitigated damage to dopaminergic neurons caused by activated astrocytes (Fig. 5D–F).
Pharmacological activation or inhibition of REV-ERBα in astrocyte affect dopaminergic neuron survival. A C8D1A astrocytes treated with DMSO or SR9009/SR8278 (20 μM) for 24 h and then cultured with supernatant from BV2 cells treated with PBS or LPS (100 ng/ml) for 24 h, and finally astrocyte supernatants used to culture SH-SY5Y cells for 48 h. B Caspase 3 and cleaved caspase 3 protein levels in SH-SY5Y cells cultured with supernatant from astrocytes treated with DMSO/SR827. C Relative expression levels of indicated proteins in (B) were determined from n = 3 independent experiments. D Caspase 3 and cleaved caspase3 protein levels in SH-SY5Y cells cultured with supernatant from astrocyte treated with DMSO/SR9009. E Relative expression levels of indicated proteins in (D) were determined from n = 3 independent experiments. F SH-SY5Y cells stained with annexin V, PI, and Hoechst 33,258 with bar representing 100 μm and ns indicating no statistical significance. **P < 0.01, *P < 0.05
Discussion
In our study, we demonstrated a downregulation of REV-ERBα in A1 astrocytes. Inhibition of REV-ERBα facilitated the conversion of astrocyte to pro-inflammatory A1 subtype by enhancing p65 phosphorylation. Conversely, activation of REV-ERBα led to the transformation of astrocytes into anti-inflammatory A2 subtype by upregulating STAT3 phosphorylation. Furthermore, the REV-ERBα agonist mitigated dopaminergic neuron damage induced by inhibiting the activation of astrocytes.
PD is commonly associated with damage to dopaminergic neurons. Nevertheless, recent research indicates that non-neuronal cell types significantly contribute to PD pathogenesis (Brandebura et al. 2023). The activation of numerous astrocytes compromises their capacity to support neuronal survival and growth, leading to neurotoxicity and dopaminergic neurodegeneration in PD (Patani et al. 2023). Altered circadian rhythms have been associated with delayed PD diagnosis, underscoring their relevance in the disease. Both astrocyte activation and circadian rhythm disruption are pivotal factors contributing to PD (Ishii et al. 2019). Nevertheless, the relationship between these factors and PD pathogenesis remains inadequately explored. Core circadian clock proteins regulate the diurnal activity of astrocytes. Dysregulated astrocytic clocks (due to Bmal1 deletion or lifestyle-related disruptions) may contribute to neurodegenerative pathways by impairing the clearance and metabolism of toxic brain metabolites (Hastings et al. 2023). Targeted Bmal1 deletion induces astrocyte activation and upregulates inflammatory gene expression both in vitro and in vivo (Lananna et al. 2018). Loss of Bmal1 typically leads to astrocyte hyperplasia. Consequently, we anticipate that REV-ERBα would suppress astrocyte proliferation due to its inhibitory effect on Bmal1 expression.
REV-ERBα has emerged as a key player in the pathogenesis and advancement of neurodegenerative diseases. Pharmacological activation of REV-ERBα inhibits LPS-induced microglial activation (Guo et al. 2019). REV-ERBα mitigated neuroinflammation in PD by regulating the NLRP3 inflammasome in microglia (Kou et al. 2022). Microglial REV-ERBα deletion exacerbates inflammation, tau aggregation, and droplet formation (Lee et al. 2023). REV-ERBα antagonist SR8278 or genetic suppression of REV-ERBα expedited microglial uptake of Aβ (Lee et al. 2020). Earlier studies documented that REV-ERBα deficiency resulted in elevated expression of GFAP and C3, along with microglial activation in mice (Griffin et al. 2019, 2020).
Previous research primarily concentrated on the role of REV-ERBα in neurodegenerative diseases within the context of microglia. Our investigation specifically addressed the impact of REV-ERBα on astrocyte activation. Within our study, we observed downregulation of REV-ERBα expression in A1 astrocytes exposed to LPS-treated BV2 supernatant. Inhibition of REV-ERBα in astrocytes resulted in a pro-inflammatory A1 phenotype, elevated levels of complement C3, iNOS and chemokine CXCL10, as well as increased phosphorylation of p65. Inhibition of REV-ERBα led to the conversion of astrocytes into an anti-inflammatory A2 phenotype, upregulated S100A10 and GDNF expression, and enhanced STAT3 phosphorylation. REV-ERBα may have potentially beneficial effects. Pharmacological activation of REV-ERB in astrocyte rescued the death of dopaminergic neurons Fig. 6.
This binary classification does not fully represent the diversity of reactive astrocytes. In reality, astrocytes can exist in various reactive profiles beyond A1 and A2. Additionally, the dynamic nature of astrocyte responses, influenced by time and varying stimuli, is not adequately captured by the static A1 and A2 classification (Escartin et al. 2021). Existing research lacks comprehensive descriptions of the morphological features distinguishing A1 and A2 reactive astrocytes. Relying solely on GFAP expression and morphology is inadequate for categorizing astrocytes as reactive. In pathological contexts, astrocytes exhibit substantial morphological alterations beyond mere hypertrophy, including elongation and process extension towards injury sites, as well as three-dimensional structural overlap (Escartin et al. 2021; Stanca et al. 2023). The morphological characteristics of astrocytes do not necessarily correlate with their functional phenotype or their effects on other cell types. In future classifications of reactive astrocytes, it is essential to incorporate various criteria, such as transcriptomics, proteomics, morphology, specific cell functions, and pathological markers (Escartin et al. 2021; Endo et al. 2022).
Our study provides insights into the pathogenesis of astrocyte activation mediated by REV-ERBα. Circadian clock regulation and neuroinflammation represent burgeoning areas of interest in PD research, each with unique and pioneering features (Colwell 2021). Our endeavor involved elucidating the mechanism by which astrocyte dysfunction, mediated by the circadian clock protein REV-ERBα, impacts dopaminergic neuron injury. Additionally, we explored the therapeutic implications of targeting circadian clock proteins and glial cells in PD.
Although this study reveals the critical role of REV-ERBα in regulating astrocyte function and the pathogenesis of Parkinson’s disease (PD), there are some limitations. Firstly, the study primarily relies on in vitro cell line experiments, which may not fully reflect the complex physiological environment in vivo, necessitating further in vivo studies to validate these findings. Secondly, the use of different cell lines may introduce variability, affecting the interpretation and reproducibility of the results; hence, future studies should consider using primary cells or animal models. Additionally, technical limitations, such as the specificity and efficacy of REV-ERBα agonists and inhibitors, may impact the understanding of their mechanisms of action, requiring further optimization of experimental conditions. In summary, while this study provides important insights, further research is needed to overcome these limitations and validate its clinical relevance.
Data Availability
No datasets were generated or analysed during the current study.
References
Adlanmerini M, Nguyen HC, Krusen BM, Teng CW, Geisler CE, Peed LC, Carpenter BJ, Hayes MR, Lazar MA (2021) Hypothalamic REV-ERB nuclear receptors control diurnal food intake and leptin sensitivity in diet-induced obese mice. J Clin Investig 131:e140424
Araujo B, Caridade-Silva R, Soares-Guedes C, Martins-Macedo J, Gomes ED, Monteiro S, Teixeira FG (2022) Neuroinflammation and Parkinson's disease-from neurodegeneration to therapeutic opportunities. Cells 11(18):2908
Brandebura AN, Paumier A, Onur TS, Allen NJ (2023) Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat Rev Neurosci 24:23–39
Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA (2014) Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 71:589–595
Chen J, Zhang DM, Feng X, Wang J, Qin YY, Zhang T, Huang Q, Sheng R, Chen Z, Li M, Qin ZH (2018) TIGAR inhibits ischemia/reperfusion-induced inflammatory response of astrocytes. Neuropharmacology 131:377–388
Colombo E, Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37:608–620
Colwell CS (2021) Defining circadian disruption in neurodegenerative disorders. J Clin Invest 131(19):e148288
Endo F, Kasai A, Soto JS, Yu X, Qu Z, Hashimoto H, Gradinaru V, Kawaguchi R, Khakh BS (2022) Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science. 378:eadc9020
Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhauser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen WT, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Diaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Gotz M, Gutierrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai KK, Norris CM, Okada S, Oliet SHR, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Perez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein JD, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner IB, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24:312–325
Everett LJ, Lazar MA (2014) Nuclear receptor Rev-erbalpha: up, down, and all around. Trends Endocrinol Metab 25:586–592
Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, Hayes ME, Cedeno MR, Nadarajah CJ, Ezerskiy LA, Colonna M, Zhang J, Bauer AQ, Burris TP, Musiek ES (2019) Circadian clock protein Rev-erbalpha regulates neuroinflammation. Proc Natl Acad Sci USA 116:5102–5107
Griffin P, Sheehan PW, Dimitry JM, Guo C, Kanan MF, Lee J, Zhang J & Musiek ES (2020) REV-ERBalpha mediates complement expression and diurnal regulation of microglial synaptic phagocytosis, eLife. 9. https://doi.org/10.7554/eLife.58765
Guo DK, Zhu Y, Sun HY, Xu XY, Zhang S, Hao ZB, Wang GH, Mu CC, Ren HG (2019) Pharmacological activation of REV-ERBalpha represses LPS-induced microglial activation through the NF-kappaB pathway. Acta Pharmacol Sin 40:26–34
Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci U S A 97:2875–2880
Hastings MH, Brancaccio M, Gonzalez-Aponte MF, Herzog ED (2023) Circadian rhythms and astrocytes: the good, the bad, and the ugly. Ann Rev Neurosci 46:123–143
Hayashi A, Matsunaga N, Okazaki H, Kakimoto K, Kimura Y, Azuma H, Ikeda E, Shiba T, Yamato M, Yamada K, Koyanagi S, Ohdo S (2013) A disruption mechanism of the molecular clock in a MPTP mouse model of Parkinson’s disease. NeuroMol Med 15:238–251
Ikegami K, Refetoff S, Van Cauter E, Yoshimura T (2019) Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol 15:590–600
Ishii T, Warabi E, Mann GE (2019) Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radical Biol Med 133:169–178
Koronowski KB, Sassone-Corsi P (2021) Communicating clocks shape circadian homeostasis. Science 371(6530):eabd0951
Kou L, Chi X, Sun Y, Han C, Wan F, Hu J, Yin S, Wu J, Li Y, Zhou Q, Zou W, Xiong N, Huang J, Xia Y, Wang T (2022) The circadian clock protein Rev-erbalpha provides neuroprotection and attenuates neuroinflammation against Parkinson’s disease via the microglial NLRP3 inflammasome. J Neuroinflammation 19:133
Lananna BV, Nadarajah CJ, Izumo M, Cedeno MR, Xiong DD, Dimitry J, Tso CF, McKee CA, Griffin P, Sheehan PW, Haspel JA, Barres BA, Liddelow SA, Takahashi JS, Karatsoreos IN, Musiek ES (2018) Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep 25(1–9):e5
Lee J, Kim DE, Griffin P, Sheehan PW, Kim DH, Musiek ES, Yoon SY (2020) Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer’s disease. Aging Cell 19:e13078
Lee HG, Wheeler MA, Quintana FJ (2022) Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discovery 21:339–358
Lee J, Dimitry JM, Song JH, Son M, Sheehan PW, King MW, Travis Tabor G, Goo YA, Lazar MA, Petrucelli L, Musiek ES (2023) Microglial REV-ERBalpha regulates inflammation and lipid droplet formation to drive tauopathy in male mice. Nat Commun 14:5197
Leone MJ, Marpegan L, Bekinschtein TA, Costas MA, Golombek DA (2006) Suprachiasmatic astrocytes as an interface for immune-circadian signalling. J Neurosci Res 84:1521–1527
Liang P, Zhang X, Zhang Y, Wu Y, Song Y, Wang X, Chen T, Liu W, Peng B, Yin J, He F, Fan Y, Han S, He X (2023) Neurotoxic A1 astrocytes promote neuronal ferroptosis via CXCL10/CXCR3 axis in epilepsy. Free Radical Biol Med 195:329–342
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487
McKee CA, Lananna BV, Musiek ES (2020) Circadian regulation of astrocyte function: implications for Alzheimer’s disease. Cell Mole Life Sci : CMLS 77:1049–1058
Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS, Yoo ID, Moon JS (2021) NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol 41:101947
Patani R, Hardingham GE, Liddelow SA (2023) Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Rev Neurol 19:395–409
Roth RH, Ding JB (2020) From neurons to cognition: technologies for precise recording of neural activity underlying behavior. BME Front 2020:7190517
Shkodina AD, Tan SC, Hasan MM, Abdelgawad M, Chopra H, Bilal M, Boiko DI, Tarianyk KA, Alexiou A (2022) Roles of clock genes in the pathogenesis of Parkinson’s disease. Ageing Res Rev 74:101554
Stanca S, Rossetti M, Bongioanni P (2023) Astrocytes as Neuroimmunocytes in Alzheimer’s disease: a biochemical tool in the neuron-glia crosstalk along the pathogenetic pathways. Int J Mol Sci. 24:13880
Uriz-Huarte A, Date A, Ang H, Ali S, Brady HJM, Fuchter MJ (2020) The transcriptional repressor REV-ERB as a novel target for disease. Bioorg Med Chem Lett 30:127395
Xu F, Jiang Y, Wang X, Shen L, Yan Y, Guo D, Wang C (2023) Sodium aescinate inhibits microglia activation through NF-kappaB pathway and exerts neuroprotective effect. Front Pharmacol 14:1086429
Funding
This work was supported by the National Natural Science Foundation of China (82001255, 32300799), the Natural Science Foundation of Jiangsu Province (SBK20200213), and the Suzhou Science and Technology Plan Project (SKYD2023090, SKYD2023091, SKYD2023180).
Author information
Authors and Affiliations
Contributions
Xiaoyu Wang performed most of the experiments and drafted manuscript. Zongqin Zhang and Hui Zhi analyzed the data; Jingwei Li revised manuscript. Dongkai Guo edited and revised manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences.
Consent for publication
The authors confirm that the work described has not been published before.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhi, H., Zhang, Z. et al. REV-ERBα Mitigates Astrocyte Activation and Protects Dopaminergic Neurons from Damage. J Mol Neurosci 74, 84 (2024). https://doi.org/10.1007/s12031-024-02264-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12031-024-02264-w