Abstract
Microglia are resident macrophages within the central nervous system, serving as the first responders to neuroinflammation. Glucocorticoids (GCs) may cause damage to brain tissue, but the specific mechanism remains unclear. This study was divided into two parts: a glucocorticoid receptor (GR) mitochondrial translocation intervention experiment and a mitochondrial oxidative stress inhibition experiment. BV-2 microglia were stimulated with dexamethasone (DEX) and treated with either tubastatin-A or mitoquinone (MitoQ) for 24 h. Our results showed that DEX increased the translocation of GRs to mitochondria, and this effect was accompanied by decreases in the expression of mitochondrially encoded cytochrome c oxidase 1 (MT-CO1) and mitochondrially encoded cytochrome c oxidase 3 (MT-CO3) and increases in the expression of NOD-like receptor thermal protein domain–associated protein 3 (NLRP3), caspase-1, and Gasdermin D (GSDMD). The level of mitochondrial respiratory chain complex IV (MRCC IV) and adenosine triphosphate (ATP) was decreased. An elevation in the level of mitochondrial oxidative stress and the opening of the mitochondrial permeability transition pore (mPTP) was also observed. Mechanistically, tubastatin-A significantly suppressed the mitochondrial translocation of GRs, improved the expression of mitochondrial genes, promoted the restoration of mitochondrial function, and inhibited pyroptosis. MitoQ significantly prevented mitochondrial oxidative stress, improved mitochondrial function, and reduced apoptosis and pyroptosis. Both tubastatin-A and MitoQ suppressed DEX-induced pyroptosis. This study substantiates that the increase in the mitochondrial translocation of GRs mediated by GCs exacerbates oxidative stress and pyroptosis in microglia, which indicates that the regulation of mitochondrial pathways by GCs is pathogenic to microglia.
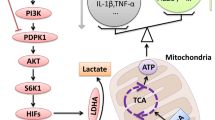
Graphical Abstract
The increase in mitochondrial translocation of GRs mediated by GCs aggravates mitochondrial dysfunction and oxidative stress, leading to pyroptosis in BV-2 microglia. Tubastatin-A and MitoQ can inhibit GR translocation and oxidative stress in mitochondria, respectively, and these effects can inhibit pyroptosis and other damage induced by GCs to microglia.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids (GCs) are a crucial class of steroid hormones that exert significant physiological and pharmacological effects on the body. As essential stress hormones, GCs play pivotal roles in various physiological and pathological processes, including metabolic homeostasis, cognition, development, reproduction, and inflammation. Moreover, as one of the remarkable medical discoveries of the twentieth century, GCs have found extensive clinical applications as prescription drugs and continue to serve as indispensable and irreplaceable agents in the treatment of autoimmune diseases, inflammation, and allergic conditions (Vandewalle et al. 2018). The long-term clinical application of GCs has been associated with a wide range of adverse effects, including osteoporosis, cutaneous atrophy, secondary infections, Cushing’s syndrome, and other drug-related side effects. These complications significantly hinder the widespread use of GCs in clinical settings (Oray et al. 2016; Vandewalle et al. 2018). Indeed, the precise mechanism underlying the side effects of GCs remains incompletely understood, and their empirical usage persists in clinical settings. Understanding the underlying mechanisms of GCs is crucial to maximize their therapeutic benefits while minimizing their adverse metabolic effects and is the current focus of researchers.
GCs have been reported to induce damage to brain tissue (Drakulić et al. 2015). Research suggests that GCs are linked to the development and progression of age-related neurodegenerative diseases such as Alzheimer disease and Parkinson disease (Choi et al. 2018; Claros et al. 2021; Canet et al. 2022; Du et al. 2023). Furthermore, GC treatment is recognized as a significant risk factor for neurological complications linked to autoimmune diseases (Tarr et al. 2017). For example, approximately 2–60% of patients with systemic lupus erythematosus (SLE) experience emotional, behavioral, and cognitive impairment, memory decline, and other psychiatric symptoms as a result of GC administration (Bolanos et al. 2004; Hanly et al. 2019). However, the specific mechanism underlying this association remains unclear. Therefore, it is crucial to elucidate the mechanisms by which GCs impair brain tissue to subsequently alleviate the adverse reactions caused by GCs.
Oxidative stress plays a pivotal role in the pathogenesis of numerous brain disorders, including neurodegenerative diseases (Salim 2017; Akyuva et al. 2021; Forman and Zhang 2021; Rostami et al. 2022; Caruso et al. 2023). Brain tissue, which is a high oxygen- and energy-demanding tissue, is also abundant in lipids. Reactive oxygen species (ROS) readily form lipid oxides with brain lipids and thus perpetuate damage to brain tissue (Salim 2017). The production of ROS is closely associated with neuroinflammation, mitochondrial dysfunction in microglia, and pathological changes in neurodegenerative diseases themselves, and they mutually influence each other (Teleanu et al. 2022). GCs have been shown to induce oxidative stress in various tissues, including the brain (Camm et al. 2011). Excessive ROS during oxidative stress can increase blood-brain barrier permeability, alter brain morphology, induce neuroinflammation, and lead to microglia and other brain cell death (Wu et al. 2020; Liu et al. 2020; Akyuva et al. 2021). Investigating the oxidative stress damage mediated by GCs may offer novel insights for understanding the mechanisms underlying GC-mediated brain tissue injury.
Mitochondria are the main source of intracellular ROS and play a critical role in oxidative stress damage (Tayanloo-Beik et al. 2022; Yıldızhan and Nazıroğlu 2023). Mitochondria produce a substantial amount of ATP through oxidative phosphorylation in the mitochondrial respiratory chain (MRC) and thereby provide vital energy for cellular functions. Concurrently, the leakage of free electrons from the MRC combines with oxygen to generate ROS, leading to oxidative damage of cellular biomacromolecules and impairing cell and organ functionality (Kowalska et al. 2020). Research has also demonstrated that impaired mitochondrial function manifests as the opening of the mitochondrial permeability transition pore (mPTP) and an increase in the mitochondrial membrane permeability (MMP), which leads to the release of cytochrome C (Cyt-C) from mitochondria and the induction of cellular apoptosis (Bonora et al. 2022; Flores-Romero et al. 2023). Oxidized fragments of mitochondrial DNA (Ox-mtDNA) can escape from the mitochondria via the mPTP-VDAC channel, which results in the activation of cytoplasmic NLRP3 and the triggering of pyroptosis (Qiu et al. 2022; Xian et al. 2022). Both apoptosis and pyroptosis are forms of programmed cell death. Abnormal mitochondrial function can exacerbate organ damage by inducing apoptosis and pyroptosis and thereby initiate and aggravate various diseases.
GRs are present in almost all human cells. The anti-inflammatory effects of GCs primarily occur through the translocation of GRs to the nucleus and the subsequent regulation of nuclear genes (Garside et al. 2004; Heitzer et al. 2007). DEX shows a stronger and longer-lasting affinity for GRs than cortisol, which may be related to its higher biological activity and longer half-life (Shimojo et al. 1995). Furthermore, emerging evidence suggests that GRs can also translocate to mitochondria, but their precise role remains unclear (Psarra and Sekeris 2009; Panettieri et al. 2019). Therefore, investigating the impact of the mitochondrial translocation of GRs may help elucidate the mechanisms underlying the adverse reactions in brain tissue induced by GCs.
Microglia are resident macrophages within the central nervous system that play a crucial immune role in supporting brain development, maintenance, homeostasis, and repair (Mehl et al. 2022). They also serve as commonly utilized cellular models to study the pathogenesis of neurodegenerative diseases (Grubman et al. 2016; Yıldızhan and Nazıroğlu 2019). The immortalized murine microglial cell line BV-2 has been used frequently as a substitute for primary microglia (Henn et al. 2009). Microglia represent the immune system of the brain. Their role is central in two phenomena, neuroinflammation and oxidative stress (Block et al. 2007; Yıldızhan and Nazıroğlu 2020; Caruso et al. 2023), which are at the roots of different pathologies related to the central nervous system. Some studies have shown that prolonged exposure to elevated levels of GCs may contribute to brain inflammation, including the pro-inflammatory activation of microglia, which could potentially increase susceptibility to neurodegenerative diseases (Pedrazzoli et al. 2019; Madore et al. 2020). In spite of significant advances in the understanding of microglial functions in neurodegenerative diseases in recent years, the exact role of microglia in various pathological states still needs to be better clarified. In this study, we focused on the role of GCs in the mitochondrial regulatory pathway of microglia to explore the potential novel mechanisms of GC-induced brain tissue damage.
Materials and Methods
Cell Culture and Treatments
BV-2 mouse microglia (SNL-155) were obtained from SUNNCELL (Wuhan, China). The cells were cultured at 37 °C in 5% CO2 in RPMI 1640 (#C11875500BT, Gibco, USA) supplemented with 10% fetal bovine serum (#FSP500, ExCell Bio, China), 105 units/l penicillin, and 100 g/l streptomycin (#15140-122, Gibco, USA). Only cells in the logarithmic phase of growth were used for the experiment. When the cell density per well reached 80–90%, interventions with DEX (#D4902, Sigma‒Aldrich, USA), tubastatin-A (#HY-13271A, MedChemExpress, USA), and MitoQ (#HY-100116A, MedChemExpress, USA) were performed for 24 h. The experimental groups were assigned based on the principle of randomization. No blind method was established in this experiment.
Cell Viability Assays
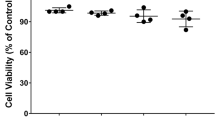
Cell viability was determined using a CCK-8 kit (#GK10001, Glpbio, USA). Based on our pre-test, BV-2 microglia were prepared as a cell suspension, counted using a cell counter (#Cellometer AUTO 1000, Nexcelom, USA), and then seeded in 96-well plates at a density of 5000 cells per well. The cells were treated with different concentrations of DEX, tubastatin-A, or MitoQ for a duration of 24 h. The cell morphology was subsequently observed. After incubation with CCK-8 reagent (10 µL) for 1 h, the optical density (OD) values were detected at 450 nm using a full-wavelength microplate reader (#Multiskan™ FC, Thermo Fisher, USA). The cell proliferation rates after exposure to different drug concentrations were determined, and the optimal concentrations for each experimental group were selected.
Mitochondrial and Nuclear Extraction
BV-2 microglia were seeded in 10-cm culture dishes and incubated for the specified duration. The cells were treated with the designated substances according to the specified protocol. After digestion with 0.25% trypsin/EDTA solution (#25200056, Thermo Fisher, USA), the cells were centrifuged at 200 × g for 10 min at room temperature for collection. Following the manufacturer’s instructions, mitochondria and nuclei were separately extracted from the cells using a cell mitochondrial separation kit (#C3601, Beyotime, China) and a nuclear protein and cytoplasmic protein extraction kit (#P0027, Beyotime, China). The isolated mitochondria and nuclei were then cleaved in RIPA buffer (#WB3100, NCM Biotech, China), which contained a combination of protease and phosphatase inhibitors. These steps were performed in preparation for subsequent western blot analysis.
Western Blot
Total protein was extracted using a lysis buffer (RIPA to PMSF = 100:1) and denatured by boiling at 100 °C for 8 min. In brief, the proteins were separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (SDS‒PAGE) (#PG112 and #PG114, EpiZyme, China) and then electrotransferred onto a polyvinylidene fluoride (PVDF) membrane (#10600021, Cytiva, USA). The membrane was blocked with 5% skim milk and then incubated overnight at 4 °C with the primary antibody. Subsequently, the membrane was incubated with an HRP-labeled secondary antibody and analyzed using a highly sensitive ECL chemiluminescence kit (#P10100, NCM Biotech, China). The protein bands were visualized using chemiluminescent imagers (#Tanon 5200, Tanon, China). For quantitative analysis, gray values were determined using ImageJ software.
The following antibodies were used: MT-CO1 (#ab203912, RRID: AB_2801537, Abcam, UK, 1:1000, 37kDa), MT-CO3 (#ab110259, RRID: AB_10859925, Abcam, UK, 1:1000, 30 kDa), GR (#ab183127, RRID: AB_2833234, Abcam, UK, 1:2000, 83 to ~86 kDa), VDAC1 (#ab15895, RRID: AB_2214787, Abcam, UK, 1:1000, 31 kDa), NLRP3 (#ab263899, RRID: AB_2889890, Abcam, UK, 1:1000, 118 kDa), pro Caspase-1 + p10 + p12 (#ab179515, RRID: AB_2884954, Abcam, UK, 1:1000, 45/42/35/12 kDa), GSDMD (#ab209845, RRID: AB_2783550, Abcam, UK, 1:1000, 53/32 kDa), GAPDH (#ab8245, RRID: AB_2107448, Abcam, UK, 1:10000, 36 kDa), Histone H3 (#BF9211, RRID: AB_2839427, Affinity Biosciences, USA, 1:1000, 15 kDa), BAX (#sc-20067, RRID: AB_626726, Santa Cruz, USA, 1:500, 23 kDa), BCL-2 (#T40056S, RRID: AB_2929011, Abmart, China, 1:1000, 26 kDa), HRP-labeled goat anti-rabbit IgG (#AS014, RRID: AB_2769854, ABclonal, China, 1:2500), and HRP-labeled goat anti-mouse IgG (#AS003, RRID: AB_2769851, ABclonal, China, 1:10000).
Quantitative Real-Time PCR (qRT‒PCR) Analysis
Total RNA extraction was performed using RNA-easy Isolation Reagent (#R701-02, Vazyme, China). The HiScript III 1st-Strand cDNA Synthesis Kit (#R323-01, Vazyme, China) was utilized for cDNA synthesis. The qPCRs were conducted with ChamQ™ Universal SYBR® qPCR Master Mix (#Q711-02, Vazyme, China) following the manufacturer’s instructions. The gene sequences published in NCBI GenBank were consulted for primer design, and Primer Premier 5.0 software was utilized (see Table 1). The relative expression was calculated using the 2^−ΔΔCt method with β-actin serving as the internal reference sequence.
Detection of MRCC IV and Mn-SOD
The cells were lysed using phosphate-buffered saline (PBS) (#C20012500BT, Gibco, USA), and the supernatants were obtained through centrifugation. Furthermore, the mouse MRCC IV ELISA kit (#MM-44753M1, MEIMIAN, China) and the CuZn/Mn-SOD activity detection kit (#S0103, Beyotime, China) were used according to the manufacturer’s instructions. MRCC IV and Mn-SOD were extracted from the cells, and their OD values were detected at 450 nm using a full-wavelength microplate reader.
Detection of ATP
ATP was extracted from the cells using the ATP detection kit (#S0026, Beyotime, China) according to the manufacturer’s instructions. RLU measurements were detected using the luminometric measurement module of the spectral scanning multimode reader (#Varioskan Flash, Thermo Fisher, New York, USA).
Detection of mtROS
The MitoSOX Red superoxide indicator (#M36008, Thermo Fisher, USA) is a recently developed fluorescent probe that specifically targets the mitochondria of live cells. This probe can permeate through the cell membrane and generate a vibrant red fluorescence after oxidation by mitochondrial superoxide. According to the manufacturer’s instructions, after 24 h of drug treatment, the supernatant was discarded, and an appropriate amount of Hoechst 33342 live cell dye (5 µg/ml) (#C1025, Beyotime, China) diluted with serum-free medium was added to the cells for nuclear staining. The cells were then incubated in the dark at 37 °C for 10 min and washed 3 times with warm PBS. A solution of MitoSOX Red (2.5 µM) diluted with serum-free medium was then added to the cells, and the cells were then incubated for 10 min at 37 °C in the absence of light. After 3 washes with warm PBS, the fluorescence intensity of mtROS was observed and documented by laser confocal microscopy (#LSM880, Zeiss, Germany). The MitoSOX Red excitation wavelength is 396 nm, and the emission wavelength is 610 nm. The Hoechst 33342 excitation wavelength was 350 nm, and the emission wavelength was 461 nm. Analyses of the average fluorescence intensities of the images were performed using ImageJ.
Detection of mPTP
The mPTP assay kit (#C2009S, Beyotime, China) was utilized to assess the opening of mPTP. According to the manufacturer’s guidelines, the supernatant was discarded after 24 h of drug treatment. Subsequently, the cells were stained with an appropriate amount of calcein AM staining solution and incubated at 37 °C for 40 min in the presence of cobalt chloride (CoCl2, 1 mmol/l). The fluorescence intensity of the mPTP was measured using an inverted fluorescence microscope (#DMi8, Leica, Germany) with an excitation wavelength of 494 nm and an emission wavelength of 517 nm. An analysis of the average fluorescence intensities of the images was performed using ImageJ.
Detection of Apoptosis
Annexin V-FITC, which is labeled with the fluorescent probe FITC (exhibits green fluorescence under fluorescence microscopy), enables very simple and direct detection of phosphatidylserine ectropion, an important feature of apoptosis. Propidium iodide (PI) can stain necrotic cells or cells that have lost their cell membrane integrity in late apoptosis, which makes them exhibit red fluorescence. Double staining with Annexin V-FITC and PI can further distinguish between apoptotic and necrotic cells. Compared to flow cytometry, the in situ fluorescence detection of adherent cells can avoid the interference of trypsin on apoptosis results.
Apoptosis was detected using the Annexin V-FITC apoptosis detection kit (#C1062M, Beyotime, China). According to the manufacturer’s instructions, after treatment of the cells for 24 h, the supernatant was removed, and the cells were washed once with PBS as described above. Then, 195 µl of Annexin V-FITC binding solution, 5 µl of Annexin V-FITC, and 10 µl of propyl iodide stain solution were successively added and gently mixed. The mixture was then incubated at room temperature for 20 min in the dark. The level of apoptosis was observed by inverted fluorescence microscopy. The FITC excitation wavelength was 488 nm, and the emission wavelength was 525 nm. The PI excitation wavelength was 535 nm, and the emission wavelength was 617 nm. An analysis of the average fluorescence intensities of the images was performed using ImageJ.
Statistical Analysis
The statistical differences among groups were evaluated using GraphPad Prism 9.0 software (GraphPad Software Inc., USA). All experiments were performed at least in triplicate, and the data are shown as the means ± standard deviations (means ± SDs). Differences among groups were assessed using Student’s t test (two groups) or ANOVA (multiple groups). P < 0.05 was considered to determine statistical significance.
Results
Tubastatin-A can Inhibit the DEX-Mediated Mitochondrial Translocation of GRs
The CCK-8 assay was adopted to identify the optimal intervention concentration of the drugs. The individual use of DEX (0–2 µM) or tubastatin-A (0–10 µM) did not have an impact on cell viability. Therefore, DEX and tubastatin-A concentrations of 1.5 µM (Fig. 1a) and 10 µM (Fig. 1b), respectively, were chosen for subsequent experiments. Tubastatin-A is a highly specific inhibitor of HDAC6 (Shen et al. 2020), which is closely associated with GR translocation. We employed tubastatin-A to inhibit the translocation of GRs. BV-2 microglia were separately treated with DEX or tubastatin-A. Mitochondria and nuclei were then extracted using mitochondrial separation kits, nuclear protein extraction kits, and cytoplasmic protein extraction kits, and a western blot analysis was subsequently conducted. Interference with DEX enhanced the translocation of GRs to the mitochondria and nucleus in BV-2 microglia, whereas tubastatin-A treatment suppressed the translocation of GRs to the mitochondria but did not induce a significant decrease in GRs in the nucleus (Fig. 1c).
Tubastatin-A inhibits the DEX-mediated mitochondrial translocation of GRs in BV-2 microglia. a The cell viability under DEX intervention was assessed with a CCK-8 assay. b The cell viability under tubastatin-A intervention was assessed with a CCK-8 assay. c The protein expression levels of mitochondrial and nuclear GRs were assessed by western blot analysis. All data are presented as the means ± SDs. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA. DEX, dexamethasone
Tubastatin-A Alleviates Mitochondrial Dysfunction Induced by DEX
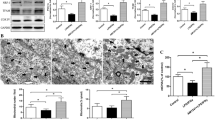
First, we investigated whether the translocation of GRs to the mitochondria directly impacts mitochondrial function. We observed a reduction in both the mRNA and protein levels of the mitochondrial genes MT-CO1 and MT-CO3 after DEX intervention (Fig. 2a and b). Because the proteins encoded by these genes are essential components of MRCC IV and both MRCC IV and ATP are integral to mitochondrial function, we detected the expression of MRCC IV and ATP. The levels of both MRCC IV (Fig. 2c) and ATP (Fig. 2d) in BV-2 microglia decreased after DEX intervention.
Tubastatin-A alleviates the mitochondrial functional disorders by inhibiting the DEX-mediated mitochondrial translocation of GRs in BV-2 microglia. a The mRNA expression levels of Mt-co1 and Mt-co3 were assessed by qRT‒PCR. b The protein expression levels of MT-CO1 and MT-CO3 were assessed by western blot analysis. c The MRCC IV level was assessed using an MRCC IV ELISA kit. d The ATP level was assessed using an ATP detection kit. e The mtROS level was assessed using the MitoSOX Red superoxide indicator and observed by laser confocal microscopy (n = 4 independent experiments). Stronger red fluorescence indicates higher levels of mtROS. Scale bar = 50 µm. f The Mn-SOD level was assessed using a CuZn/Mn-SOD activity detection kit. All data are presented as the means ± SDs. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA (the mean fluorescence intensity was analyzed by two-way ANOVA). DEX, dexamethasone
We observed an increase in the level of mtROS in the mitochondria of BV-2 microglia after DEX intervention by laser confocal microscopy (Fig. 2e), which suggests an increase in oxidative stress levels within the mitochondria. Mn-SOD is primarily expressed in mitochondria and plays a major role in antioxidant defense (Zelko et al. 2002; Bhaskaran et al. 2023). We found that the Mn-SOD activity decreased in the DEX group (Fig. 2f). To validate the hypothesis that the translocation of GRs to the mitochondria affects mitochondrial function, we treated the cells with tubastatin-A to affect GR translocation. Tubastatin-A treatment inhibited a series of mitochondrial functional disorders induced by DEX (Fig. 2), which include decreases in mitochondrial gene expression, MRCC IV levels, ATP levels, and an oxidative/antioxidative imbalance.
Tubastatin-A Inhibits the DEX-Induced Increase of the MMP
Mitochondrial dysfunction can lead to an increase in the MMP, which in turn induces apoptosis. This process is associated with the opening of the mPTP. To detect the opening of the mPTP, we utilized an mPTP assay kit. After calcein acetoxymethyl ester (calcein AM) enters the cell, it can be hydrolyzed by intracellular esterases to remove AM, generating a polarity fluorescent dye called calcein that lacks membrane permeability, which causes the cytoplasm, including the mitochondria, to exhibit strong green fluorescence. However, when calcein combines with CoCl2, it loses its fluorescence. The opening of the mPTP has two effects: on the one hand, it allows partial release of calcein into the cytoplasm, where it combines with CoCl2 and causes loss of cytoplasmic calcein fluorescence, and on the other hand, it allows CoCl2 to enter the mitochondria, leading to the partial or complete quenching of the green fluorescence of calcein in the mitochondria. We observed a reduction in the green fluorescence intensity in BV-2 microglia in the DEX group, as revealed by fluorescence microscopy. This reduction suggests substantial opening of the mPTP. Furthermore, treatment with tubastatin-A partially inhibited the DEX-induced opening of the mPTP (Fig. 3).
Tubastatin-A inhibits the opening of the mPTP induced by DEX in BV-2 microglia. The fluorescence of the mPTP opening was assessed using an mPTP assay kit and observed with an inverted fluorescence microscope. Weaker green intensity indicates an increased opening of the mPTP. Scale bar = 50 µm. The data are presented as the means ± SDs (n = 4 independent experiments). *P < 0.05, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA. DEX, dexamethasone
Tubastatin-A Suppresses DEX-Induced Pyroptosis
Although the opening of the mPTP induces apoptosis, it also causes the release of mtDNA, which potentially initiates pyroptosis. To investigate pyroptosis, we assessed the mRNA and protein levels of NLRP3, caspase-1, and GSDMD, which are key molecules involved in cellular pyroptosis. The expression levels of NLRP3, caspase-1, and GSDMD were all elevated in the DEX group. Conversely, tubastatin-A inhibited DEX-induced pyroptosis (Fig. 4a and b).
Tubastatin-A suppresses pyroptosis induced by DEX in BV-2 microglia. a The mRNA expression levels of Nlrp3, caspase-1, and Gsdmd were assessed by qRT‒PCR. b The protein expression levels of NLRP3, caspase-1, and GSDMD were assessed by western blot analysis. All data are presented as the means ± SDs. ***P < 0.001 and ****P < 0.0001 by one-way ANOVA. DEX, dexamethasone
MitoQ Alleviates DEX-Induced Mitochondrial Dysfunction
MitoQ, a mitochondria-targeted antioxidant, exerts specific and targeted antioxidant effects on mitochondria (Xiao et al. 2017; Kang et al. 2020). We utilized MitoQ to inhibit mitochondrial oxidative stress and validated the hypothesis that reducing this stress might reverse cellular apoptosis and pyroptosis caused by mitochondrial dysfunction. The MitoQ concentration of 500 nM was chosen for subsequent experiments after assessment using the CCK-8 assay (Fig. 5a). We initially confirmed the effective alleviation of DEX-induced mitochondrial oxidative stress by MitoQ. MitoQ treatment alleviated the increase in mtROS levels (Fig. 5b) and the decrease in Mn-SOD levels (Fig. 5c) induced by DEX in BV-2 microglia. Moreover, we further verified that MitoQ intervention can inhibit DEX-induced mitochondrial dysfunction. Treatment with MitoQ improved the decreases in the expression of the mitochondrial genes Mt-co1 and Mt-co3 (Fig. 5d), the expression of MRCC IV (Fig. 5e), and ATP level (Fig. 5f) induced by DEX exposure.
MitoQ alleviates mitochondrial dysfunction by inhibiting DEX-induced mitochondrial oxidative stress in BV-2 microglia. a The cell viability under MitoQ intervention was assessed via a CCK-8 assay. b The mtROS level was assessed using the MitoSOX Red superoxide indicator and observed by laser confocal microscopy (n = 4 independent experiments). Stronger red fluorescence indicates higher levels of mtROS. Scale bar = 20 µm. c The Mn-SOD level was assessed using a CuZn/Mn-SOD activity detection kit. d The mRNA expression levels of Mt-co1 and Mt-co3 were assessed by qRT‒PCR. e The MRCC IV level was assessed using an MRCC IV ELISA kit. f The ATP level was assessed using an ATP detection kit. All data are presented as the means ± SDs. *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA (the mean fluorescence intensity was analyzed by two-way ANOVA). DEX, dexamethasone; MitoQ, mitoquinone
MitoQ Can Reduce DEX-Induced Apoptosis and Pyroptosis in BV-2 Microglia
Our results suggest that DEX has the potential to induce apoptosis and pyroptosis through mitochondrial oxidative stress. To further investigate this hypothesis, we treated BV-2 microglia with MitoQ. The increase in cells positive for Annexin V-FITC staining indicated a significant increase in apoptotic responses in the DEX group (Fig. 6a), and this increase was accompanied by elevated levels of the pro-apoptotic molecule BAX and decreased levels of the anti-apoptotic molecule BCL-2 (Fig. 6b and c). Treatment with MitoQ inhibited the increases in apoptosis and pyroptosis levels induced by DEX in BV-2 microglia (Fig. 6).
MitoQ inhibits DEX-induced apoptosis and pyroptosis in BV-2 microglia. a The apoptosis level of BV-2 microglia under different drug interventions was observed using a fluorescence-inverted microscope (n = 4 independent experiments). Stronger green fluorescence indicates increased cell apoptosis. Scale bar = 50 µm. b The mRNA expression levels of Bax and Bcl-2 were assessed by qRT‒PCR. c The protein expression levels of BAX and BCL-2 were assessed by western blot analysis. d The mRNA expression levels of Nlrp3, caspase-1, and Gsdmd were assessed by qRT‒PCR. e The protein expression levels of NLRP3, caspase-1, and GSDMD were assessed by western blot analysis. All data are presented as the means ± SDs. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA (the mean fluorescence intensity was analyzed by two-way ANOVA). DEX, dexamethasone; MitoQ, mitoquinone
Discussion
Population aging has intensified in the twenty-first century, and challenges related to aging diseases such as neurodegenerative diseases are becoming increasingly prominent. Therefore, there is an urgent need to investigate the underlying mechanisms responsible for inducing potential damage to brain cells under normal conditions. GCs have been widely used in clinical practice as drugs, and the physiological secretion of GCs may also be abnormal under the influence of various stimuli. GCs have been identified as pathogenic factors for neurodegenerative diseases (Choi et al. 2018; Claros et al. 2021; Canet et al. 2022; Du et al. 2023). The literature (Garrud and Giussani 2019; Yang et al. 2022) and our previous studies (Zhao et al. 2022) consistently demonstrate that GCs can induce oxidative stress damage in tissues. GCs have been shown to contribute to the pathogenesis of neurodegenerative diseases by affecting microglia (Pedrazzoli et al. 2019; Madore et al. 2020). Neuroinflammation is a hallmark of many neurodegenerative diseases and plays a fundamental role in mediating the onset and progression of disease. Microglia are the first responders to neuroinflammation. Dysregulation of microglial function is closely related to the occurrence and development of neurodegenerative diseases (Yıldızhan and Nazıroğlu 2019). Our study focused on exploring the potential adverse effects of GCs on microglia and elucidating the possible pathogenic mechanisms through which GCs induce brain damage via the mitochondrial pathway.
Our findings demonstrate an increase in the mitochondrial translocation of GRs in BV-2 microglia following DEX intervention, suggesting that DEX may have an influence on brain cells through this mitochondrial pathway. GRs are nuclear receptors. Previous studies have shown that GCs bind to GRs in the cytoplasm, and after undergoing structural transformation and changes in activity, GRs can be translocated to the nucleus and regulate the expression of genes to exert powerful anti-inflammatory effects (Garside et al. 2004; Heitzer et al. 2007). In the past, researchers have primarily focused on the nuclear regulatory effects and outcomes resulting from the translocation of GRs into the nucleus. Relatively few studies have investigated the translocation of GRs into mitochondria induced by GCs. Psarra et al. found that after activation by GCs, GRs can translocate not only into the nucleus but also into mitochondria (Psarra and Sekeris 2009; Panettieri et al. 2019).
Additionally, Choi et al. found that the GC-mediated translocation of GR to mitochondria causes instability in neuronal microtubule dynamics and may contribute to Alzheimer disease pathogenesis (Choi et al. 2018), but the specific regulatory mechanism remains unclear. Mitochondria are the primary cellular organelles responsible for oxygen consumption and energy production. Healthy mitochondrial function is crucial for the normal functioning of brain tissue (Trigo et al. 2022). Therefore, investigating the impact of the GC-mediated mitochondrial translocation of GRs on mitochondrial function and its pathological and physiological effects on brain tissue is of scientific significance for elucidating the pathogenic mechanisms of GCs and preventing neurodegenerative diseases.
We observed that the translocation of GRs to mitochondria affects mitochondrial gene expression. Specifically, our findings demonstrate that intervention with DEX enhances the translocation of GRs to mitochondria while reducing the expression of MT-CO1 and MT-CO3. GCs primarily exert their effects through gene regulation. The GC nuclear regulatory pathway refers to the process by which GCs promote the translocation of GRs to the cell nucleus. Once in the nucleus, GRs bind to glucocorticoid response elements (GREs) on nuclear genes and thereby exert their pharmacological effects by upregulating or downregulating gene transcription. Additionally, six GREs, including three for MT-CO1 (GRE I, GRE II, and GRE III) and one for MT-CO3 (GRE IV), were distributed in mtDNA (Kokkinopoulou and Moutsatsou 2021). Our results suggest that DEX negatively regulates mitochondrial genes by promoting the translocation of GR to mitochondria. Furthermore, our results reveal a decrease in MRCC IV level and reduced expression levels of MT-CO1 and MT-CO3. We are concerned about the core role played by MT-CO1 and MT-CO3 in the MRC because their encoded proteins are components of MRCC IV (Kokkinopoulou and Moutsatsou 2021). ATP synthesis is an essential function carried out by the respiratory chain within mitochondria. Our findings indicate a systematic decline in MRC function after GC intervention that encompasses gene expression, MRCC IV level, and ATP level. This result suggests that the regulation of mitochondria by GCs is pathogenic and leads to dysfunction in neuromicroglia.
As a critical functional structure within mitochondria, MRC is a key process in mitochondrial energy metabolism and plays a crucial role in the maintenance of cellular homeostasis and normal function. The impairment of MRC function can induce oxidative stress damage to body cells, which is the fundamental pathogenesis of major human diseases and promotes disease progression (Morán et al. 2012; Forman and Zhang 2021). Our findings demonstrate that the DEX-induced mitochondrial translocation of GRs not only impairs the function of the MRC but also elevates the mtROS levels while reducing the Mn-SOD levels. MtROS and Mn-SOD directly reflect the degree of oxidative stress in mitochondria. The MRC serves as the primary source for ROS generation in cells. MtDNA lacking nucleosome protection is highly susceptible to damage by ROS, leading to mutations. Mutated mtDNA further exacerbates the functional impairments in MRC, resulting in increased production of ROS. This vicious cycle formed by abnormal downregulation of mtDNA and elevation of mtROS contributes to progressive cellular senescence and dysfunction (Zhao et al. 2021). Our results suggest that the increased mitochondrial translocation of GRs induced by GCs may aggravate neuromicroglia damage through multiple mechanisms. In addition to exogenous GC intervention, the human adrenal cortex in a stress state can secrete GC levels far beyond physiological doses. The pathogenic regulation of GCs on mitochondrial function indicates a new pathway for the onset and progression of brain tissue–related diseases, which may occur slowly but progressively worsen over time. This disease characteristic is consistent with the features of brain dysfunction diseases, such as Alzheimer disease and Parkinson disease, and deserves our attention and further research.
Our results also demonstrated that GC-mediated mitochondrial dysfunction leads to an increase in the opening of the mPTP and subsequent elevation in the apoptosis levels. The mPTP is located at the junction between the inner and outer membranes of mitochondria. The opening of the mPTP enhances mitochondrial membrane permeability, leading to increased release of the mitochondrial contents through the mPTP. Furthermore, intervention with DEX increased the expression of the cellular pyroptosis markers NLRP3, caspase-1, and GSDMD. This result suggests that DEX could induce both cell apoptosis and pyroptosis by regulating mitochondria. Mitochondria as a whole originate from endosymbiotic bacteria and gradually form a symbiotic relationship with other organelles within a cell during the lengthy process of endosymbiosis. However, these cells show significant differences in their genomes and metabolic characteristics compared with prokaryotic cells. Research has shown that decreased cellular mitochondrial function and increased oxidative stress can lead to the opening of the mPTP and the release of Cyt-C from mitochondria, which results in the initiation of cell apoptosis (Bonora et al. 2022; Flores-Romero et al. 2023). In contrast, the release of mtDNA initiates pyroptosis (Qiu et al. 2022; Xian et al. 2022). Unlike apoptosis, which does not involve inflammation, pyroptosis is associated with the release of many inflammatory factors upon cell membrane rupture. Leaked inflammatory factors can cause damage to surrounding cells and activate immune cells, which exacerbates the inflammatory response and leads to organ damage (Kesavardhana et al. 2020). Increased mitochondrial permeability resulting from the opening of mPTP has been demonstrated to play a crucial role in controlling cell death and inflammatory pathways (Flores-Romero et al. 2023). We found that NLRP3 was upregulated by GCs during the regulation of mitochondria in microglia and thereby exhibited a proinflammatory effect distinct from the anti-inflammatory effect mediated by the regulation of the nucleus. Currently, some studies have demonstrated that DEX can induce pyroptosis through the activation of related signaling pathways, but the specific mechanism remains unclear (Oh et al. 2021; Wang et al. 2021). The precise mechanism underlying the GC-mediated inflammatory response in neuromicroglia via the mitochondrial pathway has not been fully elucidated and warrants further investigation to determine its potential relevance to chronic immune inflammation associated with neurodegenerative diseases.
Our results suggest that the proinflammatory effects associated with regulation of the mitochondrial pathway by GCs are distinct from the anti-inflammatory effects associated with the nuclear regulation of GCs. To investigate both the pathogenicity of the regulation of the mitochondrial pathway by GCs and the underlying proinflammatory mechanism and to elucidate the distinction between GC nuclear regulation and GC mitochondrial regulation in immune inflammation, we utilized tubastatin-A for specific inhibition of GR translocation. Tubastatin-A is a potent and selective inhibitor of histone deacetylase 6 (HDAC6) (Shen et al. 2020). Inactivation of HDAC6 results in the disruption of the heat shock protein 90 (Hsp90)-p23-GR complex, thereby inhibiting the binding of GC to GR and the subsequent translocation of GR (Kovacs et al. 2005; Kirschke et al. 2014; Li et al. 2016; Noddings et al. 2022). We observed that tubastatin-A significantly inhibited the GC-induced mitochondrial translocation of GRs and significantly ameliorated the systemic decline in mitochondrial function resulting from increased GR translocation to mitochondria, which included a reduction in the ATP levels and an increase in mitochondrial oxidative stress. Additionally, the apoptosis and pyroptosis induced by GCs were suppressed.
In the past, the focus of attention on mitochondria was primarily centered around the generation of the energy molecule ATP. In recent years, oxidative stress damage associated with mitochondrial dysfunction has also become a hot topic of concern (Teleanu et al. 2022; Rehman et al. 2023). The generation of ATP involves the participation of highly energetic protons and free electrons, which are extremely active. When they are not effectively regulated and leaked from the MRC, they can trigger excessive production of free radicals and ROS, leading to oxidative stress. Moreover, the cell’s antioxidant defense system may be suppressed, which would make it unable to effectively remove excess free radicals and ROS. These effects lead to a vicious cycle of continuous oxidative stress that ultimately results in cellular and organismal dysfunction (Morán et al. 2012). Research has confirmed that oxidative stress damage is involved in the occurrence and development of major diseases (Forman and Zhang 2021), but insufficient attention has been given to the regulatory pathways of various endogenous molecules and commonly used drugs on mitochondria. Our results suggest that the regulatory pathways of endogenous molecules and drugs on mitochondria may be the mechanisms underlying age-related diseases and drug side effects. In-depth research on these pathways could be beneficial for addressing the challenges associated with drug treatments, such as GCs.
Currently, efforts are being made to mitigate the impact of mitochondrial dysfunction on cells and organisms to slow the aging process and delay the progression of major diseases associated with oxidative stress damage. Targeted antioxidant therapy focusing on mitochondria is a common approach to aid the restoration of the imbalance between oxidation and antioxidation, which serves as a common strategy for inhibiting mitochondrial dysfunction (Xiao et al. 2017; Kang et al. 2020; Forman and Zhang 2021; Yıldızhan and Nazıroğlu 2023).
MitoQ, a widely used drug for targeted mitochondrial antioxidant therapy, exerts evident antioxidative effects within mitochondria and has been extensively studied in both research and clinical treatment (Xiao et al. 2017; Kang et al. 2020). In our study, we utilized MitoQ to inhibit mitochondrial oxidative stress. The results demonstrated that MitoQ effectively alleviated the declines in the expression of MT-CO1 and MT-CO3 and the reductions in the levels of MRCC IV and Mn-SOD induced by GCs. Furthermore, MitoQ inhibited mitochondrial oxidative stress and suppressed pyroptosis. The results suggest that MitoQ can alleviate the GC-induced impairment of MRC function in microglia through its antioxidative effects within the mitochondria, which helps reduce oxidative stress-induced damage, subsequently inhibits cell apoptosis and pyroptosis, and thereby mitigates the pathogenic effects of GC-mediated mitochondrial regulation. In theory, MitoQ mitochondrial-targeted antioxidant therapy holds promising potential for mitigating the side effects of GCs on the brain. Further research is warranted to explore this avenue in depth.
Conclusion
Our study not only demonstrates the pathogenicity of GC-mediated mitochondrial pathways from a mechanistic perspective but also proposes a novel approach to utilize mitochondrial-targeted antioxidant therapy to prevent and treat this pathogenicity. In clinical practice, GCs are widely used due to their remarkable anti-inflammatory efficacy, but their significant adverse effects have a serious impact on the safety and sustainability of their application. Our research suggests that combining GCs with targeted mitochondrial antioxidant therapy as a synergistic treatment for anti-immune inflammation may offer a safer and more sustainable therapeutic approach. This new drug strategy has the potential to optimize the existing management of various immunoinflammatory diseases and helps alleviate the adverse effects of factors associated with inducing neurodegenerative diseases.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Akyuva Y, Nazıroğlu M, Yıldızhan K (2021) Selenium prevents interferon-gamma induced activation of TRPM2 channel and inhibits inflammation, mitochondrial oxidative stress, and apoptosis in microglia. Metab Brain Dis 36:285–298. https://doi.org/10.1007/s11011-020-00624-0
Bhaskaran S, Kumar G, Thadathil N, Piekarz KM, Mohammed S, Lopez SD, Qaisar R, Walton D, Brown JL, Murphy A, Smith N, Saunders D, Beckstead MJ, Plafker S, Lewis TL Jr, Towner R, Deepa SS, Richardson A, Axtell RC, Van Remmen H (2023) Neuronal deletion of MnSOD in mice leads to demyelination, inflammation and progressive paralysis that mimics phenotypes associated with progressive multiple sclerosis. Redox Biol 59:102550–102550. https://doi.org/10.1016/j.redox.2022.102550
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. https://doi.org/10.1038/nrn2038
Bolanos SH, Khan DA, Hanczyc M, Bauer MS, Dhanani N, Brown ES (2004) Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol 92:500–505. https://doi.org/10.1016/s1081-1206(10)61756-5
Bonora M, Giorgi C, Pinton P (2022) Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol 23(4):266–285. https://doi.org/10.1038/s41580-021-00433-y
Camm EJ, Tijsseling D, Richter HG, Adler A, Hansell JA, Derks JB, Cross CM, Giussani DA (2011) Oxidative stress in the developing brain: effects of postnatal glucocorticoid therapy and antioxidants in the rat. PLoS One 6:e21142. https://doi.org/10.1371/journal.pone.0021142
Canet G, Zussy C, Hernandez C, Chevallier N, Marchi N, Desrumaux C, Givalois L (2022) Chronic glucocorticoids consumption triggers and worsens experimental Alzheimer’s disease-like pathology by detrimental immune modulations. Neuroendocrinology 112:982–997. https://doi.org/10.1159/000521559
Caruso G, Di Pietro L, Caraci F (2023) Gap junctions and connexins in microglia-related oxidative stress and neuroinflammation: perspectives for drug discovery. Biomolecules 13:505–505. https://doi.org/10.3390/biom13030505
Choi GE, Oh JY, Lee HJ, Chae CW, Kim JS, Jung YH, Han HJ (2018) Glucocorticoid-mediated ER-mitochondria contacts reduce AMPA receptor and mitochondria trafficking into cell terminus via microtubule destabilization. Cell Death Dis 9(11):1137. https://doi.org/10.1038/s41419-018-1172-y
Claros S, Gil A, Martinelli M, Valverde N, Lara E, Boraldi F, Pavia J, Martín-Montañez E, Garcia-Fernandez M (2021) Impact of glucocorticoid on a cellular model of Parkinson’s disease: oxidative stress and mitochondrial function. Brain Sci 11:1106. https://doi.org/10.3390/brainsci11081106
Drakulić D, Stanojlović M, Nedeljković N, Grković I, Veličković N, Guševac I, Mitrović N, Buzadžić I, Horvat A (2015) Upregulation of nucleoside triphosphate diphosphohydrolase-1 and ecto-5′-nucleotidase in rat hippocampus after repeated low-dose dexamethasone administration. J Mol Neurosci 55:959–967. https://doi.org/10.1007/s12031-014-0452-y
Du F, Qian Y, Swerdlow RH, Waites CL (2023) Glucocorticoid-driven mitochondrial damage stimulates Tau pathology. Brain 146:4378–4394. https://doi.org/10.1093/brain/awad127
Flores-Romero H, Dadsena Shashank, García-Sáez AJ (2023) Mitochondrial pores at the crossroad between cell death and inflammatory signaling. Mol Cell 83:843–856. https://doi.org/10.1016/j.molcel.2023.02.021
Forman HJ, Zhang H (2021) Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20:689–709. https://doi.org/10.1038/s41573-021-00233-1
Garrud TAC, Giussani DA (2019) Combined antioxidant and glucocorticoid therapy for safer treatment of preterm birth. Trends Endocrinol Metab 30:258–269. https://doi.org/10.1016/j.tem.2019.02.003
Garside H, Stevens A, Farrow S, Normand C, Houle B, Berry A, Maschera B, Ray D (2004) glucocorticoid ligands specify different interactions with NF-κB by allosteric effects on the glucocorticoid receptor DNA binding domain. J Biol Chem 279:50050–50059. https://doi.org/10.1074/jbc.m407309200
Grubman A, Kanninen KM, Malm T (2016) Multitasking microglia and Alzheimer’s disease: diversity, tools and therapeutic targets. J Mol Neurosci 60:390–404. https://doi.org/10.1007/s12031-016-0825-5
Hanly JG, Kozora E, Beyea SD, Birnbaum J (2019) Nervous system disease in systemic lupus erythematosus: current status and future directions. Arthritis Rheumatol 71:33–42. https://doi.org/10.1002/art.40591
Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB (2007) Glucocorticoid receptor physiology. Rev Endocr Metab Disord 8:321–330. https://doi.org/10.1007/s11154-007-9059-8
Henn A, Lund S, Hedtjärn M, Schrattenholz A, Pörzgen P, Leist M (2009) The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 26(2):83–94. https://doi.org/10.14573/altex.2009.2.83
Kang L, Liu S, Li J, Tian Y, Xue Y, Liu X (2020) The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif 53(3):e12779. https://doi.org/10.1111/cpr.12779
Kesavardhana S, Malireddi RKS, Kanneganti TD (2020) Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol 38:567–595. https://doi.org/10.1146/annurev-immunol-073119-095439
Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA (2014) Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157:1685–1697. https://doi.org/10.1016/j.cell.2014.04.038
Kokkinopoulou I, Moutsatsou P (2021) Mitochondrial glucocorticoid receptors and their actions. Int J Mol Sci 22:6054. https://doi.org/10.3390/ijms22116054
Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18:601–607. https://doi.org/10.1016/j.molcel.2005.04.021
Kowalska M, Piekut T, Prendecki M, Sodel A, Kozubski W, Dorszewska J (2020) Mitochondrial and nuclear DNA oxidative damage in physiological and pathological aging. DNA Cell Biol 39(8):1410–1420. https://doi.org/10.1089/dna.2019.5347
Li ZY, Li QZ, Chen L, Chen BD, Zhang C, Wang X, Li WP (2016) HPOB, an HDAC6 inhibitor, attenuates corticosterone-induced injury in rat adrenal pheochromocytoma PC12 cells by inhibiting mitochondrial GR translocation and the intrinsic apoptosis pathway. Neurochem Int 99:239–251. https://doi.org/10.1016/j.neuint.2016.08.004
Liu Z, Yao X, Jiang W, Li W, Zhu S, Liao C, Zou L, Ding R, Chen J (2020) Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J Neuroinflammation 17(1):90. https://doi.org/10.1186/s12974-020-01751-2
Madore C, Yin Z, Leibowitz J, Butovsky O (2020) Microglia, lifestyle stress, and neurodegeneration. Immunity 52:222–240. https://doi.org/10.1016/j.immuni.2019.12.003
Mehl LC, Manjally AV, Bouadi O, Gibson EM, Tay TL (2022) Microglia in brain development and regeneration. Development 149: dev200425. https://doi.org/10.1242/dev.200425
Morán M, Moreno-Lastres D, Marín-Buera L, Arenas J, Martín MA, Ugalde C (2012) Mitochondrial respiratory chain dysfunction: implications in neurodegeneration. Free Radic Biol Med 53:595–609. https://doi.org/10.1016/j.freeradbiomed.2012.05.009
Noddings CM, Wang RY-R, Johnson JL, Agard DA (2022) Structure of Hsp90–p23–GR reveals the Hsp90 client-remodelling mechanism. Nature 601:465–469. https://doi.org/10.1038/s41586-021-04236-1
Oh S, Yang J, Park C, Son K, Byun K (2021) Dieckol attenuated glucocorticoid-induced muscle atrophy by decreasing NLRP3 inflammasome and pyroptosis. Int J Mol Sci 22:8057. https://doi.org/10.3390/ijms22158057
Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS (2016) Long-term side effects of glucocorticoids. Expert Opin Drug Saf 15:457–465. https://doi.org/10.1517/14740338.2016.1140743
Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O (2019) Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci 40:38–49. https://doi.org/10.1016/j.tips.2018.11.002
Pedrazzoli M, Losurdo M, Paolone G, Medelin M, Jaupaj L, Cisterna B, Slanzi A, Malatesta M, Coco S, Buffelli M (2019) Glucocorticoid receptors modulate dendritic spine plasticity and microglia activity in an animal model of Alzheimer’s disease. Neurobiol Dis 132:104568. https://doi.org/10.1016/j.nbd.2019.104568
Psarra A-MG, Sekeris CE (2009) Glucocorticoid receptors and other nuclear transcription factors in mitochondria and possible functions. Biochim Biophys Acta 1787:431–436. https://doi.org/10.1016/j.bbabio.2008.11.011
Qiu Y, Huang Y, Chen M, Yang Y, Li X, Zhang W (2022) Mitochondrial DNA in NLRP3 inflammasome activation. Int Immunopharmacol 108:108719. https://doi.org/10.1016/j.intimp.2022.108719
Rehman MU, Sehar N, Dar NJ, Khan A, Arafah A, Rashid S, Rashid SM, Ganaie MA (2023) Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: an update on current advances and impediments. Neurosci Biobehav Rev 144:104961. https://doi.org/10.1016/j.neubiorev.2022.104961
Rostami A, Taleahmad F, Haddadzadeh-Niri N, Joneidi E, Afshin-Majd S, Baluchnejadmojarad T, Roghani M (2022) Sinomenine attenuates trimethyltin-induced cognitive decline via targeting hippocampal oxidative stress and neuroinflammation. J Mol Neurosci 72(8):1609–1621. https://doi.org/10.1007/s12031-022-02021-x
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360:201–205. https://doi.org/10.1124/jpet.116.237503
Shen S, Svoboda M, Zhang G, Cavasin MA, Motlova L, McKinsey TA, Eubanks JH, Bařinka C, Kozikowski AP (2020) Structural and in vivo characterization of tubastatin A, a widely used histone deacetylase 6 inhibitor. ACS Med Chem Lett 11:706–712. https://doi.org/10.1021/acsmedchemlett.9b00560
Shimojo M, Hiroi N, Yakushiji F, Ueshiba H, Yamaguchi N, Miyachi Y (1995) Differences in down-regulation of glucocorticoid receptor mRNA by cortisol, prednisolone and dexamethasone in HeLa cells. Endocr J 42:629–636. https://doi.org/10.1507/endocrj.42.629
Tarr T, Papp G, Nagy N, Cserép E, Zeher M (2017) Chronic high-dose glucocorticoid therapy triggers the development of chronic organ damage and worsens disease outcome in systemic lupus erythematosus. Clin Rheumatol 36:327–333. https://doi.org/10.1007/s10067-016-3492-6
Tayanloo-Beik A, Kiasalari Z, Roghani M (2022) Paeonol ameliorates cognitive deficits in streptozotocin murine model of sporadic Alzheimer’s disease via attenuation of oxidative stress, inflammation, and mitochondrial dysfunction. J Mol Neurosci 72(2):336–348. https://doi.org/10.1007/s12031-021-01936-1
Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, Costăchescu B, Grumezescu AM, Teleanu RI (2022) An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci 23:5938. https://doi.org/10.3390/ijms23115938
Trigo D, Avelar C, Fernandes M, Sá J, da Cruz E, Silva O (2022) Mitochondria, energy, and metabolism in neuronal health and disease. FEBS Lett 596:1095–1110. https://doi.org/10.1002/1873-3468.14298
Vandewalle J, Luypaert A, De Bosscher K, Libert C (2018) Therapeutic mechanisms of glucocorticoids. Trends Endocrinol Metab 29:42–54. https://doi.org/10.1016/j.tem.2017.10.010
Wang L, Jiao XF, Wu C, Li XQ, Sun HX, Shen XY, Zhang KZ, Zhao C, Liu L, Wang M, Bu YL, Li JW, Xu F, Chang CL, Lu X, Gao W (2021) Trimetazidine attenuates dexamethasone-induced muscle atrophy via inhibiting NLRP3/GSDMD pathway-mediated pyroptosis. Cell Death Discov 7(1):251. https://doi.org/10.1038/s41420-021-00648-0
Wu T, Liang X, Liu X, Li Y, Wang Y, Kong L, Tang M (2020) Induction of ferroptosis in response to graphene quantum dots through mitochondrial oxidative stress in microglia. Part Fibre Toxicol 17(1):30. https://doi.org/10.1186/s12989-020-00363-1
Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, Ying W, Hoffman HM, Shadel GS, Karin M (2022) Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 55:1370-1385.e8. https://doi.org/10.1016/j.immuni.2022.06.007
Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, Greka A, Zhou Z, Liu F, Dong Z, Sun L (2017) The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11:297–311. https://doi.org/10.1016/j.redox.2016.12.022
Yang L, Zhou H, Huang L, Su Y, Kong L, Ji P, Sun R, Wang C, Li W, Li W (2022) Stress level of glucocorticoid exacerbates neuronal damage and Aβ production through activating NLRP1 inflammasome in primary cultured hippocampal neurons of APP-PS1 mice. Int Immunopharmacol 110:108972–108972. https://doi.org/10.1016/j.intimp.2022.108972
Yıldızhan K, Nazıroğlu M (2019) Microglia and its role in neurodegenerative diseases. J Cell Neurosci Oxid Stress 11:861–873. https://doi.org/10.37212/jcnos.683407
Yıldızhan K, Nazıroğlu M (2020) Glutathione depletion and parkinsonian neurotoxin MPP+-induced TRPM2 channel activation play central roles in oxidative cytotoxicity and inflammation in microglia. Mol Neurobiol 57:3508–3525. https://doi.org/10.1007/s12035-020-01974-7
Yıldızhan K, Nazıroğlu M (2023) NMDA receptor activation stimulates hypoxia-induced TRPM2 channel activation, mitochondrial oxidative stress, and apoptosis in neuronal cell line: modular role of memantine. Brain Res 1803:148232. https://doi.org/10.1016/j.brainres.2023.148232
Zelko IN, Mariani TJ, Folz RJ (2002) Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33:337–49. https://doi.org/10.1016/s0891-5849(02)00905-x
Zhao Y, Liu B, Xu L, Yu S, Fu J, Wang J, Yan X, Su J (2021) ROS-induced mtDNA release: the emerging messenger for communication between neurons and innate immune cells during neurodegenerative disorder progression. Antioxidants 10:1917–1917. https://doi.org/10.3390/antiox10121917
Zhao X, Dang R, Cheng X, Song X, Huang X, Huang C, Liu S, Wang X, Yan P, Yang Y, Fan P, Zhou Y, Zhang S (2022) Effects of compound Ziyin Qingre prescription, NAC and mitoQ on abnormal renal mitochondrial function in dexamethasone-induced MRL/lpr mice. Int J Immunol 45(6):564–574. https://doi.org/10.3760/cma.j.issn.1673-4394.2022.06.002
Acknowledgements
The authors would like to acknowledge all members of the State Key Laboratory of Respiratory Disease at Guangzhou Medical University for their invaluable support and stimulating discussions.
Funding
Guangzhou City's Construction Project for Tier-Three Famous Traditional Chinese Medicine Clinics, 3208203801, Zhongnanshan Medical Foundation of Guangdong Province, ZNSA-2020013, National Natural Science Foundation of China, 81673983, 82074172, Natural Science Foundation of Guangdong Province, 02820005
Author information
Authors and Affiliations
Contributions
DRN, HXY, HXL, CXP, and XXH designed the experiments. DRN, HXY, and HXL performed the experiments and wrote the manuscript. HCF, ZXQ, WXR, ZN, and YYQ provided experimental technical support and assisted in completing the study. LN, LS, and YP analyzed the data. ZSY and DYQ participated in the review and coordination. FP, SXH, CXP, and XXH provided the resources. CXP and XXH supervised the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Informed Consent
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dang, R., Hou, X., Huang, X. et al. Effects of the Glucocorticoid-Mediated Mitochondrial Translocation of Glucocorticoid Receptors on Oxidative Stress and Pyroptosis in BV-2 Microglia. J Mol Neurosci 74, 30 (2024). https://doi.org/10.1007/s12031-024-02192-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12031-024-02192-9