Abstract
Malignant astrocytomas presenting in humans of any age group are a challenge to diagnose and treat. Hence, there is a quest for new markers to ascertain their grades and predict disease outcomes. Proline, glutamic acid, and leucine-rich protein 1 (PELP1), a nuclear receptor co-regulator, is an oncogene found in various cancers. We postulate that by screening for PELP1, its correlation with survival outcomes of patients across various grades can indicate a plausible novel diagnostic marker and a potential therapeutic target in gliomas. Immunostaining of 100 cases of astrocytomas for PELP1 was performed on paraffin-embedded sections. Results showed that PELP1 expression increases with higher grades; the mean H-score of PELP1 in grade-I astrocytomas was determined to be 112.3, whereas in grade-IV it was 235.1 (P value = 0.0001). Survival analysis of patients with H-score of 200–300 was only 8.8% and 68.8% in patients with scores of 0–100. PELP1 expression in high-grade astrocytomas is an important factor in determining the outcomes.

Evaluation of molecular expression of PELP1 along with Ki-67 LI signifies a linear increase in its expression pattern among different grades of astrocytomas from low- to high-grade tumors, which can serve as a potential prognostic molecular marker in differentiating various types of astrocytomas in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytomas are one of the recurrent intracranial tumors representing more than 60% of primary brain cancers. As formulated by the World Health Organization (WHO), they are graded and classified based on histomorphological criteria into grade-I (pilocytic astrocytomas), grade-II (diffuse astrocytomas), grade-III (anaplastic astrocytomas), and grade-IV (glioblastomas) (Daumas-Duport et al. 1988). The pilocytic astrocytomas are slow growing and circumscribed lesions that deserve grade-I due to their distinct characteristics and being benign in nature (Fernandez et al. 2003), whereas glioblastomas are applied to cytologically malignant, mitotically active, necrosis-prone primary brain tumors (Davis 2016). Extensive research was conducted to ascertain various prognostic factors for individuals with astrocytomas such as age, proliferative activity, apoptotic index, micro-vascular proliferation, micro-vascular density, molecular parameters, and cancer stem cell analysis (Verhaak et al. 2010). The histomorphological grade corresponds directly with the age of the individuals (Sukheeja et al. 2015). There is an urgent need for novel therapeutic agents to ameliorate survival rates and eventually to heal individuals of this lethal disease. Recent expansion of knowledge in integrated genomics has aided in the recognition of specific molecular networks implicated in the pathogenesis of malignant gliomas (Altieri et al. 2014). The receptor tyrosine kinase signaling through Ras-MAPK and P13K-AKT-mToR pathways, Wnt signaling, cell cycle regulating pRb and p53 pathways are some of the extensively studied pathways (Cairncross et al. 1998; Huang et al. 1997; James et al. 1988; Libermann et al. 1985; Louis 1994). Recognizing the various molecular subtypes showing aberrations in any of these pathways may help in developing different targeted therapies. Despite many years of intense investigations and recent advancements in neuroimaging, surgery, radiotherapy, chemotherapy, and molecular biology, high-grade astrocytomas remain incurable.
Proline, glutamic acid, and leucine-rich protein 1 (PELP1) is a novel nuclear receptor co-regulator (Vadlamudi et al. 2001) that acts as a large scaffolding protein, which facilitates interactions among different signaling molecules (De Luca et al. 2017). It is expressed ubiquitously in most of the cell types with highest expression levels observed in tissues such as brain, testes, ovaries, and uterus. PELP1 is mostly localized to the nuclear compartment of a cell with little expression observed even in the cytoplasm (Rajhans et al. 2007). PELP1 has high tumorigenic potential and interacts with and modulates the activities of various proto-oncogenes (Sareddy and Vadlamudi 2016). The deregulation of PELP1 expression and localization results in different cancers such as breast, endometrial, prostate, ovarian, salivary, pancreas, colon, lung, etc. (Vadlamudi and Kumar 2007; Vadlamudi et al. 2005). Recent reports also suggested that PELP1 acquires a remarkable position in astrocytic tumor formation.
Mitotically active tumors are thought to be more malignant and thus have poor prognosis than the inactive ones (Chirieac 2016). Biological markers of cellular proliferation and nuclear morphometry were investigated extensively to offer high prognostic value in predicting the clinical course of the disease. A wide variety of techniques were employed, of which Ki-67 has emerged as one of the best proliferation markers (Aziz et al. 2016). Ki-67 labeling index (Ki-67 LI) reflects total percentage of MIB-1 labeled cells, and is widely adopted due to its ability to be utilized in formalin fixed and paraffin embedded (FFPE) tissue sections. It has been proven to reflect mitotic activity more accurately than counting the mitoses (Ladstein et al. 2010) but has to be validated within each individual institution. The overall disease-free survival correlates with low Ki-67 LI, and has prognostic importance, independent of clinicopathological features (Ambroise et al. 2011).
In the present investigation, we proposed to explore the molecular expression pattern of PELP1 in various grades of astrocytomas and correlate with mitotic marker Ki-67 expression; evaluate PELP1 expression with several clinicopathological profiles of the subjects including survival analysis.
Methods
Tissue Samples
For the retrospective study, a total number of 100 cases of astrocytic tumors were selected and retrieved from the files of the Department of Human Pathology, SRMC (Sri Ramachandra Medical College, Chennai, India) between January 2010 and July 2013. This study included 41 subjects of grade-IV, 16 of grade-III, 30 of grade-II, and 13 cases of grade-I astrocytomas, which were reviewed and classified based on the WHO grading method with the aid of hematoxylin and eosin stained (H&E) sections. Institutional Ethics Committee approval was obtained for the clinical study protocol along with the patients’ consent for both the study participation and the provision of tumor samples (IEC: CSP-MED/12/DEC/05/47). The clinical sample data were acquired from the patients’ medical history records, and demographic details were noted.
Immunohistochemistry (IHC)
For the immunohistochemical analysis, approximately 5-μ sized formalin fixed, paraffin embedded (FFPE) tissue sections were made and mounted on coated glass slides. These slides were immunostained for PELP1 using IHC-grade anti-PELP1 antibody procured from Bethyl Laboratories Inc. (Texas, USA), for Ki-67 using anti-Ki-67 antibody (BioGenex), and for negative controls using anti-IgG antibody by standard Streptavidin-Biotin conjugated peroxidase technique. In brief, the processed tissue sections mounted on the glass slides were subjected to de-paraffinization followed by antigen retrieval using standard protocol that follows blocking and primary antibody incubation overnight. The following day, after incubating with HRP-conjugated secondary antibody, the sections were subjected to incubation with peroxide/DAB (diaminobenzidine) substrate solution followed by counter staining with hematoxylin. Scoring was done by the quickscore method.
Quickscore (H-score) Calculation
The immunohistochemically stained sections were evaluated and scored manually by two independent, highly experienced neuro pathologists blinded to the histopathological characteristics of the tissue sections. Initially, the slides were scanned at a magnification of 100× to observe the distribution of tumor cells, and the immunostained cells were assessed semi-quantitatively at 200× and 400× magnifications before assigning final scores. The quickscores were accredited based on their intensity and the quantity of brown stain present in the cells. PELP1 immunostaining was observed exclusively in the nucleus of the tumor cells. We counted the nuclear positivity of immunostain for 1000 cells and converted the count to percentage of cells (P), and a score for the intensity of staining (I) was assigned (0 = no staining, 1 = weak, 2 = moderate, 3 = strong staining) to each tumor.
Score | 0 | 1+ | 2+ | 3+ |
Positive cells | <25% | 25–50% | 50–75% | >75% |
Staining intensity | Negative | Weak | Moderate | Strong |
Ki-67 Labeling Index (Ki-67 LI)
Ki-67 immunostaining was performed using the anti-Ki-67 antibody, and these immunostained slides were utilized to calculate the Ki-67 labeling index (Ki-67 LI) according to the manual method. Initially, the slides were scanned for the most densely labeled areas at a magnification of 40×, and only the distinctly labeled tumor nuclei were evaluated subsequently at a higher magnification (100×). The labeling index (LI) was determined by counting at least 1000 tumor cells and is defined as the percentage of total immunostained nuclei present in the tumor cell population. The results were evaluated by two independent human pathologists blinded to the clinical features of tissue sections, and the statistical significance was further analyzed.
Statistical Analysis
Statistical analysis was performed by either GraphPad Prism 6.0 software (San Diego, USA) or SPSS v16.0 (SPSS Inc. Chicago, USA) and the data was represented as mean + SEM. The significance of the data was evaluated using one-way or two-way ANOVA, and defined as statistically significant at a P-value of <0.05 levels. Pearson chi-square test was utilized for correlation studies of various data, and Kaplan–Meier survival analysis was performed for evaluating survival data of individuals using SPSS v16.0.
Results
Clinicopathological Characteristics
This retrospective study was supervised on a total number of 100 cases diagnosed with different grades of Astrocytomas in SRMC Research University (Sri Ramachandra Medical College, Chennai, India) over a time period from January 2010 to July 2013. In this study, the patient sample was composed in the following manner: 13% with grade-I, 30% – grade-II, 16% – grade-III, and 41% – grade-IV tumors. The age distribution of patients with various grades of astrocytomas was from 13 to 95 years with the mean age of 36.69, 37.43, 46.875, and 47.07 years for grades-I, II, III, and IV, respectively (Fig. 1). The clinicopathological features of the subjects are presented in Table 1. The mean age of the patients showed an increase with an increase in the grade of the tumors. The gender ratio of the cases studied was observed to be 1.5:1 for males:females.
Ki-67 Labeling Index (Ki-67 LI)
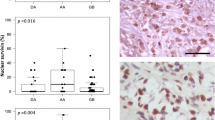
Out of the 100 diseased cases, 98 cases were immunostained for Ki-67 expression. The Ki-67 LI was observed to be varied in different grades of astrocytomas. The mean values were 1.84, 2.73, 21.68, and 34.18 for grades-I, II, III, and IV, respectively (Table 2, Fig. 2). We achieved a statistically significant difference among Ki-67 labeling indices of different grades of astrocytomas (p = 0.05) using one way analysis of variance (ANOVA). A multiple comparison test among each of the four grades and mean Ki-67 LI was performed to assess the statistically significant difference in Ki-67 values across different grades of astrocytomas. Our analysis proved that there was a statistically significant difference between grades-IV and I (p = 0.000), grades-IV and II (p = 0.000), grades-III and II (p = 0.000), grades-III and I (p = 0.000), and grades-III and IV (p = 0.007). However, no countable difference was observed between grades-II and I.
Graphical presentation of Ki-67 LI across various astrocytic tumor grades evaluated by the percentage of total immunostained nuclei from random number of 1000 tumor cells present in the tumor cell population scored by two independent human pathologists blinded to clinical parameters. N = 98 and the statistical significance was analyzed by one-way ANOVA. *P value <0.05
PELP1 Immunostaining
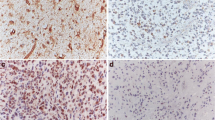
PELP1 expression was noted exclusively in the nuclei of tumor cells, and there was no cytoplasmic positivity in any of the studied cases. PELP1 staining was found to increase with an increase in the grades of astrocytomas. The mean values of quickscore for grades-I, II, III, and IV are represented as 112.30, 139.50, 168.13, and 235.12, respectively (Table 3, Fig. 3). On statistical evaluation, we analyzed that there was a substantial difference in PELP1 expression between grades-IV and III (p = 0.033), grades-IV and II (p = 0.000), and grades-IV and I (p = 0.000). However, we were unable to show any statistically significant differences between grades-III and II, grades-III and I, and grades-I and II. To ascertain the specificity of the immunohistochemical staining, IHC was performed on glioblastoma sections using IgG control. The photomicrograph for IgG control is shown in Supplementary Fig. 1. Further, to confirm the PELP1 positivity in glioblastoma tissue sections, immunofluorescence was performed using PELP1 primary antibody and Alexa 488 conjugated secondary Ab & counter stained with DAPI. IF staining occurred on eight samples with different scores (grades-I to IV) — 2 cases per grade and the staining intensity was quantified by a blindfolded pathologist. Results showed that IF quantification correlated well with IHC scoring. These results are included as Supplementary Fig. 2.
Graphical representation of PELP1 H-score calculated from the total percentage and intensity of positively immunostained cells, scored by two independent human pathologists blinded to pathological aspects across various grades of astrocytomas and analyzed using one-way ANOVA in GraphPad Prism 6.0 software. N = 100 and * indicates a P value of <0.05
Correlation between Expression Pattern of Ki-67 and PELP1
Multiple comparisons were made to see if there was any statistically notable correlation between Ki-67 LI and PELP1 expression. We noticed an adequate correlation between Ki-67 LI and PELP1 H-score in astrocytomas (P = 0.000) by Pearson correlation studies (Table 4 (i) & (ii)). Figure 4 represents the bivariate scattered plot of PELP1 H-score with Ki-67 LI for its correlation. Figure 5 constitutes microphotographic representative images that demonstrate the staining criteria across different grades of astrocytomas presenting H&E Staining, Ki-67 expression, and PELP1 expression by IHC.
Survival Analysis
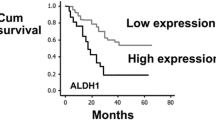
Comparison of PELP1 H-score with survival data of the patients at the end of the study period (2009–2013) portrayed a statistically significant difference. Survival data was available in 79% of the patients included in the study, and the rest were lost to follow-up. The case-processing summary of samples is presented in Table 5. On evaluation, cases with 20–49 H-score had 100.0% survival, those with H-score of 50–99 had 60.0% survival, those with H-score of 100–199 had 65.7% survival, and those with H-score of 200–300 had 12.5% survival. This indicates a negative correlation between PELP1 H-score and patient survival among different grades of astrocytomas. The significant difference between two variables was calculated by cross-tabulation of PELP1 H-score versus survival frequency using Pearson chi-squared test that is presented in Table 5. Kaplan–Meier survival analysis was done on 79 clinical samples with PELP1 H-scores, and survival status of individuals was done using SPSS v16.0 software. The survival curves are depicted in Fig. 6.
Discussion
Astrocytomas are the highly prevalent primary cancers of the nervous system (Ohgaki 2009; Wen and Kesari 2008). As classified by the World Health Organization, glioblastoma multiforme (grade-IV) neoplasm is the most frequent and destructive astrocytoma type with only 12–15 months of median survival rate. Grade-I tumors are histologically benign and can be surgically resected in early stage, whereas grade-II tumors are low-grade malignant tumors that may follow a long course, and grade-III tumors are malignant, which may lead to patient death within a few years. To date, only age and histological grade stand out as independent predictors of survival (Burger and Green 1987) in which younger patients have better prognosis than the older ones (Bostrom et al. 2001). We analyzed the demographic profile of patients with astrocytomas and found that the mean age of patients increased with increasing grade, which was found to be statistically significant. Male preponderance was noted, and was consistent with the earlier reports (Bostrom et al. 2001).
The WHO grading system used worldwide to classify brain tumors utilizes factors such as cellularity, vascular proliferation, nuclear morphometry, mitosis, and necrosis to allocate a definite grade. Techniques that can measure and quantitate the proliferative activity of tumor cells may form a useful adjunct while grading them. Quantitative assessment of tumor proliferation can be achieved using flow cytometry, cell cycle analysis or the IHC technique that labels cell division associated with nuclear antigens. Cellular markers such as proliferating nuclear antigen (PCNA), incorporation of thymidine analog, and Ki-67 labeling have demonstrated promising results in the assessment of gliomas in tissue sections (Coons et al. 1997). Ki-67 is a nuclear antigen that represents actively dividing cells in all phases of the cell cycle except for the resting cell phase (G0 and early part of G1).
In our study, we collected immunohistochemical staining data of Ki-67 LI from 98 cases of astrocytomas. Grade-I gliomas uniformly revealed a very low Ki-67 LI of <2%, confirming it to be a lower-grade tumor. This was consistent with a similar study conducted by Kim et al., where they found a mean Ki-67 LI of 1.8, which is almost equal to our mean value. Ki-67 LI is extremely useful in cases of pilocytic astrocytomas, which are grade-I tumors but they may exhibit misleading appearance of a pleomorphic, infiltrating neoplasm on routine histopathology observations (Tihan et al. 2000). The mean Ki-67 LI of grade-II gliomas in our study was 2.7, whereas that of grade-III and grade-IV was 21.6 and 34.17, respectively. This manifests a significant increase in labeling index as the grade increases. On multiple comparisons, the difference in Ki-67 LI was reported to be statistically significant among grades-I, III, and IV but not between grades-I and II, which highlights the difficulty in differentiating grade-I from grade-II, based on the Ki-67 index. Thomas et al. proposed that proliferative index would be of little utility in differentiating high-grade lesions (Montine et al. 1994). Hence, there is a pressing demand to discover a molecular marker that can be employed as a potential candidate to distinguish among various grades.
Several advances have been made recently in understanding the tumorigenesis behavior and progression of astrocytomas. Many molecular and genetic alterations were reported in these tumors, but recognition of the aberrant signaling pathways that lead to tumorigenesis and specific markers that are used to identify them at a cellular level will go a long way in designing an effective therapeutic protocol. PELP1 is a novel molecular marker, and any alteration in its expression leads to various malignant tumors such as breast, endometrium, prostate, and ovary. Habashy et al., in their study, described PELP1 as an independent prognostic marker of short disease free interval in breast carcinoma (Habashy et al. 2010), in which its over-expression was directly correlated with poor patient outcome. Additionally, Grivas et al. described that there was PELP1 over-expression in colonic carcinoma cells (Grivas et al. 2009), and in an another independent study Garban et al. indicated a defective PELP1 regulation in lung cancers (Marquez-Garban et al. 2007). Kefalopoulou et al., in their study on astrocytic tumors, reported deregulation of PELP1 expression in tumor cells (Kefalopoulou et al. 2012). With this available literature, this study was undertaken to evaluate the expression of PELP1 protein in astrocytomas. SaReddy and Vadlamudi have shown that PELP1 is upregulated in glioma cells and signals via the β-catenin pathway (Sareddy and Vadlamudi 2016).
We evaluated the expression of PELP1 in 100 cases of astrocytomas, and the evaluation was conducted using a semi-quantitative quick scoring (H-score) method, which is a well-established and widely accepted technique in both clinical and experimental parts of medical science (Detre et al. 1995). It is interesting to note that PELP1 immunostaining had manifested a highly specific nuclear positivity in tumor cells of all 100 cases of astrocytomas, whereas the control brain cells (normal glial cells) exhibited completely negative staining. The mean H-score of PELP1 showed a clear increase with an increase in the tumor grade. This difference of PELP1 expression was statistically significant, except between grades-II & I astrocytomas, and this result was similar to the results of another study reported by Zinovia et al., which explained that the over-expression of PELP1 was significantly higher in high-grade gliomas compared to low-grade tumors (Kefalopoulou et al. 2012). In our study, the H-score of grade-I gliomas ranged between 45 and 190, whereas that of grade-II ranged between 30 and 210, grade-III was between 70 and 270, and grade-IV was between 60 and 300, which is a distinct indication of substantial increase in H-score with an increase in the tumor grade. The mean H-score of grade-IV astrocytomas was the highest followed by grade-III, II, and I, and an explicit statistically significant difference in PELP1 expression was noticed between grades-III and IV. Additionally, the correlation of the two variables, namely PELP1 H-score and Ki-67 LI, was detected to be positively correlated with an increase in the tumor grade. Moreover, our study confirmed that the tumor cases with a higher PELP1 H-score had an inferior prognosis value; for example, a group of subjects with a score range of 200–300 had only a survival percentage of 12.5%. This was also consistent with the report published by Zinovia et al., which stated that the positive PELP1 expression predicted a shorter overall survival (Kefalopoulou et al. 2012).
In conclusion, this study underlines the importance of clinicopathological characteristics of individuals in understanding different grades of astrocytomas. In addition, the determination of PELP1 molecular expression pattern and its correlation with Ki-67 LI may serve as a potential marker in establishing the clinical course of various astrocytomas. This report also signifies the prominence of PELP1 in tumorigenesis and prognosis of various astrocytic cancers. We strongly believe that this study will encourage further investigations to determine the molecular mechanisms behind PELP1 over-expression in astrocytic tumors, and explore its diverse interacting partners that in turn will aid in the development of new therapeutics.
Conclusions
In this case control study, we found that grade-IV tumors were the most common among all astrocytic tumors accounting for approximately 41% of total cases, and the high-grade gliomas were observed to occur with an increase in age where the peak incidence was seen between 51 and 60 years of age. Grade-I tumors exhibited an equal sex occurrence, whereas the other grades displayed a male predominance. PELP1 staining on normal glial cells of control brain tissues revealed a complete negativity for its expression, although there was a statistically significant staining detected between various grades of glioma. The difference in Ki-67 LI was remarkable across various grades, except between grades-I and II. There was no notable correlation between PELP 1 expression and Ki67 LI in grades-I, II, and III; however, grade-IV showed substantial correlation. In addition, the PELP1 expression and patient survival outcome showed a strong negative correlation, which was statistically significant. Hence, the increase in the PELP1 expression has a potential role in manifesting pathogenesis and prognosis of astrocytic tumors that can be further verified with future investigations.
Abbreviations
- ANOVA:
-
Analysis of variance
- FFPE:
-
Formalin fixed paraffin embedded
- IHC:
-
Immuno histo chemistry
- PELP1:
-
Proline, glutamic acid, leucine rich protein 1
- WHO:
-
World Health Organization
References
Altieri R, Agnoletti A, Quattrucci F, Garbossa D, Calamo Specchia FM, Bozzaro M et al (2014) Molecular biology of gliomas: present and future challenges. Transl Med UniSa 10:29–37
Ambroise MM, Khosla C, Ghosh M, Mallikarjuna VS, Annapurneswari S (2011) Practical value of MIB-1 index in predicting behavior of astrocytomas. Ind J Pathol Microbiol 54(3):520–525. https://doi.org/10.4103/0377-4929.85085
Aziz S, Wik E, Knutsvik G, Klingen TA, Chen Y, Davidsen B et al (2016) Evaluation of tumor cell proliferation by Ki-67 expression and mitotic count in lymph node metastases from breast Cancer. PLoS One 11(3):e0150979. https://doi.org/10.1371/journal.pone.0150979
Bostrom J, Meyer-Puttlitz B, Wolter M, Blaschke B, Weber RG, Lichter P et al (2001) Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol 159(2):661–669. https://doi.org/10.1016/S0002-9440(10)61737-3
Burger PC, Green SB (1987) Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer 59(9):1617–1625
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90(19):1473–1479
Chirieac LR (2016) Tumor cell proliferation, proliferative index and mitotic count in lung cancer. Transl Lung Cancer Res 5(5):554–556. https://doi.org/10.21037/tlcr.2016.10.10
Coons SW, Johnson PC, Pearl DK (1997) The prognostic significance of Ki-67 labeling indices for oligodendrogliomas. Neurosurgery 41(4):878–884, discussion 884–875
Daumas-Duport C, Scheithauer B, O'Fallon J, Kelly P (1988) Grading of astrocytomas. A simple and reproducible method. Cancer 62(10):2152–2165
Davis ME (2016) Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs 20(5 Suppl):S2–S8. https://doi.org/10.1188/16.CJON.S1.2-8
De Luca A, Avena P, Sirianni R, Chimento A, Fallo F, Pilon C et al (2017) Role of scaffold protein proline-, glutamic acid-, and leucine-rich protein 1 (PELP1) in the modulation of adrenocortical Cancer cell growth. Cells 6(4):42. https://doi.org/10.3390/cells6040042
Detre S, Saclani Jotti G, Dowsett M (1995) A "quickscore" method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 48(9):876–878. https://doi.org/10.1136/jcp.48.9.876
Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A, Lena G (2003) Pilocytic astrocytomas in children: prognostic factors--a retrospective study of 80 cases. Neurosurgery 53(3):544–553, discussion 554–545
Grivas PD, Tzelepi V, Sotiropoulou-Bonikou G, Kefalopoulou Z, Papavassiliou AG, Kalofonos H (2009) Expression of ERalpha, ERbeta and co-regulator PELP1/MNAR in colorectal cancer: prognostic significance and clinicopathologic correlations. Cell Oncol 31(3):235–247. https://doi.org/10.3233/CLO-2009-0467
Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, Ellis IO (2010) The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat 120(3):603–612. https://doi.org/10.1007/s10549-009-0419-9
Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD et al (1997) The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 272(5):2927–2935
James CD, Carlbom E, Dumanski JP, Hansen M, Nordenskjold M, Collins VP, Cavenee WK (1988) Clonal genomic alterations in glioma malignancy stages. Cancer Res 48(19):5546–5551
Kefalopoulou Z, Tzelepi V, Zolota V, Grivas PD, Christopoulos C, Kalofonos H et al (2012) Prognostic value of novel biomarkers in astrocytic brain tumors: nuclear receptor co-regulators AIB1, TIF2, and PELP1 are associated with high tumor grade and worse patient prognosis. J Neuro-Oncol 106(1):23–31. https://doi.org/10.1007/s11060-011-0637-y
Ladstein RG, Bachmann IM, Straume O, Akslen LA (2010) Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer 10:140. https://doi.org/10.1186/1471-2407-10-140
Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H et al (1985) Amplification and overexpression of the EGF receptor gene in primary human glioblastomas. J Cell Sci Suppl 3:161–172
Louis DN (1994) The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol 53(1):11–21
Marquez-Garban DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ (2007) Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 72(2):135–143. https://doi.org/10.1016/j.steroids.2006.11.019
Montine TJ, Vandersteenhoven JJ, Aguzzi A, Boyko OB, Dodge RK, Kerns BJ, Burger PC (1994) Prognostic significance of Ki-67 proliferation index in supratentorial fibrillary astrocytic neoplasms. Neurosurgery 34(4):674–678, discussion 678–679
Ohgaki H (2009) Epidemiology of brain tumors. Methods Mol Biol 472:323–342. https://doi.org/10.1007/978-1-60327-492-0_14
Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK (2007) Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res 67(11):5505–5512. https://doi.org/10.1158/0008-5472.CAN-06-3647
Sareddy GR, Vadlamudi RK (2016) PELP1: structure, biological function and clinical significance. Gene 585(1):128–134. https://doi.org/10.1016/j.gene.2016.03.017
Sukheeja D, Singhvi S, Rai NN, Midya M (2015) A comparative study of histopathology of Astrocytomas with intraoperative cytology with special reference to MIB-1 labelling index. J Clin Diagn Res 9(8):EC01–EC03. https://doi.org/10.7860/JCDR/2015/12372.6281
Tihan T, Davis R, Elowitz E, DiCostanzo D, Moll U (2000) Practical value of Ki-67 and p53 labeling indexes in stereotactic biopsies of diffuse and pilocytic astrocytomas. Arch Pathol Lab Med 124(1):108–113. https://doi.org/10.1043/0003-9985(2000)124<0108:PVOKAP>2.0.CO;2
Vadlamudi RK, Kumar R (2007) Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal 5:e004. https://doi.org/10.1621/nrs.05004
Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A, Kumar R (2001) Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem 276(41):38272–38279. https://doi.org/10.1074/jbc.M103783200
Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, Kumar R (2005) Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res 65(17):7724–7732. https://doi.org/10.1158/0008-5472.CAN-05-0614
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1):98–110. https://doi.org/10.1016/j.ccr.2009.12.020
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359(5):492–507. https://doi.org/10.1056/NEJMra0708126
Acknowledgements
The authors acknowledge the management of SRIHER for support & encouragement.
Funding
Indo-Russia grant DST/INT/RUS/RSF/P-26 to SKR is acknowledged. Funding for this project was partly supported by the grant from Department of Biotechnology (GV, DP & KG) vide sanction no. BT/PR5034/BRB/10/1057/2012.
Author information
Authors and Affiliations
Contributions
Study concept and design—GV and RSK; scientific guidance—DP and GV; data collection—KP, LDC, AV, HP, DP, KG; data analysis and manuscript preparation—VK, LDC and DP; statistical analysis-RSP; manuscript review—all authors.
Corresponding author
Ethics declarations
The study was approved by the IEC of Sri Ramachandra Institute of Higher Education & Research.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Padmavathy, K.P., Vuttaradhi, V.K., Venu, A. et al. Clinical Evaluation of Proline, Glutamic acid, and Leucine-Rich Protein 1 Expression in Astrocytomas and Correlations with the Proliferation Marker Ki-67. J Mol Neurosci 71, 724–733 (2021). https://doi.org/10.1007/s12031-020-01690-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01690-w