Abstract
Multiple sclerosis (MS) is a multifactorial, central nervous system, immune-mediated disease characterized by inflammation, demyelination, and neurodegeneration. Evidence suggests a steady rise in MS prevalence over the past five decades in the United States and around the world. Even with increased understanding of immunology, the specific etiological trigger of MS remains unknown. Evidence suggests that oxidative/nitroxidative stress is an important contributor to MS etiology, progression, and clinical symptoms. A multifaceted treatment approach aimed at counteracting oxidative/nitroxidative stress including MS disease–modifying medications, Mediterranean style diet, stress-relieving activities, smoking and alcohol cessation, exercise, and peer support programs is the best way to treat the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a multifactorial, central nervous system (CNS), immune-mediated disease that is characterized by demyelination, neurodegeneration, inflammation, and gliosis (Calabresi 2004; Hayes and Donald Acheson 2008; Weinshenker 1996). Evidence suggests a steady rise in MS prevalence around the world in the past five decades (Wallin et al. 2019). Indeed, over 700,000 and an estimated 900,000 adults were affected by MS in 2010 and 2017, respectively, in the United States (US) (Wallin et al. 2019).
MS is the leading progressive non-traumatic neurological disease of young adults (Zwibel and Smrtka 2011), and it reduces the sufferer’s quality of life (Zwibel and Smrtka 2011). It is an expensive chronic disease to treat and manage. Indeed, using the data from 1999 to 2008, the total all-cause healthcare direct and indirect costs for MS ranged from just under $10,000 to over $50,000 per patient per year with prescription medications accounting for the majority of direct costs (Adelman et al. 2013).
Clinically, MS normally presents in multiple subtypes including relapsing-remitting MS (RRMS), the most common subtype noted by episodes of neurological dysfunction and subsequent remission, secondary progressive MS (SPMS), primary progressive MS (PPMS) which affects about 10–15% of MS patients, and progressive relapsing MS (PRMS) (Ghasemi et al. 2017). PPMS patients have no female predominance like RRMS and unlike RRMS are older at disease onset and have a faster accumulation of disability (Antel et al. 2012). PPMS and RRMS have been found to display identical lesion morphology under ultrahigh field magnetic resonance imaging (MRI), indicating a strong similarity in many respects despite differences in disease course and clinical features (Kuchling et al. 2014). Most RRMS patients (about 65%) will over time develop SPMS which is typically considered phase 2 of the disease (Ghasemi et al. 2017). PRMS is the least common MS subtype and occurs in about 5% of patients (Ghasemi et al. 2017). Despite the differences observed in MS phenotypes, such differences are mostly quantitative (Antel et al. 2012). MS phenotypes are believed to be part of a disease spectrum, and differences are influenced by individual genetics and environmental factors (Antel et al. 2012).
The specific elements that provoke MS pathogenesis remain unknown. This makes it hard to effectively treat the disease. The objective of this narrative review is to highlight the key role of oxidative/nitroxidative stress in MS pathogenesis, pathophysiology, and clinical symptoms. This could help improve our understanding of the disease and enhance strategies of treatment.
Oxidative/Nitroxidative Stress in MS.
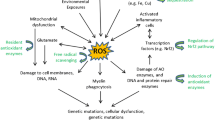
Excessive generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (such as superoxide, nitric oxide, and peroxynitrite), mitochondrial dysfunction, and poor or suboptimal antioxidant defense system lead to oxidative stress (OS) or nitroxidative stress (NOS), and these play a critical role in MS pathology (Haider 2015). Indeed, OS/NOS plays a critical role in the early phase of MS (Siotto et al. 2019) and on both the inflammatory and neurodegenerative components of the disease (Adamczyk and Adamczyk-Sowa 2016; Miller et al. 2019). Elevated levels of peroxynitrite formation in the CNS are implicated in MS pathogenesis (Cross et al. 1998), and research has found increased levels of OS markers (cholesteryl ester hydroperoxides) in plasma isolated from MS patients and impaired antioxidant mechanisms (Ferretti et al. 2005). The antioxidant, melatonin, has been shown to attenuate OS and increase the activity of antioxidative enzymes, superoxide dismutase, and glutathione peroxide in SPMS patients’ red blood cells (Miller et al. 2013) as well as alleviate MS symptoms (Adamczyk-Sowa et al. 2014; Álvarez-Sánchez et al. 2017).
Oxidative/Nitroxidative Stress in MS Pathophysiology and Clinical Symptoms
Mitochondrial Dysfunction
Mitochondrial dysfunction plays a critical role in MS (Tobore 2019). Indeed, mitochondrial abnormalities including energy failure, DNA defects, abnormal gene expression, defective enzyme activities, and impaired DNA repair activity are involved in the development and progression of demyelination and neurodegeneration in MS (Mao and Reddy 2010). Mitochondrial DNA SNP, nt13708 G/A, is associated with a significantly increased risk of MS (Yu et al. 2008). Variants of the mitochondrial ATP6 and ND2 genes have been found to be associated with both MS and systemic lupus erythematosus (Vyshkina et al. 2008). Mitochondrial dysfunction is implicated as playing a major role in axonal degeneration in MS (Dutta et al. 2006).
OS plays a critical role in mitochondrial dysfunction in MS (Haider 2015). Indeed, OS triggers mitochondrial injury in MS patients and energy failure in the CNS of individuals susceptible to MS (Haider 2015). In an EAE model, OS mediated mitochondrial dysfunction induced by protein inactivation was critical in initiating the molecular events that resulted in apoptosis and neurodegeneration (Qi et al. 2006). Oxidative damage affects mitochondrial DNA, and oxidative damage to mitochondrial macromolecules resulted in a reduction in the activity of mitochondrial energy metabolism and enzyme complexes in chronic MS active lesions (Lu et al. 2000). Impaired permeability transition pore opening mediated by ROS and calcium dyshomeostasis play a key role in MS mitochondrial dysfunction and neurodegeneration (Su et al. 2013). Research on an EAE model found that focal intra-axonal mitochondrial injury precedes alterations in axon morphology and ROS and RNS provoked mitochondrial injury initiating focal axonal degeneration (Nikić et al. 2011). Indeed, attenuation of ROS and RNS reversed mitochondrial injury and axonal degeneration (Nikić et al. 2011).
Demyelination, Neurodegeneration, and Axonal Damage in MS.
OS plays a major role in MS demyelination and neurodegeneration (Lassmann and van Horssen 2016) and mediates MS neurodegeneration initiated by microglial activation (Gonsette 2008). In MS brain, OS triggers demyelination and neurodegeneration through oxidation of proteins, lipids, and DNA and by inducing mitochondrial injury, resulting in energy failure and further generation of ROS (Lassmann and van Horssen 2016). OS damages all types of glial cells and neurons, although neurons and oligodendrocytes are most sensitive to its effect (Lassmann and van Horssen 2016). Indeed, oligodendrocytes are highly susceptible to OS/NOS than astrocytes and microglia, due to their weak antioxidant defense and other risk factors resulting in selective oligodendrocyte death, and demyelination (Dutta et al. 2006). OS damage of oligodendrocytes and neurons is quite significant and is associated with MS active demyelination and axonal injury (Haider et al. 2011). Indeed, inhibiting peroxynitrite formation may protect oligodendrocyte against OS-induced toxicity (Li et al. 2011).
Diet
Diet and nutritional status play a role in the etiopathogenesis of MS (Armon-Omer et al. 2019; Jahromi et al. 2012). Antioxidant-rich food has been found to reduce the risks of MS while prooxidant food increases it. Indeed, MS is higher in people who consume a diet low in whole grains and high in animal fats, potato, sugars, meat products, and hydrogenated fats (Jahromi et al. 2012). Serum total antioxidant capacity is significantly lower in the MS patients and associated with disease severity (Armon-Omer et al. 2019). Antioxidant-rich diets from plants including fruits, vegetables, and plant protein, dietary fiber, vitamin C, cereal fiber, thiamin, calcium, riboflavin, and potassium significantly reduce the risk of MS (Ghadirian et al. 1998). Vitamin A, E, and B1 deficiencies are involved in MS pathophysiology, and vitamin B9, B12, A, B1, and B3 may improve MS relapses, clinical symptoms, neurodegeneration, and inflammation (Khosravi-Largani et al. 2018). Also, potent antioxidants and immunomodulators such as vitamin D and B12 have been implicated in MS pathogenesis and severity (Bagur et al. 2017; Tobore 2020a). Indeed, elevated homocysteine level is believed to contribute to MS pathogenesis (Dardiotis et al. 2017) and vitamin B12 is known to attenuate homocysteine induced OS (van de Lagemaat et al. 2019). Supplementation of antioxidant-rich omega-3 and fish oils alleviates MS relapsing rate, the activity of pro-inflammatory agents, and improves MS patients’ quality of life (AlAmmar et al. 2019).
Ketogenic diet (KD) attenuates OS by modulating uncoupling proteins and elevating levels of antioxidant enzymes (e.g., catalase and glutathione) via inhibiting histone deacetylases and activating the Nrf2 pathway (Storoni and Plant 2015). It also improves mitochondrial function and has been suggested to be potentially useful in attenuating neurodegeneration in MS (Storoni and Plant 2015). A pilot study found that it alleviates fatigue and depression, promotes weight loss, and attenuates serologic pro-inflammatory adipokines in patients with RRMS (Brenton et al. 2019). A murine model of experimental autoimmune encephalomyelitis (EAE) found that KD improves cognitive ability and motor function and attenuates pro-inflammatory agents and OS (Kim et al. 2012).
Also, gut microbial dysbiosis is implicated in MS pathology (Cekanaviciute et al. 2017; Chen et al. 2016; Ochoa-Reparaz et al. 2017). Indeed, MS patients have a special or different gut microbiota (Chen et al. 2016; Schepici et al. 2019). Evidence suggests that Parabacteroides distasonis, which promoted anti-inflammatory IL-10 cells expressing IL-10+FoxP3+ Tregs in mice and human CD4+CD25+ T cells, were reduced in MS patients and Akkermansia muciniphila and Acinetobacter calcoaceticus, which provoked increased proinflammatory activity in monocolonized mice and human peripheral blood mononuclear cells, were elevated in MS patients (Cekanaviciute et al. 2017). MS patients have also been found to have elevated gut microbial populations of Streptococcus, Flavobacterium, Dorea, Pedobacteria, Mycoplana, Pseudomonas, Eggerthella, and Blautia and reduced microbial populations of Prevotella, Coprobacillus, Bacteroides, Sutterella, Adlercreutzia, Lactobacillus, and Faecalibacterium (Schepici et al. 2019). Transfer of microbiota from MS patients into germ-free mice led to heightened severity of symptoms of EAE and reduced quantity of anti-inflammatory IL-10+ Tregs (Cekanaviciute et al. 2017). Restoring the microbial population in patients with RRMS attenuated inflammation and reactivated the immune system (Schepici et al. 2019).
Importantly, diet has a profound effect on the composition of the gut microbiome (Schepici et al. 2019; Singh et al. 2017). Indeed, diet modulates health partly through its effect on gut microbiome (Wu et al. 2011). Mediterranean diet is an antioxidant-rich food, and adherence reduces the risk of developing MS (Sedaghat et al. 2016). In addition, Mediterranean diet modulates gut microbiome and adherence is associated with increased levels of certain bacteria taxa including fecal SCFAs, Prevotella, other Firmicutes, Lactobacillus, and Bifidobacterium and decreases in Clostridium (De Filippis et al. 2016; Singh et al. 2017).
Also, diet plays a role in obesity and a high BMI and obesity are associated with increased risks of MS (Ghadirian et al. 1998; Langer-Gould et al. 2013; Mokry et al. 2016). Evidence suggests that OS is a cause, mediator, and consequence of obesity (Tobore 2020b) and OS has been suggested to be the underlying mechanism that links obesity to MS (Tobore 2020a).
Psychiatric Disturbance and Cognitive Dysfunction
Neuropsychiatric symptoms are prevalent in MS patient population (Murphy et al. 2017b; Skokou et al. 2012). Both biological and psychosocial factors play a role in neuropsychiatric symptoms in MS, and risk factors include female sex, family history of major depression, age (less than 35 years), and stress (Skokou et al. 2012). Psychiatric disorders particularly depression are strongly associated with reduced quality of life for MS patients (Amato et al. 2001; Biernacki et al. 2019; Fruewald et al. 2001).
Increased inflammation and OS/NOS play a critical causative role in the pathophysiology of MS and major depression (Morris et al. 2018a). Indeed, significant correlation between elevated OS/NOS and the initiation of MS relapses and the number of MS relapses as well as correlation between OS/NOS levels and severity of major depression indicate an underlying causative role of OS/NOS in both diseases (Morris et al. 2018a). OS plays a critical role in the etiology and pathophysiology of psychiatric disorders (Hassan et al. 2016; Moniczewski et al. 2015; Ng et al. 2008; Salim 2014; Smaga et al. 2015; Tsaluchidu et al. 2008) and contributes to depression in MS (Katarina et al. 2018). Coenzyme Q10 (CoQ10) supplementation in RRMS patients treated with interferon-β1a 44 μg is associated with attenuating OS and inflammation and improving depressive symptoms (Moccia et al. 2019).
Also, cognitive impairment, particularly impairment in processing speed, executive functions, attention, visuospatial perception, and episodic memory, is prevalent and considered a core feature of MS pathology (Di Filippo et al. 2018; Rao 1995). Indeed, about 45 to 65% of MS sufferers are affected by cognitive problems at some time in the disease course (Rao 1995). In MS patients, N-acetyl-cysteine has been found to positively modulate cerebral glucose metabolism, which is associated with improvements in cognition and attention (Monti et al. 2020). Reduced potent CNS antioxidant, glutathione, has been found in different MS types and is associated with cognitive impairment severity (processing speed and learning and memory deficits) (Hughes 2014). Similarly, reduced glutathione is associated with a decline in executive function with aging in healthy adults (Hajjar et al. 2018). In schizophrenia, OS-induced prefrontal oligodendrocyte precursor cell dysfunctioning is theorized to be the causal factor that drives the etiology of cognitive symptoms (Maas et al. 2017). In an experimental model of MS, vitamin D3 using its antioxidant properties attenuated OS and improved cognitive deficits (spatial learning and memory deficits) (Tarbali and Khezri 2016).
Neuroinflammation
MS is strongly associated with neuroinflammation (Rosenberg 2002). Inflammation promotes OS and vice versa, suggesting a self-perpetuating vicious cycle (Ortiz et al. 2013). Levels of ROS and RNS can dramatically increase under inflammation conditions, overcoming the antioxidant defenses within lesions, and may result in damage in proteins, cell mitochondria, nucleic acids, and lipids, and cell death (Park et al. 2010). Indeed, oxidative damage to mitochondrial macromolecules develops concomitantly with inflammation in the CNS and plays a role in the reduction of mitochondrial enzyme complex activity and energy metabolism in chronic active lesions of MS, potentially resulting in cell death or degeneration (Lu et al. 2000). OS is implicated in the initiation of inflammation in the acute phase of MS (Adamczyk et al. 2017), and in RRMS patients, melatonin has been shown to promote an insulated cytokine microenvironment and mitigate inflammatory response by decreasing Th1 and Th22 responses in patients (Álvarez-Sánchez et al. 2017).
Inflammation-induced OS in activated microglia and macrophages plays an important role in demyelination and OS-induced tissue injury that results in MS pathogenesis (Fischer et al. 2012; Ortiz et al. 2013), and OS is implicated in inflammation-induced MS demyelination and neurodegeneration (Wang et al. 2014). ROS may damage myelin sheaths and promote macrophage activity on myelin sheaths (Smith et al. 2006). ROS and NO released during inflammation have been implicated in the damage from OS to DNA in MS lesions and in nearby neurons around these lesions contributing to the development of MS clinical disability (Vladimirova et al. 1998). Macrophages and microglia express myeloperoxidase and promote ROS production during myelin phagocytosis in the white matter, and demyelination in MS has been found to be associated with significantly increased myeloperoxidase activity in homogenates of MS white matter, indicating an intricate relationship between inflammation and OS in contributing to axonal injury within plaques (Gray et al. 2008).
Sleep Disturbances
Sleep disturbance is prevalent in MS (Bøe Lunde et al. 2012), and it is a predictor of poor quality of life (Merlino et al. 2009). Sleep disturbance in MS is strongly associated with depression, anxiety, fatigue, and pain (Vitkova et al. 2016, 2014). Inflammation and OS/NOS play a role in the pathophysiology of poor sleep and circadian abnormalities in neuropsychiatric disorders including MS and sleep disturbance promotes further inflammation, OS/NOS in a vicious cycle (Morris et al. 2018b). Evidence suggests that MS patients have higher serum levels of total oxidant status and melatonin uses its antioxidant properties to improve poor sleep quality in MS patients (Adamczyk-Sowa et al. 2014). Also, sleep is an important resting state with antioxidant properties, responsible for attenuating OS produced during wakefulness (Teixeira et al. 2019; Villafuerte et al. 2015). Indeed, poor sleep in MS could amplify OS/NOS and worsen disease severity (Tobore 2019).
Pain
Pain is common in MS and accounts for a significant portion (approximately 30%) of all drugs uses to treat MS symptoms (Solaro et al. 2013; Solaro and Messmer Uccelli 2011). MS patients can experience different types of pain simultaneously and at any interval in the disease (Solaro and Messmer Uccelli 2011). Chronic neuropathic pain in MS is one of the most frequent symptoms associated with reduced quality of life (Murphy et al. 2017a).
OS/NOS plays a critical role in different types of pain (Little et al. 2012; Salvemini et al. 2011) including neuropathic pain (Kim et al. 2004; Park et al. 2006; SINISCALCO et al. 2007; Yowtak et al. 2013, 2011). Research indicates that lipoic acid, a potent antioxidant, can improve whole-brain atrophy rate in SPMS patients (Spain et al. 2017), suppress matrix metallopeptidase 9 activity, and disrupt T cell migration into the CNS in MS patients (Yadav et al. 2005) and it has been found in a case study to be effective in the treatment of MS-induced neuropathic pain (Kulaklı 2018). US Government approved medication for MS, dimethyl fumarate, has been found to attenuate nociceptive hypersensitivity triggered by peripheral nerve injury by activating antioxidant response-nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, attenuating neuroinflammation, and mitochondrial OS mechanisms involved in promoting nociceptive hypersensitivity (Li et al. 2020). CoQ10 supplementation in RRMS patients treated with interferon-β1a 44 μg is associated with attenuating OS and inflammation and with clinical improvement in pain symptoms (Moccia et al. 2019).
Conclusions and Clinical Recommendations
MS is a complex, multifactorial disease, and OS/NOS play a critical role in the disease pathogenesis and progression. Aside from the factors described above, OS/NOS has been implicated as the underlying mechanism in many factors involved in MS including neurotransmitters alteration, Epstein-Barr Virus (EBV), human herpes 6 (HHV6), smoking, mycoplasma pneumonia, lower insulin-like growth factor-1 (IGF-I), lower growth hormone (GH), MS relapse and disability, visual impairment, thyroid dysfunction, sex hormones, and altered hypothalamic pituitary adrenal (HPA) axis (Tobore 2020b; Tobore 2019). Armed with the understanding of the critical role of OS/NOS in MS, an integrated or heterogeneous approach to attenuate OS/NOS is likely to confer the best therapeutic benefit. This includes sleep quality improvement; exercise; disease-modifying drugs; stress-relieving activities including yoga, meditation, and social or peer support programs; smoking and alcohol cessation; and antioxidant-rich diet.
Regular exercise boosts endogenous antioxidant defense systems and is linked with improvement in fatigue and quality of life of MS patients (Motl and Gosney 2008; Pilutti et al. 2013; White and Castellano 2008). As adjunct therapy, stress-relieving activities including yoga and meditation should be recommended as they attenuate OS (Tobore 2020a), reduce fatigue (Shohani et al. 2020) and pain, and improve quality of life in MS patients (Tavee et al. 2011). More MS peer support programs should be created and expanded, and patients should be encouraged to participate as it may help improve quality of life and MS-related psychological functions including depression, anxiety, and stress (Ng et al. 2013). Melatonin uses its antioxidant properties to improve sleep and depression in MS patients (Adamczyk-Sowa et al. 2014) and should be recommended for MS patients with reported sleep-related problems.
Pro-oxidant foods (Tobore 2020a) should be avoided, and adherence to antioxidant-rich diet particularly the Mediterranean diet, which decreases OS (Tobore 2020b), reduces MS risk (Sedaghat et al. 2016), and improves the quality of life and severity of the disease (Katz Sand et al. 2019), is strongly recommended. Supplementation of antioxidant-rich omega-3 and fish oils should be encouraged as it has been found to reduce MS relapsing rate, pro-inflammatory agents, and improve MS patient’s quality of life (AlAmmar et al. 2019). Antioxidant supplementation including vitamins A, E, B1, B9, B12, and B3 should be recommended in cases of deficiency as this may improve MS relapses, clinical symptoms, neurodegeneration, and inflammation (Khosravi-Largani et al. 2018). CoQ10 supplementation (500 mg/day) is recommended to reduce MS-related fatigue and depression (Sanoobar et al. 2016). Importantly, caution must be applied in the use of supplements and preference should be given to antioxidant-rich diet because excessive antioxidant supplementation could promote OS. Indeed, excessive vitamin C can aggravate MS symptoms because of the promotion of Fenton reaction (Khosravi-Largani et al. 2018).
In conclusion, OS/NOS plays a critical role in MS etiopathogenesis, and progression and treatment strategies should employ an integrated approach aimed at reducing OS/NOS. However, MS remains a complex disease that involves other factors including genetics that cannot be accounted for by OS/NOS. So, more and continued research is necessary to better and more effective treatment of the disease.
References
Adamczyk-Sowa M, Pierzchala K, Sowa P, Mucha S, Sadowska-Bartosz I, Adamczyk J, Hartel M (2014) Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem Res 39:1585–1593. https://doi.org/10.1007/s11064-014-1347-6
Adamczyk B, Adamczyk-Sowa M (2016) New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxidative Med Cell Longev 2016:1–18. https://doi.org/10.1155/2016/1973834
Adamczyk B, Niedziela N, Adamczyk-Sowa M (2017) Novel approaches of oxidative stress mechanisms in the multiple sclerosis pathophysiology and therapy, in: multiple sclerosis: perspectives in treatment and pathogenesis. Codon Publications, pp. 155–171. https://doi.org/10.15586/codon.multiplesclerosis.2017.ch10
Adelman G, Rane SG, Villa KF (2013) The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ 16:639–647. https://doi.org/10.3111/13696998.2013.778268
AlAmmar WA, Albeesh FH, Ibrahim LM, Algindan YY, Yamani LZ, Khattab RY (2019) Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: a systematic review. Nutr Neurosci:1–11. https://doi.org/10.1080/1028415X.2019.1659560
Álvarez-Sánchez N, Cruz-Chamorro I, Díaz-Sánchez M, Sarmiento-Soto H, Medrano-Campillo P, Martínez-López A, Lardone PJ, Guerrero JM, Carrillo-Vico A (2017) Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J Pineal Res 63:e12442. https://doi.org/10.1111/jpi.12442
Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L (2001) Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler J 7:340–344. https://doi.org/10.1177/135245850100700511
Antel J, Antel S, Caramanos Z, Arnold DL, Kuhlmann T (2012) Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol 123:627–638. https://doi.org/10.1007/s00401-012-0953-0
Armon-Omer A, Waldman C, Simaan N, Neuman H, Tamir S, Shahien R (2019) New insights on the nutrition status and antioxidant capacity in multiple sclerosis patients. Nutrients 11:427. https://doi.org/10.3390/nu11020427
Bagur MJ, Murcia MA, Jiménez-Monreal AM, Tur JA, Bibiloni MM, Alonso GL, Martínez-Tomé M (2017) Influence of diet in multiple sclerosis: a systematic review. Advances in Nutrition: An International Review Journal 8:463–472. https://doi.org/10.3945/an.116.014191
Biernacki T, Sandi D, Kincses ZT, Füvesi J, Rózsa C, Mátyás K, Vécsei L, Bencsik K (2019) Contributing factors to health-related quality of life in multiple sclerosis Brain and Behavior:9. https://doi.org/10.1002/brb3.1466
Bøe Lunde HM, Aae TF, Indrevåg W, Aarseth J, Bjorvatn B, Myhr K-M, Bø L (2012) Poor sleep in patients with multiple sclerosis. PLoS One 7:e49996. https://doi.org/10.1371/journal.pone.0049996
Brenton JN, Banwell B, Bergqvist AGC, Lehner-Gulotta D, Gampper L, Leytham E, Coleman R, Goldman MD (2019) Pilot study of a ketogenic diet in relapsing-remitting MS. Neurology - Neuroimmunology Neuroinflammation 6:e565. https://doi.org/10.1212/NXI.0000000000000565
Calabresi PA (2004) Diagnosis and management of multiple sclerosis. Am Fam Physician 70:1935–1944
Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, Crabtree-Hartman E, Katz I, Gacias M, Zhu Y, Casaccia P, Cree BAC, Knight R, Mazmanian SK, Baranzini SE, Zhu Y (2017) Correction for "gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci 10:10713–10718. https://doi.org/10.1073/pnas.1711235114
Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, Weinshenker BG, Rodriguez M, Kantarci OH, Nelson H, Murray JA, Mangalam AK (2016) Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 6:28484. https://doi.org/10.1038/srep28484
Cross AH, Manning PT, Keeling RM, Schmidt RE, Misko TP (1998) Peroxynitrite formation within the central nervous system in active multiple sclerosis. J Neuroimmunol 88:45–56. https://doi.org/10.1016/S0165-5728(98)00078-2
Dardiotis E, Arseniou S, Sokratous M, Tsouris Z, Siokas V, Mentis AFA, Michalopoulou A, Andravizou A, Dastamani M, Paterakis K, Bogdanos D, Brotis A (2017) Vitamin B12, folate, and homocysteine levels and multiple sclerosis: a meta-analysis. Multiple Sclerosis and Related Disorders 17:190–197. https://doi.org/10.1016/j.msard.2017.08.004
De Filippis, F., Pellegrini, N., Vannini, L., Jeffery, I.B., La Storia, A., Laghi, L.I, Serrazanetti, D., Di Cagno, R., Ferrocino, I., Lazzi, C., Turroni, S., Cocolin, L., Brigidi, P., Neviani, E., Gobbetti, M., O’Toole, P.W., Ercolini, D., 2016. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome Gut 65. https://doi.org/10.1136/gutjnl-2015-309957
Di Filippo M, Portaccio E, Mancini A, Calabresi P (2018) Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci 19:599–609. https://doi.org/10.1038/s41583-018-0053-9
Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59:478–489. https://doi.org/10.1002/ana.20736
Ferretti G, Bacchetti T, Principi F, Di Ludovico F, Viti B, Angeleri VA, Danni M, Provinciali L (2005) Increased levels of lipid hydroperoxides in plasma of patients with multiple sclerosis: a relationship with paraoxonase activity. Mult Scler J 11:677–682. https://doi.org/10.1191/1352458505ms1240oa
Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, Van Horssen J, Lassmann H (2012) NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. A JOURNAL OF NEUROLOGY 135:886–899. https://doi.org/10.1093/brain/aws012
Fruewald S, Loeffler-Stastka H, Eher R, Saletu B, Baumhacki U (2001) Depression and quality of life in multiple sclerosis. Acta Neurol Scand 104:257–261. https://doi.org/10.1034/j.1600-0404.2001.00022.x
Ghadirian, P., Jain, M., Ducic, S., Shatenstein, B., Morisset, R. (Epidemiology R.U.R.C.C.P.H.-D. 3850 rue S.U.M.Q.H. 1T8 (Canada)), 1998. Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada. International Journal of Epidemiology (United Kingdom)
Ghasemi N, Razavi S, Nikzad E (2017) Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J 19:1–10. https://doi.org/10.22074/cellj.2016.4867
Gonsette RE (2008) Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci 274:48–53. https://doi.org/10.1016/j.jns.2008.06.029
Gray E, Thomas TL, Betmouni S, Scolding N, Love S (2008) Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci Lett 444:195–198. https://doi.org/10.1016/j.neulet.2008.08.035
Haider L (2015) Inflammation. Iron, Energy Failure, and Oxidative Stress in the Pathogenesis of Multiple Sclerosis 2015:1–10. https://doi.org/10.1155/2015/725370
Haider L, Fischer MT, Frischer JM, Bauer J, Hö R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H (2011) Oxidative damage in multiple sclerosis lesions. A JOURNAL OF NEUROLOGY 134:1914–1924. https://doi.org/10.1093/brain/awr128
Hajjar I, Hayek SS, Goldstein FC, Martin G, Jones DP, Quyyumi A (2018) Oxidative stress predicts cognitive decline with aging in healthy adults: an observational study. J Neuroinflammation 15:17. https://doi.org/10.1186/s12974-017-1026-z
Hassan W, Noreen H, Castro-Gomes V, Mohammadzai I, da Rocha JBT, Landeira-Fernandez J (2016) Association of Oxidative Stress with Psychiatric Disorders. Curr Pharm Des 22:2960–2974
Hayes CE, Donald Acheson E (2008) A unifying multiple sclerosis etiology linking virus infection, sunlight, and vitamin D, through viral interleukin-10. Med Hypotheses 71:85–90. https://doi.org/10.1016/j.mehy.2008.01.031
Hughes, A.J., 2014. Glutathione as a predictor of neuropsychological impairment in patients with relapsing remitting, secondary progressive, and primary progressive multiple sclerosis
Jahromi SR, Toghae M, Jahromi MJR, Aloosh M (2012) Dietary pattern and risk of multiple sclerosis. Iranian journal of neurology 11:47–53
Katarina V, Gordana T, Svetlana MD, Milica B (2018) Oxidative stress and neuroinflammation should be both considered in the occurrence of fatigue and depression in multiple sclerosis. Acta Neurol Belg. https://doi.org/10.1007/s13760-018-1015-8
Katz Sand, I., Benn, E.K.T., Fabian, M., Fitzgerald, K.C., Digga, E., Deshpande, R., Miller, A., Gallo, S., Arab, L., 2019. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: A pilot study Multiple Sclerosis and Related Disorders 36. https://doi.org/10.1016/j.msard.2019.101403
Khosravi-Largani M, Pourvali-Talatappeh P, Rousta AM, Karimi-Kivi M, Noroozi E, Mahjoob A, Asaadi Y, Shahmohammadi A, Sadeghi S, Shakeri S, Ghiyasvand K, Tavakoli-Yaraki M (2018) A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalSci 10:37–44 https://doi.org/10.1016/j.ensci.2018.01.007
Kim DY, Hao J, Liu R, Turner G, Shi F-D, Rho JM (2012) Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One 7:e35476. https://doi.org/10.1371/journal.pone.0035476
Kim HK, Park SK, Zhou J-L, Taglialatela G, Chung K, Coggeshall RE, Chung JM (2004) Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111:116–124. https://doi.org/10.1016/j.pain.2004.06.008
Kuchling J, Ramien C, Bozin I, Dörr J, Harms L, Rosche B, Niendorf T, Paul F, Sinnecker T, Wuerfel J (2014) Identical lesion morphology in primary progressive and relapsing-remitting MS -an ultrahigh field MRI study. Mult Scler J 20:1866–1871. https://doi.org/10.1177/1352458514531084
Kulaklı F (2018) The effect of alpha lipoic acid in the treatment of multiple sclerosis induced neuropathic pain: a case report. Eurasian Journal of Medicine and Oncology. https://doi.org/10.14744/ejmo.2017.37929
Langer-Gould A, Brara SM, Beaber BE, Koebnick C (2013) Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology 80:548–552. https://doi.org/10.1212/WNL.0b013e31828154f3
Lassmann H, van Horssen J (2016) Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta Mol basis Dis 1862:506–510. https://doi.org/10.1016/j.bbadis.2015.09.018
Li J, Ma J, Lacagnina MJ, Lorca S, Odem MA, Walters ET, Kavelaars A, Grace PM (2020) Oral dimethyl fumarate reduces peripheral neuropathic pain in rodents via NFe2L2 antioxidant signaling. Anesthesiology 132:343–356. https://doi.org/10.1097/ALN.0000000000003077
Li S, Vana AC, Ribeiro R, Zhang Y (2011) Distinct role of nitric oxide and peroxynitrite in mediating oligodendrocyte toxicity in culture and in experimental autoimmune encephalomyelitis. Neuroscience 184:107–119. https://doi.org/10.1016/j.neuroscience.2011.04.007
Little JW, Doyle T, Salvemini D (2012) Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids 42:75–94. https://doi.org/10.1007/s00726-010-0633-0
Lu F, Selak M, O’Connor J, Croul S, Lorenzana C, Butunoi C, Kalman B (2000) Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 177:95–103. https://doi.org/10.1016/S0022-510X(00)00343-9
Maas DA, Vallès A, Martens GJM (2017) Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry 7:e1171. https://doi.org/10.1038/tp.2017.138
Mao P, Reddy PH (2010) Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta (BBA) - Mol Basis Dis 1802:66–79. https://doi.org/10.1016/J.BBADIS.2009.07.002
Merlino G, Fratticci L, Lenchig C, Valente M, Cargnelutti D, Picello M, Serafini A, Dolso P, Gigli GL (2009) Prevalence of “poor sleep” among patients with multiple sclerosis: an independent predictor of mental and physical status. Sleep Med 10:26–34. https://doi.org/10.1016/j.sleep.2007.11.004
Miller E, Walczak A, Majsterek I, Kędziora J (2013) Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol 257:97–101. https://doi.org/10.1016/j.jneuroim.2013.02.012
Miller ED, Dziedzic A, Saluk-Bijak J, Bijak M (2019) A review of various antioxidant compounds and their potential utility as complementary therapy in multiple sclerosis. Nutrients. 11. https://doi.org/10.3390/nu11071528
Moccia M, Capacchione A, Lanzillo R, Carbone F, Micillo T, Perna F, De Rosa A, Carotenuto A, Albero R, Matarese G, Palladino R, Brescia Morra V (2019) Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-β1a-treated multiple sclerosis. Ther Adv Neurol Disord 12:1756286418819074. https://doi.org/10.1177/1756286418819074
Mokry LE, Ross S, Timpson NJ, Sawcer S, Davey Smith G, Richards JB (2016) Obesity and multiple sclerosis: a Mendelian randomization study. PLoS Med 13:e1002053. https://doi.org/10.1371/journal.pmed.1002053
Moniczewski A, Gawlik M, Smaga I, Niedzielska E, Krzek J, Przegaliński E, Pera J, Filip M (2015) Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 1. Chemical aspects and biological sources of oxidative stress in the brain. Pharmacol Rep 67:560–568. https://doi.org/10.1016/j.pharep.2014.12.014
Monti DA, Zabrecky G, Leist TP, Wintering N, Bazzan AJ, Zhan T, Newberg AB (2020) N-acetyl cysteine administration is associated with increased cerebral glucose metabolism in patients with multiple sclerosis: an exploratory study. Front Neurol 11:88. https://doi.org/10.3389/fneur.2020.00088
Morris G, Reiche EMV, Murru A, Carvalho AF, Maes M, Berk M, Puri BK (2018a) Multiple immune-inflammatory and oxidative and Nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Mol Neurobiol 55:6282–6306. https://doi.org/10.1007/s12035-017-0843-5
Morris G, Stubbs B, Köhler CA, Walder K, Slyepchenko A, Berk M, Carvalho AF (2018b) The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med Rev 41:255–265. https://doi.org/10.1016/j.smrv.2018.03.007
Motl RW, Gosney JL (2008) Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler J 14:129–135. https://doi.org/10.1177/1352458507080464
Murphy KL, Bethea JR, Fischer R (2017a) Neuropathic pain in multiple sclerosis–current therapeutic intervention and future treatment perspectives, in: multiple sclerosis: perspectives in treatment and pathogenesis. Codon Publications, pp. 53–69. https://doi.org/10.15586/codon.multiplesclerosis.2017.ch4
Murphy R, O’Donoghue S, Counihan T, McDonald C, Calabresi PA, Ahmed MA, Kaplin A, Hallahan B (2017b) Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry 88:697–708. https://doi.org/10.1136/JNNP-2016-315367
Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11:851–876. https://doi.org/10.1017/S1461145707008401
Ng L, Amatya B, Khan F (2013) Outcomes of a peer support program in multiple sclerosis in an Australian community cohort: a prospective study. Journal of Neurodegenerative Diseases 2013:1–7
Nikić I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Brück W, Bishop D, Misgeld T, Kerschensteiner M (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17:495–499. https://doi.org/10.1038/nm.2324
Ochoa-Reparaz J, Magori K, Kasper LH (2017) The chicken or the egg dilemma: intestinal dysbiosis in multiple sclerosis. Annals of Translational Medicine 5:145. https://doi.org/10.21037/atm.2017.01.18
Ortiz GG, Pacheco-Moisés FP, Bitzer-Quintero OK, Ramírez-Anguiano AC, Flores-Alvarado LJ, Ramírez-Ramírez V, Macias-Islas MA, Torres-Sánchez ED (2013) Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clinical & developmental immunology 2013:708659–708614. https://doi.org/10.1155/2013/708659
Park CW, Macinnis DJ, Priester J, Eisingerich AB, Iacobucci D (2010) Brand attachment and brand attitude strength: conceptual and empirical differentiation of two critical brand equity drivers. J Mark 74:1–17
Park E-S, Gao X, Chung JM, Chung K (2006) Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett 391:108–111. https://doi.org/10.1016/j.neulet.2005.08.055
Pilutti LA, Greenlee TA, Motl RW, Nickrent MS, Petruzzello SJ (2013) Effects of exercise training on fatigue in multiple sclerosis. Psychosom Med 75:575–580. https://doi.org/10.1097/PSY.0b013e31829b4525
Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J (2006) Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J Biol Chem 281:31950–31962. https://doi.org/10.1074/jbc.M603717200
Rao SM (1995) Neuropsychology of multiple sclerosis. Curr Opin Neurol 8:216–220. https://doi.org/10.1097/00019052-199506000-00010
Rosenberg GA (2002) Matrix metalloproteinases and neuroinflammation in multiple sclerosis. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 8:586–595. https://doi.org/10.1177/1073858402238517
Salim S (2014) Oxidative stress and psychological disorders. Curr Neuropharmacol 12:140–147. https://doi.org/10.2174/1570159X11666131120230309
Salvemini D, Little JW, Doyle T, Neumann WL (2011) Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med 51:951–966. https://doi.org/10.1016/j.freeradbiomed.2011.01.026
Sanoobar M, Dehghan P, Khalili M, Azimi A, Seifar F (2016) Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: a double blind randomized clinical trial. Nutr Neurosci 19:138–143. https://doi.org/10.1179/1476830515Y.0000000002
Schepici G, Silvestro S, Bramanti P, Mazzon E (2019) The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant 28:1507–1527. https://doi.org/10.1177/0963689719873890
Sedaghat F, Jessri M, Behrooz M, Mirghotbi M, Rashidkhani B (2016) Mediterranean diet adherence and risk of multiple sclerosis: a case-control study. Asia Pac J Clin Nutr 25:377–384. https://doi.org/10.6133/apjcn.2016.25.2.12
Shohani, M., Kazemi, F., Rahmati, S., Azami, M., 2020. The effect of yoga on the quality of life and fatigue in patients with multiple sclerosis: A systematic review and meta-analysis of randomized clinical trials Complementary Therapies in Clinical Practice 39. https://doi.org/10.1016/j.ctcp.2020.101087
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15:73. https://doi.org/10.1186/s12967-017-1175-y
Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth K, Maione S, Denovellis V (2007) Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res 55:158–166. https://doi.org/10.1016/j.phrs.2006.11.009
Siotto M, Filippi MM, Simonelli I, Landi D, Ghazaryan A, Vollaro S, Ventriglia M, Pasqualetti P, Rongioletti MCA, Squitti R, Vernieri F (2019) Oxidative stress related to Iron metabolism in relapsing remitting multiple sclerosis patients with low disability. Front Neurosci 13:86. https://doi.org/10.3389/fnins.2019.00086
Skokou M, Soubasi E, Gourzis P (2012) Depression in multiple sclerosis: a review of assessment and treatment approaches in adult and pediatric populations. International scholarly research network ISRN neurology 2012. https://doi.org/10.5402/2012/427102
Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegaliński E, Pera J, Filip M (2015) Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Rep 67:569–580. https://doi.org/10.1016/J.PHAREP.2014.12.015
Smith KJ, Kapoor R, Felts PA (2006) Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 9:69–92. https://doi.org/10.1111/j.1750-3639.1999.tb00212.x
Solaro C, Messmer Uccelli M (2011) Management of pain in multiple sclerosis: a pharmacological approach. Nat Rev Neurol 7:519–527. https://doi.org/10.1038/nrneurol.2011.120
Solaro C, Trabucco E, Messmer Uccelli M (2013) Pain and multiple sclerosis: pathophysiology and treatment topical collection on demyelinating disorders. Current Neurology and Neuroscience Reports 13:1–9. https://doi.org/10.1007/s11910-012-0320-5
Spain R, Powers K, Murchison C, Heriza E, Winges K, Yadav V, Cameron M, Kim E, Horak F, Simon J, Bourdette D (2017) Lipoic acid in secondary progressive MS. Neurology: Neuroimmunology and NeuroInflammation 4:e374. https://doi.org/10.1212/NXI.0000000000000374
Storoni M, Plant GT (2015) The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int 2015:681289–681289. https://doi.org/10.1155/2015/681289
Su K, Bourdette D, Forte M (2013) Mitochondrial dysfunction and neurodegeneration in multiple sclerosis. Front Physiol 4:169. https://doi.org/10.3389/fphys.2013.00169
Tarbali S, Khezri S (2016) Vitamin D3 attenuates oxidative stress and cognitive deficits in a model of toxic demyelination. Iranian Journal of Basic Medical Sciences 19:80–88. https://doi.org/10.22038/ijbms.2016.6418
Tavee J, Rensel M, Planchon SM, Butler RS, Stone L (2011) Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy. International Journal of MS Care 13:163–168. https://doi.org/10.7224/1537-2073-13.4.163
Teixeira KRC, dos Santos CP, de Medeiros LA, Mendes JA, Cunha TM, De Angelis K, Penha-Silva N, de Oliveira EP, Crispim CA (2019) Night workers have lower levels of antioxidant defenses and higher levels of oxidative stress damage when compared to day workers. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-40989-6
Tobore TO (2019) On elucidation of the role of mitochondria dysfunction and oxidative stress in multiple sclerosis. Neurology and Clinical Neuroscience 7:305–317. https://doi.org/10.1111/ncn3.12335
Tobore TO (2020a) Towards a comprehensive etiopathogenetic and pathophysiological theory of multiple sclerosis. International journal of neuroscience 130. https://doi.org/10.1080/00207454.2019.1677648
Tobore TO (2020b) Towards a comprehensive theory of obesity and a healthy diet: the causal role of oxidative stress in food addiction and obesity. Behav Brain Res 384:112560. https://doi.org/10.1016/j.bbr.2020.112560
Tsaluchidu S, Cocchi M, Tonello L, Puri BK (2008) Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry 8:S5. https://doi.org/10.1186/1471-244X-8-S1-S5
van de Lagemaat EE, de Groot LCPGM, van den Heuvel EGHM (2019) Vitamin B 12 in relation to oxidative stress: a systematic review. Nutrients. 11. https://doi.org/10.3390/nu11020482
Villafuerte, G., Miguel-Puga, A., Murillo Rodríguez, E., Machado, S., Manjarrez, E., Arias-Carrión, O., 2015. Sleep deprivation and oxidative stress in animal Models: A Systematic Review. https://doi.org/10.1155/2015/234952
Vitkova M, Gdovinova Z, Rosenberger J, Szilasiova J, Nagyová I, Mikula P, Krokavcova M, Groothoff JW, Van Dijk JP (2014) Factors associated with poor sleep quality in patients with multiple sclerosis differ by disease duration. Disability and Health Journal 7:466–471. https://doi.org/10.1016/j.dhjo.2014.05.004
Vitkova M, Rosenberger J, Gdovinova Z, Szilasiova J, Mikula P, Groothoff JW, Reijneveld SA, van Dijk JP (2016) Poor sleep quality in patients with multiple sclerosis: gender differences. Brain and Behavior 6:e00553. https://doi.org/10.1002/brb3.553
Vladimirova, O., O’Connor, J., Cahill, A., Alder, H., Butunoi, C., Kalman, B., 1998. Oxidative damage to DNA in plaques of MS brains. Multiple sclerosis (Houndmills, Basingstoke, England) 4, 413–8. https://doi.org/10.1177/135245859800400503
Vyshkina T, Sylvester A, Sadiq S, Bonilla E, Canter JA, Perl A, Kalman B (2008) Association of common mitochondrial DNA variants with multiple sclerosis and systemic lupus erythematosus. Clin Immunol 129:31–35. https://doi.org/10.1016/j.clim.2008.07.011
Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, Cutter GR, Kaye WE, Wagner L, Tremlett H, Buka SL, Dilokthornsakul P, Topol B, Chen LH, Larocca NG (2019) The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 92:E1029–E1040. https://doi.org/10.1212/WNL.0000000000007035
Wang P, Xie K, Wang C, Bi J (2014) Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and Neurodegeneration in multiple sclerosis. Eur Neurol 72:249–254. https://doi.org/10.1159/000363515
Weinshenker BG (1996) Epidemiology of multiple sclerosis. Neurol Clin 14:291–308
White LJ, Castellano V (2008) Exercise and brain health ??? Implications for multiple sclerosis. Sports Med 38:91–100. https://doi.org/10.2165/00007256-200838020-00001
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. https://doi.org/10.1126/science.1208344
Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, Shinto L, Morris C, Bourdette D (2005) Lipoic acid in multiple sclerosis: a pilot study. Mult Scler J 11:159–165. https://doi.org/10.1191/1352458505ms1143oa
Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM (2011) Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain 152:844–852. https://doi.org/10.1016/j.pain.2010.12.034
Yowtak J, Wang J, Kim HY, Lu Y, Chung K, Chung JM (2013) Effect of antioxidant treatment on spinal GABA neurons in a neuropathic pain model in the mouse. Pain 154:2469–2476. https://doi.org/10.1016/j.pain.2013.07.024
Yu X, Koczan D, Sulonen AM, Akkad DA, Kroner A, Comabella M, Costa G, Corongiu D, Goertsches R, Camina-Tato M, Thiesen HJ, Nyland HI, Mørk SJ, Montalban X, Rieckmann P, Marrosu MG, Myhr KM, Epplen JT, Saarela J, Ibrahim SM (2008) mtDNA nt13708A variant increases the risk of multiple sclerosis. PLoS ONE 3. https://doi.org/10.1371/journal.pone.0001530
Zwibel, H.L., Smrtka, J., 2011. Improving quality of life in multiple sclerosis: an unmet need. The American journal of managed care 17 Suppl 5 improving, S139-45
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tobore Onojighofia Tobore: Independent scholar.
Rights and permissions
About this article
Cite this article
Tobore, T.O. Oxidative/Nitroxidative Stress and Multiple Sclerosis. J Mol Neurosci 71, 506–514 (2021). https://doi.org/10.1007/s12031-020-01672-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01672-y