Abstract
So far no evidence is available as to whether TGFβ and Wnt signaling pathways cooperatively modulate dopaminergic differentiation of the adult stem cells. To investigate the interaction between the two pathways in early dopaminergic differentiation, we cultured the newly introduced unrestricted somatic stem cells (USSCs) in neuron differentiation media followed by treatments with inducers and inhibitors of Wnt and TGF beta pathways either alone or in combinations. Our results showed that the level of Nurr-1 as a marker for dopaminergic neuron precursors and that of the nuclear β-catenin as the key effector of the active Wnt pathway were significantly elevated following the treatment with either TGFβ or BIO (the Wnt pathway inducer). Conversely, Nurr-1 expression was significantly reduced following the combined treatments with SB431542 (the TGFβ inhibitor) plus BIO or with TGFβ plus Dkk1 (the specific Wnt inhibitor). Nuclear β-catenin was also significantly reduced following combined treatments with SB431542 plus either BIO or TGFβ. Altogether, our results imply that Wnt and TGFβ signaling pathways cooperatively ensure the early dopaminergic differentiation of the USSC adult stem cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is increasing evidence for the involvement of signaling pathways like transforming growth factor beta (TGFβ) and Wnt in differentiation and maintenance of the embryonic midbrain dopaminergic (DA) neurons (Andersson et al. 2013; Angbohang et al. 2016; Dastjerdi et al. 2012; Dun et al. 2012; Falk et al. 2008; Fujimori et al. 2017; Galli et al. 2014; Gollamudi et al. 2012; Hegarty et al. 2014; Kashima and Hata 2017; Massey et al. 2018; Massey et al. 2019; Meyers and Kessler 2017; Ribeiro et al. 2011; Roussa et al. 2009; Roussa et al. 2006; Song et al. 2018; Stephano et al. 2018; Tesseur et al. 2017; Zhang et al. 2015). Wnt ligands including Wnt3a, Wnt1, and Wnt5a play important roles in development of dopaminergic phenotype (Andersson et al. 2013); Wnt1 and Wnt3a regulate the proliferation of dopaminergic precursors, and Wnt5a regulates differentiation of Nurr-1 precursor cells to tyrosine hydroxylase (TH) cells (Castelo-Branco et al. 2003). Both β catenin-dependent canonical (main) and β catenin-independent non-canonical Wnt pathways play important roles in development of DA neurons (Andersson et al. 2013). Due to the crosstalk between Wnt and other signaling pathways and also its complexity, controversies on the role of Wnt pathway in proliferation/differentiation of stem cells exist (Prakash and Wurst 2006). One important Wnt interacting pathway is the TGFβ pathway reported to be active in stem cell niches and essential for the maintenance and differentiation of neural stem cells both in vitro and in vivo (Falk et al. 2008). Increasing evidence indicates that transforming growth factor beta (TGFβ) is involved in differentiation and maintenance of midbrain dopaminergic neurons (Gadue et al. 2006; Hegarty et al. 2014; Kashima and Hata 2017; Li et al. 2006; Nishita et al. 2000; Roussa et al. 2009; Roussa et al. 2006; Tesseur et al. 2017; Zhang et al. 2015; Zhou et al. 2004). Members of the TGFβ family bind to the complex receptors including type I and II receptor serine/threonine kinases. Type II receptor activates type I receptor which then phosphorylates Smads 2/3 that oligomerizes with Smad 4 leading their translocation to the nucleus where the transcription of the TGFβ target genes are triggered (Shi and Massague 2003). Moreover, TGFβ is known to synergistically interact with other growth factors and thus increase migration, proliferation, differentiation, and survival of dopaminergic neurons (Roussa et al. 2006). Cai et al. (2013) also showed that the interaction between Wnt, TGFβ, and BMP pathways induces the embryonic stem cells towards dopaminergic differentiation (Cai et al. 2013). Wnt and TGFβ pathways are also known to differentially regulate proliferation and differentiation of mesenchymal stem cells (MSCs) towards non-neural lineages such as cartilage, bone, and fat cells (Zhang et al. 2015; Zhou et al. 2004). For example, while activation of Wnt and TGFβ pathways triggers the differentiation of MSCs towards the chondrocytes, that towards the adipocytes and osteocytes is inhibited (Zhang et al. 2015; Zhou et al. 2004). Despite all the abovementioned evidence on the interaction between Wnt and TGF in dopaminergic differentiation of the embryonic stem cells, limited knowledge however is available if they also interact in neural differentiation of the adult stem cells. In the present study, we therefore used the unrestricted somatic stem cells (USSCs) as an experimental model to examine if Wnt and TGFβ interaction enhances the dopaminergic potential of USSCs. The activation of the Wnt pathway was performed by using 6-bromoindirubin-3′-oxime (BIO) known to specifically inhibit the β catenin degrading enzyme, glycogen synthase kinase (GSK-3β) (Meijer et al. 2003). Wnt inhibition was performed by using recombinant Dickkopf-1 (Dkk1) as the specific Wnt inhibitor (Glinka et al. 1998). The activation and inhibition of the TGFβ pathway were induced by the recombinant TGFβ protein and the small molecule SB431542 (Inman et al. 2002), respectively. Using combinations of treatments, we analyzed the levels of Nurr-1 as a dopamineric neuron marker (Saucedo-Cardenas et al. 1998) and nuclear β catenin as the key element in the Wnt pathway. The results in this study indicate an interactive cooperation between the two pathways towards dopaminergic differentiation of the adult stem cell USSCs.
Materials and Methods
Chemicals
Retinoic acid, IBMX (isobutylmethylxanthine), and bFGF (basic fibroblast growth factor) were purchased from Sigma (UK). Human recombinant TGFβ1 (used at final concentration 1 ng/ml) and SB431542 (used at final concentration 10 μM) were purchased from Peprotech (Germany) and TOCRIS (Germany), respectively. Dkk1 (used at final concentration 100 ng/ml) was purchased from R&D systems (Germany). Nurr-1 and β catenin antibodies were purchased from Santa Cruz (Germany) and Sigma (Germany), respectively . FITC-conjugated secondary antibody was purchased from Razi Biotech (Iran); DMEM, fetal bovine serum (FBS), penicillin-streptomycin, trypsin, and EDTA were obtained from Gibco (UK). 6-Bio was purchased from Calbiochem (Germany). All other chemicals were purchased from Merck (Germany).
USSC Isolation and Culture
Isolation, culture, and characterization of human USSCs were performed as described by Kogler et al. (2004). Umbilical cord vein of the informed consented mothers was considered as a source of cord blood (CB) from which the mononuclear cell fraction was separated by density centrifugation of a Ficoll gradient. USSCs were successfully extracted at a rate of 40% from cord blood samples, expanded in low glucose DMEM supplemented with 20% FBS (control medium) in a humidified incubator with 5% CO2 at 37 °C. Being 80% confluent, the cells were passaged and replated after detachment by 0.25% Trypsin-EDTA.
Neural Differentiation
Human USSCs were cultured at a density of 4000 cells/cm2 on coverslips and treated with neural induction medium containing DMEM, 7.5% FBS, 1% penicillin/streptomycin, 20 ng/ml bFGF, 0.5 mM IBMX, 50 μM ascorbic acid, and 10 μM retinoic acid for up to 10 days (Khanghahi et al. 2014). Culture medium was changed every 2–3 days.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde, permeabilized with Triton X-100 (0.3%), and probed using primary antibodies to Nurr-1 (1:200), β catenin (1/1000), and FITC-conjugated secondary antibodies (1:60). The nuclei were determined by using DAPI.
Quantification Method of the Intensities of Nuclear β Catenin and Nurr-1
The stained nuclei of at least 50–70 cells in each group from at least 3 repeats of experiments were quantified. Using the image J program, the intensities were measured as the following steps: The images were firstly turned into 8-bit type. In the blue channel, the Image Adjust>Threshold was chosen to “select” the nuclei. Then, the nuclei in red were marked in ROI by choosing Edit>Selection>Create Selection>and added each to the ROI Manager. To Analyze the data>Set Measurements was selected where the “Grey value” refers to the intensity. Then, the same selected cells in the blue channel were also marked in the green channel. Finally, through the Analyze>Measure, the intensities of the nuclei in the green channel were measured and the ratio to that of the blue channel was calculated. All values are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed for the ratios between different groups using ANOVA by Prism 8.0 (GraphPad Software).
RNA Isolation and RT-PCR
Total cellular RNA was isolated from USSCs using RNX-plus (Fermentas, Germany) according to the manufacturer’s instructions. cDNA was synthesized using 2 μg RNA, oligo dT-primer, and reverse transcriptase (Cinagen, Iran). cDNAs were used as templates in polymerase chain reactions (PCR) in a thermocycler PCR machine using a PCR reaction mixture (Qiagen, Germany) with primer pairs according to the following program: 30 cycles at 95 °C/15″, 59°C/25″, and 72 °C/25″. PCR products were run on 1.5% agarose gel containing the red safe and visualized under the U.V. The primer sequences were as follows: Nurr-1 forward: 5-AATGCGTTCGTGGCTTTGG-3, Nurr-1 reverse: 5-AGTTCCTTTGAA GTGCTTGGG-3. GAPDH forward: 5′CCA GGT GGT CTC CTC TGA CTT CAA CAG-3′. GAPDH reverse: 5′AGG GTC TCT CTC TTC CTC TTG TGC TCT-3′. Amplicon sizes for Nurr-1 and GAPDH were 405 and 226 bps, respectively.
Results
USSCs Acquire Neuronal Fate in Neural Differentiation Media
As we previously showed (Dastjerdi et al. 2012), USSCs show fibroblast-like morphologies in the control media containing low glucose DMEM supplemented with 20% FBS (Fig. 1a). Following 3 days of culture in neuronal differentiation media (containing FBS, DMEM, ascorbic acid, retinoic acid, bFGF, and IBMX), these cells acquired neuronal phenotypes consisting of the elongated cells with neurites (Fig. 1b).
Wnt Canonical Pathway Is Activated by 6-BIO in USSCs
In order to activate the Wnt pathway in USSCs, these were treated with 6-BIO (a specific GSK-3β inhibitor), followed by immunofluorescence analysis of β-catenin expression pattern. As shown in Fig. 2, while the expression of β–catenin in the cells of the control group was mainly cytoplasmic (Fig. 2a, g), it was weakly localized in the nucleus in those treated with the neural differentiation medium (0.7188 ± 0.06309; Fig. 2c, g). This expression though was greatly enhanced after the treatment with 2 μM BIO (1.345 ± 0.0415; Fig. 2e, g), suggesting that 6-BIO activates the Wnt canonical pathway.
Immunofluorescence photomicrographs of β-catenin expression in USSC cells treated with either the neuron differentiation media, BIO, or both for 24 h. The expression of β–catenin in the control group (a) is mainly cytoplasmic and no nuclear staining is seen, whereas in neural differentiation medium treated group (c) is localized in the nucleus though not strong. Following the treatment with Bio together with the neural differentiation medium (e), it is robustly enhanced in the nucleus, indicating that Bio activates the Wnt signaling pathway. The nuclei were localized by using DAPI (b, d, f). Quantification of the intensities of the β catenin nucleus staining (g) showed that Bio enhanced the intensity for almost 1.5-folds compared to the neural differentiation medium-treated group. ***p < 0.001. Scale bar 100 μm
BIO Induces the Differentiation of USSCs Towards Dopaminergic Fate
In order to investigate if BIO triggers the dopaminergic differentiation of USSCs, the spatial expression of Nurr-1 protein (as one of the dopaminergic differentiation markers) was investigated in neurally induced USSCs treated with BIO for 3 days compared to the untreated and neurally induced cells. As shown in Fig. 3, the expression of Nurr-1 was significantly increased after the treatment with 2 μM BIO (1.036 ± 0.053; Fig. 3e, g) compared to the control (0.058 ± 0.004; Fig. 3a, g) and the differentiation media alone treated cells (0.739 ± 0.015; Fig. 3c, g), indicating that 6-BIO enhances the differentiation of USSCs towards the dopaminergic phenotype.
Immunofluorescense of Nurr-1 expression in USSCs following treatment with Bio. While there is a weak nuclear expression of Nurr-1 in the control cells (a), more is present in those treated with the differentiation media (c) and significantly enhanced in those treated with Bio 2 μM in the presence of differentiation medium (e). The nuclei were localized by using DAPI (b, d, f). Quantification of the intensities of the Nurr-1 nuclei staining (g) showed that Bio enhanced the intensity of Nurr-1 significantly compared to those in the neural differentiation medium-treated and the control groups. ***p < 0.001. Scale bar 100 μm
Activation of TGFβ Pathway in USSCs Triggers Their Differentiation Towards the Dopaminergic Fate
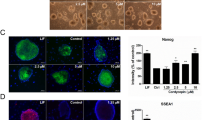
We examined the intensity of Nurr-1 protein expression as a dopaminergic differentiation marker in USSCs treated with the recombinant TGFβ1 either alone or together with its specific inhibitor SB431542. As shown in Fig. 4, Nurr-1 expression in the neurally induced USSCs (0.73 ± 0.015; Fig. 4b, i) was significantly enhanced after the TGFβ1 treatment (0.97 ± 0.022; Fig. 4c, i) as compared to the control untreated group (0.058 ± 0.004; Fig. 4a, i). Conversely, treatment with the SB431542 alone (0.7173 ± 0.04013; Fig. 4d, i) or together with TGFβ1 (0.7641 ± 0.02397; Fig. 4e, i) significantly reduced the expression of Nurr-1 compared to that in the TGFβ1-treated cells (0.97 ± 0.022; Fig. 4c, i). Altogether, these results indicate that the differentiation of USSCs into Nurr-1-positive neurons is triggered by TGFβ1 since it is antagonized by the inhibitor of TGFβ1, SB431542.
Nurr-1 localization in USSCs treated with neural differentiation media alone (a) or combined with TGFβ1 (c), SB431542 alone (e), or combined with TGFβ1 (g). As shown, while Nurr1 is weakly expressed in the neural differentiation media-treated group alone (a), it is increased after TGFβ1 treatment (c). Conversely, in the presence of SB431542 either alone (e) or combined with TGFβ1 (g), Nurr1 expression is significantly decreased. The localizations of the nuclei were determined by using DAPI (b, d, f, h). Quantification of the intensities of the Nurr-1 nuclei staining (i) showed that treatment with SB either alone or together with TGFβ1 reduced the intensity of Nurr-1 staining significantly compared to that in the TGFβ1-treated group. Scale bar 100 μm
Wnt Inhibition Reduced Nurr-1 Gene Expression Induced by Recombinant TGFβ
To investigate if inhibition of Wnt signaling pathways affects TGFβ induction of Nurr-1 gene as a marker of dopaminergic differentiation in USSCs, Dkk1 as a specific Wnt inhibitor was used. While Nurr-1 was expressed in groups of the control, neural differentiation media, and TGFβ1-treated USSCs, it was abolished after treatment with Dkk1 either alone or together with TGFβ (Fig. 5), suggesting that the active Wnt is required for TGFβ induction of dopaminergic differentiation.
Nuclear Localization of β-Catenin Requires the Activation of Both Wnt and TGFβ Pathways
To further investigate if both Wnt and TGFβ pathways cooperatively are involved in neural differentiation of USSCs, cells were treated with the Wnt activator, 6-BIO, together with TGFβ or its inhibitor SB431542 and analyzed the nuclear localization of β-catenin. The nuclear localization of β-catenin in USSCs was significantly enhanced by using either BIO (1.345 ± 0.0415; Fig. 6c, m) or the recombinant TGFβ (1.39 ± 0.03; Fig. 6e, m). The intensity was however significantly reduced following treatment with SB431542 either alone (0.102 ± 0.027; Fig. 6g, m), or together with BIO (0.74 ± 0.02; Fig. 6i, m) or TGFβ (0.8 ± 0.016; Fig. 6k, m), indicating that nuclear transfer of β-catenin requires the active Wnt and TGFβ pathways.
Spatial expression pattern of β-catenin following simultaneous activation/inhibition of Wnt and TGFβ pathways. a In USSCs treated with the neural differentiation media, the expression of β-catenin is mainly localized in cytoplasm, but weakly in the nucleus. Treatment with either BIO (c) or TGF β1 (e) however leads to a confined expression of β-catenin in the nucleus. After treatment with the neural differentiation media and SB431542 alone (g), or combined with BIO (i) or TGFβ (k), a reduction in nuclear β-catenin expression is seen. The localizations of the nuclei were determined by using DAPI (b, d, f, h, l). Quantification of the intensities of β-catenin nuclei staining (m) showed that treatment with SB alone or together with either TGFβ1 or BIO reduced the intensity of stainings significantly compared to the TGFβ1- or BIO-treated groups. Scale bar 100 μm
Discussion
In recent years, stem cell-based therapies for neural regeneration have become a special interest of many research studies. Among various sources of stem cells, the cord blood-derived unrestricted somatic stem cells (USSCs) could be considered as a proper candidate for autologous transplantation. Unraveling the mechanisms that regulate their differentiation is essential prior to clinical trials. Our present study reveals for the first time that Wnt and TGFβ pathways cooperatively regulate neural differentiation of USSCs towards the dopaminergic fate. First, we showed that each of the Wnt or TGFβ pathways had its own neural inducing potency. Treatment of USSCs with BIO as the specific activator of the Wnt pathway enhanced the nuclear localization of Nurr-1 and β catenin, also been confirmed in our previously published article (Dastjerdi et al. 2012). That 6-BIO enhances neuronal induction via β-catenin entry to the nuclei has also been shown by other researchers using other cell types. For example, Sato et al. (2004) used mouse embryonic stem cells and showed the neural inducing potency of 6-BIO (Sato et al. 2004).
We further showed that not only the Wnt pathway but also TGFβ triggered the dopaminergic differentiation of the USSC adult stem cells. Treatment with the TGFβ inhibitor SB431542 significantly reduced the nuclear localization of Nurr-1. The inducing effect of TGFβ towards the dopaminergic fate has been also evidenced by other researchers in the adult midbrain neurons (Roussa et al. 2009; Tesseur et al. 2017).
In the present study, we have also shown for the first time that the presence of both Wnt and TGFβ is required to ensure the dopaminergic differentiation of USSCs; inhibition of one reduced the differentiation potency of the other; treatment with a combined treatment of BIO and TGFβ inhibitor SB43154 or with that of TGFβ and the Wnt inhibitor Dkk1 reduced the Nurr-1 expression induced by either TGFβ or BIO. This positive cooperative interaction between TGFβ and Wnt has also been evidenced elsewhere, for example, during embryonic development, e.g., in the Spemann organizer (Nishita et al. 2000), in the non-lens ectoderm next to the retina (Grocott et al. 2011), in the embryonic stem cells (Cai et al. 2013; Song et al. 2018), and in non-neural cells (Zhang et al. 2015; Zhou et al. 2004).
On the contrary to this positive cooperation, there is evidence elsewhere suggesting that TGF beta antagonizes the Wnt pathway via interactions with other pathways (Angbohang et al. 2016; Cai et al. 2013; Falk et al. 2008). As an example, Cai et al. (2013) used the induced pluripotent stem cells (iPSCs) and showed that inhibition of TGFβ resulted in upregulation of Wnt signaling only if the BMP was constitutively inhibited. This agonistic versus antagonistic interactions between the two pathways under the same biological context could be explained by the limited available pool of Smads 2/3/4 which may alter the type of interaction between the two pathways (Nishita et al. 2000). Indeed, according to our preliminary result, the cytoplasmic level of co-immunoprecipitated Smads 2/3 and β-catenin was reduced drastically following the TGFβ treatment (data not shown), suggesting that TGFβ might have used the Smads 2/3 to transfer β-catenin to the nucleus. In other words, Smads 2/3 and β-catenin might have physically interacted with each other leading to their entry to the nucleus and as a result of the crosstalks between the two pathways. Agonizing/antagonizing the Wnt and other related pathways by TGFβ via regulation of Smads has also been evidenced by other researchers using different cell types. For example, Jian et al. (2006) showed that TGF-β1 induced a Smad3-dependent nuclear translocation of β-catenin and led to proliferation of the bone marrow-derived adult human mesenchymal stem cells (Jian et al. 2006). Calvo-Sanchez et al. (2019) showed that for growth/rest switching in hair follicle stem cells, β-catenin interacts with Smad4 in a BMP/TGFβ receptor-dependent context and both proteins act synergistically to activate TGFβ receptor (Endoglin) promoter transcription (Calvo-Sanchez et al. 2019). Also, Zhang et al. (2019) showed that Wnt/β-catenin signaling activation under the ischemic injury may be related to the TGFβ/Smad3 signaling pathway (Zhang et al. 2019). Chawla and Ghosh (2018) showed that TGFβ/Smad and Wnt/β-catenin signaling pathways are involved in differentiation of dermal fibroblasts to myofibroblasts during the scar formation (Chawla and Ghosh 2018). All these evidence highlight the interaction between β-catenin and Smads during the stem cell differentiation, specifically in our case the adult neural stem cells.
In summary, our study points at the positive interaction between the TGFβ and Wnt pathways to ensure the dopaminergic differentiation of USSCs. Thus, inhibition of one attenuates the inducing effect of the other, and the presence of both is required to fulfill the adult stem cell dopaminergic differentiation.
References
Andersson ER et al (2013) Wnt5a cooperates with canonical Wnts to generate midbrain dopaminergic neurons in vivo and in stem cells. Proc Natl Acad Sci U S A 110:E602–E610. https://doi.org/10.1073/pnas.1208524110
Angbohang A et al (2016) Downregulation of the canonical WNT signaling pathway by TGFbeta1 inhibits photoreceptor differentiation of adult human Muller glia with stem cell characteristics stem cells and development. Stem Cells Dev 25:1–12. https://doi.org/10.1089/scd.2015.0262
Cai J, Schleidt S, Pelta-Heller J, Hutchings D, Cannarsa G, Iacovitti L (2013) BMP and TGF-beta pathway mediators are critical upstream regulators of Wnt signaling during midbrain dopamine differentiation in human pluripotent stem cells. Dev Biol 376:62–73. https://doi.org/10.1016/j.ydbio.2013.01.012
Calvo-Sanchez MI, Fernandez-Martos S, Carrasco E, Moreno-Bueno G, Bernabeu C, Quintanilla M, Espada J (2019) A role for the Tgf-beta/Bmp co-receptor Endoglin in the molecular oscillator that regulates the hair follicle cycle. J Mol Cell Biol 11:39–52. https://doi.org/10.1093/jmcb/mjy051
Castelo-Branco G et al. (2003) Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a Proc Natl Acad Sci U S A 100:12747-12752 doi:https://doi.org/10.1073/pnas.1534900100
Chawla S, Ghosh S (2018) Regulation of fibrotic changes by the synergistic effects of cytokines, dimensionality and matrix: towards the development of an in vitro human dermal hypertrophic scar model. Acta Biomater 69:131–145. https://doi.org/10.1016/j.actbio.2018.01.002
Dastjerdi FV et al (2012) Inhibition of GSK-3beta enhances neural differentiation in unrestricted somatic stem cells. Cell Biol Int 36:967–972. https://doi.org/10.1042/cbi20110541
Dun Y et al (2012) Inhibition of the canonical Wnt pathway by Dickkopf-1 contributes to the neurodegeneration in 6-OHDA-lesioned rats. Neurosci Lett 525:83–88. https://doi.org/10.1016/j.neulet.2012.07.030
Falk S, Wurdak H, Ittner LM, Ille F, Sumara G, Schmid MT, Draganova K, Lang KS, Paratore C, Leveen P, Suter U, Karlsson S, Born W, Ricci R, Götz M, Sommer L (2008) Brain area-specific effect of TGF-β signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell 2:472–483. https://doi.org/10.1016/j.stem.2008.03.006
Fujimori K, Matsumoto T, Kisa F, Hattori N, Okano H, Akamatsu W (2017) Escape from pluripotency via inhibition of TGF-β/BMP and activation of Wnt signaling accelerates differentiation and aging in hPSC progeny cells Stem Cell Reports 9:1675–1691 doi:https://doi.org/10.1016/j.stemcr.2017.09.024
Gadue P, Huber TL, Paddison PJ, Keller GM (2006) Wnt and TGF-β signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci 103:16806–16811. https://doi.org/10.1073/pnas.0603916103
Galli S, Lopes DM, Ammari R, Kopra J, Millar SE, Gibb A, Salinas PC (2014) Deficient Wnt signalling triggers striatal synaptic degeneration and impaired motor behaviour in adult mice. Nat Commun 5:4992. https://doi.org/10.1038/ncomms5992
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357–362. https://doi.org/10.1038/34848
Gollamudi S, Johri A, Calingasan NY, Yang L, Elemento O, Beal MF (2012) Concordant signaling pathways produced by pesticide exposure in mice correspond to pathways identified in human Parkinson’s disease. PLoS One 7:e36191. https://doi.org/10.1371/journal.pone.0036191
Grocott T, Johnson S, Bailey AP, Streit A (2011) Neural crest cells organize the eye via TGF-beta and canonical Wnt signalling. Nat Commun 2:265. https://doi.org/10.1038/ncomms1269
Hegarty SV, Sullivan AM, O'Keeffe GW (2014) Roles for the TGFbeta superfamily in the development and survival of midbrain dopaminergic neurons. Mol Neurobiol 50:559–573. https://doi.org/10.1007/s12035-014-8639-3
Inman GJ et al (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65–74. https://doi.org/10.1124/mol.62.1.65
Jian H, Shen X, Liu I, Semenov M, He X, Wang XF (2006) Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev 20:666–674. https://doi.org/10.1101/gad.1388806
Kashima R, Hata A (2017) The role of TGF-β superfamily signaling in neurological disorders. Acta Biochim Biophys Sin 50:106–120. https://doi.org/10.1093/abbs/gmx124
Khanghahi AM, Zeynali B, Akhlaghpoor A, Tafreshi AP, Krieglstein K (2014) Activation of TGFbeta1 signaling enhances early dopaminergic differentiation in unrestricted somatic stem cells. Neurosci Lett 583:60–64. https://doi.org/10.1016/j.neulet.2014.08.055
Kogler G et al (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200:123–135. https://doi.org/10.1084/jem.20040440
Li T-F et al (2006) Transforming growth factor-beta stimulates cyclin D1 expression through activation of beta-catenin signaling in chondrocytes. J Biol Chem 281:21296–21304. https://doi.org/10.1074/jbc.M600514200
Massey J, Liu Y, Alvarenga O, Saez T, Schmerer M, Warmflash A (2018) WNT ligands stimulate transient signaling in human pluripotent cells and synergize with TGF-β ligands to stimulate sustained signaling during differentiation. bioRxiv. https://doi.org/10.1101/406306
Massey J, Liu Y, Alvarenga O, Saez T, Schmerer M, Warmflash A (2019) Synergy with TGFbeta ligands switches WNT pathway dynamics from transient to sustained during human pluripotent cell differentiation. Proc Natl Acad Sci U S A 116:4989–4998. https://doi.org/10.1073/pnas.1815363116
Meijer L et al (2003) GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol 10:1255–1266
Meyers EA, Kessler JA (2017) TGF-beta family signaling in neural and neuronal differentiation, Development, and Function. Cold Spring Harb Perspect Biol 9. https://doi.org/10.1101/cshperspect.a022244
Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW (2000) Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann’s organizer. Nature 403:781–785. https://doi.org/10.1038/35001602
Prakash N, Wurst W (2006) Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci 63:187–206. https://doi.org/10.1007/s00018-005-5387-6
Ribeiro D et al (2011) Dkk1 regulates ventral midbrain dopaminergic differentiation and morphogenesis. PLoS One 6:e15786. https://doi.org/10.1371/journal.pone.0015786
Roussa E, von Bohlen Und Halbach O, Krieglstein K (2009) TGF-beta in dopamine neuron development, maintenance and neuroprotection. Adv Exp Med Biol 651:81–90
Roussa E, Wiehle M, Dunker N, Becker-Katins S, Oehlke O, Krieglstein K (2006) Transforming growth factor beta is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: ectopic induction in dorsal mesencephalon. Stem Cells (Dayton, Ohio) 24:2120–2129. https://doi.org/10.1634/stemcells.2005-0514
Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10:55–63. https://doi.org/10.1038/nm979
Saucedo-Cardenas O et al (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A 95:4013–4018. https://doi.org/10.1073/pnas.95.7.4013
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 13:685–700
Song Y, Lee S, Jho EH (2018) Enhancement of neuronal differentiation by using small molecules modulating Nodal/Smad, Wnt/beta-catenin, and FGF signaling. Biochem Biophys Res Commun 503:352–358. https://doi.org/10.1016/j.bbrc.2018.06.033
Stephano F, Nolte S, Hoffmann J, el-Kholy S, von Frieling J, Bruchhaus I, Fink C, Roeder T (2018) Impaired Wnt signaling in dopamine containing neurons is associated with pathogenesis in a rotenone triggered Drosophila Parkinson’s disease model. Sci Rep 8:2372. https://doi.org/10.1038/s41598-018-20836-w
Tesseur I, Nguyen A, Chang B, Li L, Woodling NS (2017) Deficiency in neuronal TGF-beta signaling leads to nigrostriatal degeneration and activation of TGF-beta signaling protects against MPTP neurotoxicity in mice. J Neurosci 37:4584–4592. https://doi.org/10.1523/jneurosci.2952-16.2017
Zhang F, Ren T, Wu J, Niu J (2015) Small concentrations of TGF-beta1 promote proliferation of bone marrow-derived mesenchymal stem cells via activation of Wnt/beta-catenin pathway. Indian J Exp Biol 53:508–513
Zhang G et al (2019) Wnt/beta-catenin signaling pathway contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury and is possibly related to the transforming growth factorbeta1/Smad3 signaling pathway. Biomed Pharmacother 110:420–430. https://doi.org/10.1016/j.biopha.2018.11.143
Zhou S, Eid K, Glowacki J (2004) Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res 19:463–470. https://doi.org/10.1359/JBMR.0301239
Funding
This study was supported by Centre for International Scientific Studies and Collaboration (CISSC) under the International cooperative Research Program (ICRP; code: 1917), University of Tehran (6104040/6/028), and the National Institute of Genetic Engineering and Biotechnology (Nigeb).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All studies were accomplished in accordance with the guidelines provided by “Tehran University of Medical Sciences Research Ethics Committee.”
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akhlaghpour, A., Parvaneh Tafreshi, A., Roussa, E. et al. TGFβ and Wnt Signaling Pathways Cooperatively Enhance Early Dopaminergic Differentiation of the Unrestricted Somatic Stem Cells. J Mol Neurosci 70, 769–777 (2020). https://doi.org/10.1007/s12031-020-01487-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01487-x