Abstract
Alzheimer’s disease (AD) is the most common cause of dementia and is characterized by the presence of β-amyloid (Aβ) plaques and defective autophagy in the brain, which is believed to cause neuronal dysfunction. By using APP/PS1 transgenic AD mice, we investigated the influence of orientin (Ori) on cognitive function and its underlying mechanisms in AD models. Our data indicated that Ori improved spatial learning and memory in APP/PS1 mice, possibly through decreasing brain Aβ deposition and attenuating autophagy impairment. Ori decreased the LC3-II/I ratio, p62 and cathepsin D (Ctsd) protein levels and the number of autolysosomes, whereas the protein levels of Ulk1 and Beclin-1 were no different between the control and treatment groups, indicating increased autolysosome clearance and thus a decreased Aβ burden in the brain. Our results showed that Ori could enhance autolysosome clearance, decrease brain Aβ deposition and improve learning and memory in AD mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD), one of the greatest global public health care challenges, is the most common form of dementia and clinically manifests as progressive cognitive and behavioral impairment. The prevalence of AD approximately doubles every 5 years after age 65, and the socioeconomic burden of AD is huge, especially with its increasing prevalence (Lane et al. 2018). Although remarkable advances have been made in the understanding of AD pathogenesis, curative treatments are still not available. AD is characterized by the accumulation of deposited Aβ and neurofibrillary tangles in the brain. The pathogenesis of AD is complicated and involves multiple factors, such as lifestyle, vascular risk factors, and genetic susceptibility. Aβ is a critical factor during the progression and pathogenesis of AD (Karran et al. 2011). Mutations in the amyloid precursor protein (APP), presenilin 1 (PSEN1), presenilin 2 (PSEN2), and APOE genes leads to elevated Aβ build-up, deposition, and spread and eventually contributes to familial or sporadic forms of AD (Karch and Goate 2015; Schmechel et al. 1993).
The pathogenic proteins responsible for Aβ and tau accumulation in AD indicate the emergence of disease-related deficits in eliminating damaged proteins (Menzies et al. 2017). The clearance of Aβ and tau is closely associated with autophagy. Autophagy is a major cellular self-digestion pathway that delivers long-lived proteins, aggregate-prone proteins and damaged organelles to lysosomes for degradation (Mizushima and Komatsu 2011). Accumulated autophagosomes and high levels of lysosomal hydrolases have been found in the brains of AD patients and animal models, indicating impairment of the autophagosomal-lysosomal pathway (Bordi et al. 2016; Nixon et al. 2005). Autophagic clearance defects lead to neuronal impairment, Aβ accumulation, neuron death, and AD development (Boland et al. 2008). Attenuating autophagy dysfunction could increase the clearance of aggregation-prone neurotoxic protein and alleviate the pathological changes in AD, thus serving as an effective therapeutic strategy (Boland et al. 2018; Menzies et al. 2017). Many autophagic regulators were shown to be beneficial in both AD cells and animal model (Deng and Mi 2016; Du et al. 2013; Huang et al. 2017; Lee et al. 2014; Liu et al. 2013; Son et al. 2016; Yang et al. 2011; Zhang and Zhao 2015). Some agents, such as resveratrol and curcumin, are undergoing clinical evaluation in AD patients, although their unequivocal therapeutic efficacies need confirmation through long-term study (Boland et al. 2018).
Ori is a flavonoid compound extracted from some natural plants, such as Trollius chinensis and bamboo leaves. Ori has a variety of pharmacological activities, including the promotion of autophagy (Liu et al. 2016) and anti-inflammatory (Wang et al. 2017), anti-oxidant (Xiao et al. 2018), and neuroprotective (Liu et al. 2015; Tian et al. 2018; Wang et al. 2016) effects, and might be an underlying multitarget AD drug because of these properties, which are closely associated with AD pathogenesis. A recent study showed that Ori could attenuate cognitive impairment in Aβ-induced AD mice via decreasing the level of oxidants (Yu et al. 2015). It is uncertain whether Ori affects cognitive function, Aβ load and autophagy in transgenic AD mice. In this study, we investigated the effects of Ori on autophagy and β-amyloid in the brains of APP/PS1 transgenic mice.
Materials and Methods
Animals
This study is performed consentaneously with the recommendations of Animal Care Committee of Jinzhou Medical University and the protocol was approved by Animal Care committee of Jinzhou Medical University. Seven-month-old female APP/PS1 transgenic C57BL/6J mice and age-matched wild-type C57BL/6J mice were obtained from the Institute of Laboratory Animal Science, China Medical University. APP/PS1 transgenic mice harboring AD-mutated human APPswe and PS1ΔE9 transgenes have been shown to simulate the pathological features of AD patients (Gotz et al. 2018). All animals were housed with a reversed 12:12 h light/dark cycle and food/water available ad libitum. The mice were randomly assigned into three groups (n = 8 in each group): nontransgenic mice (NT), APP/PS1 transgenic mice (Tg) treated with saline, and APP/PS1 transgenic mice treated with orientin (Tg + Ori). The NT and Tg groups were injected intraperitoneally with 0.5 ml saline daily. The Tg + Ori group was treated intraperitoneally with 10 mg/kg orientin in 0.5 ml saline daily. All groups were injected for 30 days.
Reagents
Orientin (purity > 99%) was purchased from Chengdu Must (Chengdu, China). Rabbit anti-LC3, anti-Beclin-1, and mouse anti-LAMP2 antibody were from Proteintech (Wuhan, China). Rabbit anti-p62, anti-Ulk1 and anti-Ctsd antibody were from Wanleibio (Shenyang, China). Mouse anti-Aβ antibody was from CST (MA, USA).
Morris Water Maze Behavior Test
The spatial learning and memories of the mice were assessed by the Morris water maze test. The maze consisted of a circular pool (diameter of 125 cm) filled with water (20–22 °C) and a digital camera tracker system. A vitreous cylindrical platform (diameter of 7 cm) was placed in the center of quadrant 1. During the acquisition training phase (day 1 to day 8), each mouse carried out 4 × 120 s trials per day. The mice were released into the pool at different quadrants in each 120 s trial. If the mouse could not find the platform in 120 s, they were guided to it. Mice were allowed to sit on the platform for 15 s to enhance their memory after reaching the platform. The time from when the mice got into the water to when they found and sat on the platform was recorded as escape latency. On day 1 to day 3, the platform was set 1 cm above the surface of the water, and it was set 1 cm below the surface of the water on day 4 to day 8. Probe trials in which the platform was removed were administered 24 h after the training trials, and every mouse was given one 120-s trial to swim in the pool. The previous platform location was recognized as the target zone.
Western Blotting
Total protein lysates were extracted from mouse hippocampi in lysis buffer. Then, 30 μg of protein was separated with SDS-PAGE gels and transferred onto PVDF membranes. The membranes were blocked in 5% bovine serum albumin (BSA) and then incubated overnight at 4 °C with the following primary antibodies: anti-LC3, anti-p62, anti-Beclin-1, anti-Ctsd, and anti-Ulk1. After being washed with TBST three times, the membranes were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. The membranes were washed three times again before chemiluminescent development with ECL substrate. Images were captured with a BioSpectrum imaging system. The immunoblot bands were analyzed with ImageJ software.
Immunohistochemical Staining
Animals were perfused transcardially with normal saline (0.9% NaCl), and their brains were excised. One hemisphere was fixed in 4% paraformaldehyde and then embedded in paraffin, and the coronal sections were cut with a microtome. The other hemisphere was microdissected to separate the hippocampus and frozen in a − 80 °C refrigerator. Brain sections were dewaxed with xylene, dehydrated with an ethanol gradient, and then incubated with boiled 0.1 mmol/L sodium citrate for antigen retrieval. The sections were incubated in H2O2, permeabilized with Triton X and then blocked with normal serum prior to incubation with anti-Aβ (1:200), anti-LC3 (1:200), and anti-LAMP2 (1:100) antibodies at 4 °C overnight. The primary antibody-labeled sections were then incubated with biotin-labeled secondary antibody, and following stained with a DAB staining kit, appropriate Alexa Fluor 488- or Alexa Fluor 594-labeled secondary antibodies. Nuclei were counterstained with hematoxylin or DAPI and imaged with an epifluorescence microscope. Staining was quantified using ImageJ software.
Statistical Analysis
Data were analyzed using SPSS 16.0. Comparisons among groups were performed using one-way ANOVA followed by Tukey’s HSD or Student-Newman-Keuls post hoc test. Linear relationships between two variables were analyzed by Pearson’s correlation analysis. The results are expressed as the mean ± SD. Values of P < 0.05 indicated statistical significance.
Results
Orientin Treatment Improves Cognition in AD Mice
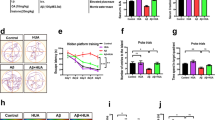
The Morris water maze test was conducted to assess spatial learning and memory. Escape latency apparently decreased over time among the three groups during the training trials. The escape latency of the NT group was significantly lower than that of the Tg group, and Ori treatment reduced the escape latency of the Tg mice (Fig. 1a). In addition, there were no significant differences in the average swimming speeds of the three groups (Fig. 1b), and the platform was removed to test spatial reference memory 24 h after the last training trials. Spatial reference memory was assessed by the number of times a mouse entered the target zone and the time the mouse spent in it. The number of times the mice entered the target zone and the time they spent in it were significantly greater in the NT and Tg + Ori groups than in the Tg group (Fig. 1c and d). Together, these data suggest that Ori treatment significantly improved spatial learning and memory in APP/PS1 mice.
Orientin treatment improves cognition in APP/PS1 mice. a Spatial learning and memory were assessed by the measurement of escape latency. b Average swimming speed was assessed. c The number of times mice entered the target zone in the probe trail. d The time mice spent in the target zone during the 120 s probe trail. e Representative swimming paths at day 8 of training. n = 8, #P < 0.05, ##P < 0.01 vs NT; *P < 0.05, **P < 0.01 vs Tg
Effects of Orientin on the Autophagy-Lysosomal Signaling Pathway in the Hippocampi of AD Mice
Autophagy is involved in AD development. To evaluate the level of autophagy in AD mice, we examined the autophagy-related proteins LC3, p62, Beclin-1, Ulk1, and Ctsd by western blotting (Fig. 2a–d). Tg mice exhibited elevated LC3-II and LC3-II/I ratios and p62 and Ctsd levels compared with those in the NT group. Ori treatment significantly decreased the ratio of LC3-II/I and the levels of LC3-II, p62, and Ctsd in APP/PS1 mice compared to those in untreated mice. The levels of Beclin-1 and Ulk1 were not significantly different between any of the groups. Immunofluorescent staining (Fig. 2e–g) showed an increased Pearson’s correlation coefficient for LC3 and LAMP2 (which are associated with the autophagosome and lysosome, respectively) and elevated levels of double-positive vesicles (LC3-LAMP2 double-positive vesicles are associated with autolysosome.) in APP/PS1 mice compared to those in control mice. Ori treatment reduced the levels of LC3-II, p62, and Ctsd; the Pearson’s correlation coefficient; and the LC3-LAMP2-positive vesicle area in APP/PS1 mice. Together, these results showed the accumulation of autolysosomes in APP/PS1 mice and that Ori treatment decreased the accumulation of autolysosomes.
Effects of orientin on the autophagy-lysosomal signaling pathway in the hippocampi of AD mice. a-d Protein expression and quantification of LC3, p62, Beclin-1, Ulk1, and Ctsd. e Immunofluorescent staining for LC3 (red) and LAMP2 (green) in CA1 hippocampal neurons (× 400). f Pearson’s correlation coefficient for LC3 with LAMP2. g Quantitative analysis of LC3 and LAMP2 double-positive vesicles (autolysosomes). n = 4–6, #P < 0.05, ##P < 0.01 vs NT; *P < 0.05, **P < 0.001 vs Tg
Orientin Treatment Reduces the Amyloid Plaques in AD Mice
We asked whether Ori could help degrade Aβ and alleviate its deposition in the brain. The expression of brain amyloid plaques was measured by immunohistochemical staining and semiquantified by determining the percentages of sections positively labeled by Aβ antibody. Aβ immunopositivity was almost absent in the cortices and hippocampi of mice in the NT group and significantly increased in those in the Tg group (Fig. 3). After 30 days of Ori treatment, the Aβ burden in the cortex and hippocampus was significantly decreased. These data suggested that Ori treatment effectively decreased the Aβ load in the cortices and hippocampi of APP/PS1 mice.
Discussion
In this study, we demonstrated that Ori treatment enhanced autophagic clearance and decreased the abnormal accumulation of autolysosomes to ameliorate autophagy dysfunction, leading to decreased brain Aβ deposition and improved learning and memory in APP/PS1 transgenic mice.
Autophagy is a key cellular self-digestion pathway that is responsible for the systematic degradation and recycling of harmful components, such as long-lived proteins, protein aggregates, and impaired organelles (Mizushima and Komatsu 2011). Healthy mammalian cells show a low basal level of highly efficient autophagy, which is essential for neuronal homeostasis (Boland et al. 2008). Autophagy is a dynamic process involving induction, autophagosome formation, the fusion of autophagosomes with lysosomes to form autolysosomes, and final digestion by proteases to generate amino acids and energy. Mounting evidence indicates that autophagy dysfunction is closely related to neurodegenerative diseases that show abnormally aggregated proteins, such as AD (Nixon et al. 2005). The accumulation of abnormal autolysosomes has been observed in AD patients and AD models (Boland et al. 2008; Bordi et al. 2016). Furthermore, the impaired autophagy pathway is increasingly regarded as an important contributor to the pathogenesis of AD. Therefore, positively regulating autophagy is a strategy for AD treatment (Boland et al. 2018).
Autophagy flux is controlled by the balance between autophagosome formation and autophagic degradation. In the healthy brain, autophagy is constitutively active, and the clearance of the autophagosomal-lysosomal pathway is highly efficient to prevent autophagic substrate accumulation (Boland et al. 2008). LC3 is a biomarker for autophagic structures. During autophagosome formation, the soluble cytoplasmic form of LC3-I is converted to LC3-II, and the latter is mainly associated with autophagic membranes and essential for cargo gathering (Karim et al. 2007). The amount of LC3-II and the ratio of LC3-II/I indicate changes in autophagy activity (Karim et al. 2007). p62 is a ubiquitin protein that serves as a bridge in autophagic protein gathering by connecting LC3-II and autophagic substrates (Bjorkoy et al. 2005). LC3-II and p62 are degraded by hydrolase at a later step in autolysosomes. In the current study, Tg mice showed an elevated ratio of LC3-II/I and the increased expression of LC3-II and p62 compared with their expression in the control group, indicating increased autophagy flux, or a block in fusion or degradation in AD animals, which is consistent with previous research (Bordi et al. 2016; Lee et al. 2014; Zhang and Zhao 2015). We next examined the level of Beclin-1, which is a component of a kinase complex responsible for autophagy induction that indicates the formation of autophagosomes (Cao and Klionsky 2007). Pickford et al. (2008) showed that Beclin-1 protein levels are decreased in AD patients; however, some other studies indicated that Beclin-1 is unchanged in AD animals and patients (Bordi et al. 2016; Lee et al. 2014; Zhang and Zhao 2015). This difference may be due to the use of different models and the procession of AD. In this study, the protein expression of Ulk1 and Beclin-1 was no different among the groups, indicating that autophagy induction and autophagosome formation were not increased in APP/PS1 transgenic mice. Thus, we evaluated autophagic degradation by measuring the expression of Ctsd, which is an aspartyl lysosomal protease and essential to autophagy substrate degradation (Vidoni et al. 2016). Abnormally elevated levels of Ctsd were reported in the hippocampi of AD patients and AD animals (Bordi et al. 2016; Lee et al. 2014; Zhang and Zhao 2015). In this study, the level of the mature form of Ctsd (approximately 31–33 kDa) (Vidoni et al. 2016) was increased in APP/PS1 mice, indicating an elevated number of lysosomes or autolysosomes. Shin et al. showed the accumulation of autophagosomes in the brains of AD animals, which was due to impaired fusion with lysosomes; thus, enhancing the fusion of autophagosomes with lysosomes could promote autophagosome clearance (Shin et al. 2014). However, others demonstrated that impaired lysosomal function impeded autolysosome clearance and led to autolysosome accumulation in AD models and patients (Bordi et al. 2016; Lee et al. 2010; Wolfe et al. 2013). In this study, immunofluorescence staining showed the accumulation of autolysosomes in AD mice. Thus, the elevated expression of LC3, p62, and Ctsd is derived from a block in the degradation of autolysosomes in APP/PS1 mice, rather than through increased autophagy flux or a block in the fusion between autophagosomes and lysosomes. AD animals showed obstructed autolysosome clearance that thus led to an accumulation of autolysosomes (Bordi et al. 2016; Lee et al. 2010; Wolfe et al. 2013). In this study, Ori treatment reduced the protein expression of LC3, p62, and Ctsd and autolysosome levels. Our results indicate that Ori treatment decreased autophagic accumulation through promoting autolysosome clearance.
Aβ deposition is one of the main pathological markers of AD (Karran et al. 2011). Aβ impairs the autophagic lysosomal pathway, decreases autophagic degradation and leads to the accumulation of autophagic vesicles (Silva et al. 2011). Meanwhile, impaired autophagic flux exacerbates Aβ production and secretion (Nilsson and Saido 2014; Yu et al. 2005), which involves a vicious cycle between Aβ generation and autophagic defects. Our results showed a great number of Aβ plaques in the cortices and hippocampi of APP/PS1 mice. After Ori treatment, the Aβ burden in the cortex and hippocampus was significantly decreased. Our data suggest that Ori could increase Aβ degradation by promoting autophagic clearance.
However, there are also some shortcomings in this study. First, multiple doses of Ori were not used, and the dose-dependent response to Ori has not been explored because transgenic mice are not easily available. In addition, the multiple pharmacological effects of Ori have not been explored. Ori exhibits anti-oxidant, anti-aging, anti-inflammatory, and antidiabetic properties. These medicinal properties closely correspond to AD pathological changes and might be responsible for the results of this study. In summary, our results demonstrated that Ori exhibited neuroprotective effects through enhancing autolysosome clearance, decreasing brain Aβ deposition, and improving learning and memory in APP/PS1 transgenic mice. Therefore, Ori might be a promising candidate agent for the treatment of AD.
References
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, … Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol, 171(4):603–614. doi: https://doi.org/10.1083/jcb.200507002
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci 28(27):6926–6937. https://doi.org/10.1523/JNEUROSCI.0800-08.2008
Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, … Millan MJ (2018) Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov, 17(9):660-688. doi:https://doi.org/10.1038/nrd.2018.109
Bordi M, Berg MJ, Mohan PS, Peterhoff CM, Alldred MJ, Che S, … Nixon RA (2016) Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12(12):2467–2483. doi:https://doi.org/10.1080/15548627.2016.1239003
Cao Y, Klionsky DJ (2007) Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 17(10):839–849. https://doi.org/10.1038/cr.2007.78
Deng H, Mi MT (2016) Resveratrol attenuates Abeta25-35 caused neurotoxicity by inducing autophagy through the TyrRS-PARP1-SIRT1 signaling pathway. Neurochem Res 41(9):2367–2379. https://doi.org/10.1007/s11064-016-1950-9
Du J, Liang Y, Xu F, Sun B, Wang Z (2013) Trehalose rescues Alzheimer’s disease phenotypes in APP/PS1 transgenic mice. J Pharm Pharmacol 65(12):1753–1756. https://doi.org/10.1111/jphp.12108
Gotz J, Bodea LG, Goedert M (2018) Rodent models for Alzheimer disease. Nat Rev Neurosci 19(10):583–598. https://doi.org/10.1038/s41583-018-0054-8
Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y (2017) Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of beta-amyloid in APP/tau/PS1 mouse model of Alzheimer's disease. Exp Gerontol 91:25–33. https://doi.org/10.1016/j.exger.2017.02.004
Karch CM, Goate AM (2015) Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77(1):43–51. https://doi.org/10.1016/j.biopsych.2014.05.006
Karim MR, Kanazawa T, Daigaku Y, Fujimura S, Miotto G, Kadowaki M (2007) Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy 3(6):553–560
Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10(9):698–712. https://doi.org/10.1038/nrd3505
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25(1):59–70. https://doi.org/10.1111/ene.13439
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, … Nixon RA (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell, 141(7):1146–1158. doi: https://doi.org/10.1016/j.cell.2010.05.008
Lee JK, Jin HK, Park MH, Kim BR, Lee PH, Nakauchi H, … Bae JS (2014) Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer’s disease. J Exp Med 211(8):1551–1570. doi:https://doi.org/10.1084/jem.20132451
Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, … Mattson MP (2013) Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging 34(6):1564–1580. doi: https://doi.org/10.1016/j.neurobiolaging.2012.11.020
Liu Y, Lan N, Ren J, Wu Y, Wang ST, Huang XF, Yu Y (2015) Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol Nutr Food Res 59(6):1130–1142. https://doi.org/10.1002/mnfr.201400753
Liu L, Wu Y, Huang X (2016) Orientin protects myocardial cells against hypoxia-reoxygenation injury through induction of autophagy. Eur J Pharmacol 776:90–98. https://doi.org/10.1016/j.ejphar.2016.02.037
Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, … Rubinsztein DC (2017) Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93(5):1015–1034. doi:https://doi.org/10.1016/j.neuron.2017.01.022
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. https://doi.org/10.1016/j.cell.2011.10.026
Nilsson P, Saido TC (2014) Dual roles for autophagy: degradation and secretion of Alzheimer’s disease Abeta peptide. Bioessays 36(6):570–578. https://doi.org/10.1002/bies.201400002
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64(2):113–122
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, … Wyss-Coray T (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest 118(6):2190–2199. doi:https://doi.org/10.1172/JCI33585
Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, … Roses AD (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 90(20):9649–9653
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, Lee PH (2014) Mesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease models. Autophagy 10(1):32–44. https://doi.org/10.4161/auto.26508
Silva DF, Esteves AR, Arduino DM, Oliveira CR, Cardoso SM (2011) Amyloid-beta-induced mitochondrial dysfunction impairs the autophagic lysosomal pathway in a tubulin dependent pathway. J Alzheimers Dis 26(3):565–581. https://doi.org/10.3233/JAD-2011-110423
Son SM, Shin HJ, Byun J, Kook SY, Moon M, Chang YJ, Mook-Jung I (2016) Metformin facilitates amyloid-beta generation by beta- and gamma-secretases via autophagy activation. J Alzheimers Dis 51(4):1197–1208. https://doi.org/10.3233/jad-151200
Tian T, Zeng J, Zhao G, Zhao W, Gao S, Liu L (2018) Neuroprotective effects of orientin on oxygen-glucose deprivation/reperfusion-induced cell injury in primary culture of rat cortical neurons. Exp Biol Med (Maywood) 243(1):78–86. https://doi.org/10.1177/1535370217737983
Vidoni C, Follo C, Savino M, Melone MA, Isidoro C (2016) The role of Cathepsin D in the pathogenesis of human neurodegenerative disorders. Med Res Rev 36(5):845–870. https://doi.org/10.1002/med.21394
Wang S, Yu Y, Feng Y, Zou F, Zhang X, Huang J, … Liu Y (2016) Protective effect of the orientin on noise-induced cognitive impairments in mice. Behav Brain Res 296:290–300. doi:https://doi.org/10.1016/j.bbr.2015.09.024
Wang X, An F, Wang S, An Z, Wang S (2017) Orientin attenuates cerebral ischemia/reperfusion injury in rat model through the AQP-4 and TLR4/NF-kappaB/TNF-alpha signaling pathway. J Stroke Cerebrovasc Dis 26(10):2199–2214. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.05.002
Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA (2013) Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci 37(12):1949–1961. https://doi.org/10.1111/ejn.12169
Xiao Q, Piao R, Wang H, Li C, Song L (2018) Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int J Biol Macromol 118(Pt A):747–755. https://doi.org/10.1016/j.ijbiomac.2018.06.130
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, … Nixon RA (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain 134(Pt 1):258–277. doi:https://doi.org/10.1093/brain/awq341
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, … Nixon RA (2005) Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol, 171(1):87–98. doi:https://doi.org/10.1083/jcb.200505082
Yu L, Wang S, Chen X, Yang H, Li X, Xu Y, Zhu X (2015) Orientin alleviates cognitive deficits and oxidative stress in Abeta1-42-induced mouse model of Alzheimer’s disease. Life Sci 121:104–109. https://doi.org/10.1016/j.lfs.2014.11.021
Zhang YD. Zhao JJ (2015) TFEB participates in the Abeta-induced pathogenesis of Alzheimer’s disease by regulating the autophagy-lysosome pathway. DNA Cell Biol, 34(11):661–668. doi: https://doi.org/10.1089/dna.2014.2738
Funding
This research was supported by grants from the Nature Science Foundation of Liaoning Province (No. 201601357).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study is performed consentaneously with the recommendations of Animal Care Committee of Jinzhou Medical University and the protocol was approved by Animal Care committee of Jinzhou Medical University.
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, Y., Zheng, Qy., Sun, Cy. et al. Orientin Improves Cognition by Enhancing Autophagosome Clearance in an Alzheimer’s Mouse Model. J Mol Neurosci 69, 246–253 (2019). https://doi.org/10.1007/s12031-019-01353-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01353-5