Abstract
It has been reported that myeloid-related protein 8/14 (MRP8/14) participates in the progression of inflammation after release from neutrophils and monocytes. This study aimed to clarify the mechanism(s) of the MRP8/14-augmented inflammatory response in mice with pneumococcal meningitis. Streptococcus pneumoniae (SP) meningitis was established by intracerebral injection of SP suspension. Balb/c mice were randomly divided into four groups and received the following injections: phosphate-buffer saline (PBS), MRP8/14 alone, SP alone, and SP plus MRP8/14. At 6 h, 24 h and 48 h postinfection, the clinical disease status was measured by the modified neurological severity score test, body weight loss and degree of cerebral edema; mice were anaesthetized, blood samples and brain samples were collected and brain inflammation was detected by haematoxylin and eosin (HE) staining; tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP) and monocyte chemoattractant protein-1 (MCP-1) levels in serum and brain homogenates were assessed by an enzyme-linked immunosorbent assay (ELISA), and the mRNA levels of the above cytokines in brain homogenates were measured by polymerase chain reaction (PCR); and the expression of nuclear factor-kappa B (NF-κB) p65 in brain tissues was determined by immunohistochemical assay. In this study, we identified that MRP8/14 substantially augmented the SP-stimulated inflammatory response, aggravated clinical disease status and exacerbated SP-induced brain edema in a murine model of pneumococcal meningitis. Exogenous administration of MRP8/14 significantly enhanced mRNA and protein expression of the proinflammatory cytokines and chemokines TNF-α, CRP, IL-6 and MCP-1 in brain homogenates and serum from mice with pneumococcal meningitis, which may be related to the NF-κB signalling pathway. We further found that MRP8/14 strongly augmented SP-induced phosphorylation of NF-κB p65 in brain tissue slices from the same model. In conclusion, our results indicated that MRP8/14 augmented the inflammatory response in mice with pneumococcal meningitis and contributed to the development of disease, which was probably through NF-κB signalling pathway activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae (S. pneumoniae, SP) is one of the main and most common pathogenic bacteria in human bacterial meningitis. Bacterial meningitis is a serious infectious disease affecting the central nervous system of children, and SP meningitis cases account for more than half of the cases of bacterial meningitis (Hoffmann et al. 2007; Brouwer et al. 2010). In recent years with the use of effective antibiotics, mortality of bacterial meningitis still remains high; nearly half of the survivors have cognitive dysfunction, visual damage, hearing damage, epilepsy and other neurological sequelae (Auburtin et al. 2006; Hoffmann et al. 2007; Barichello et al. 2010a; Thigpen et al. 2011). The adverse outcomes of SP meningitis are mainly associated with the occurrence of encephalic complications including brain oedema and cerebral vascular injury (Kastenbauer and Pfister 2003). The main pathogenic factors of SP are the SP capsule and pneumolysin (PLY), as the capsule can resist phagocyte phagocytosis and the PLY can trigger the inflammatory response, apoptosis, etc. (Hoffmann et al. 2007). After infection, the pathogen-associated molecular patterns (PAMPs) combine with the pattern recognition receptors (PRRs), such as toll-like receptor (TLR) 2, to mediate activation of nuclear factor-kappa B (NF-κB), producing a large number of proinflammatory cytokines and chemokines, which induce excessive immune damage (Yamamoto et al. 2003; Foell et al. 2007a). Mounting evidence has shown that proinflammatory cytokines including tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP) and monocyte chemoattractant protein-1 (MCP-1) play significant roles in the process of immune inflammation in bacterial meningitis (Malipiero et al. 2006; Barichello et al. 2010b).
Myeloid-related protein 8/14 (MRP8/14), a member of the calcium-binding protein superfamily, mainly comes from neutrophils and mononuclear macrophages. Recently, MRP8/14 has been found to participate in innate immunity-related inflammation after its release into the extracellular space (Foell et al. 2007b). In addition, it has been well demonstrated that MRP8/14, combined with TLR4, can stimulate the activation of NF-κB and the release of proinflammatory cytokines from monocytes and macrophages (Vogl et al. 2007; Loser et al. 2010; Rauvala and Rouhiainen 2010).
Recently, a study (Wache et al. 2015) found that in pneumococcal meningitis, MRP 14 can induce the inflammatory response after the bacteria were cleared, but the role of the heterodimer MRP8/14, a more common form of the protein complex, in meningitis inflammation is still unclear. MRP8 and MRP14, also known as S100A8 and S100A9, tend to form stable heterodimers. Their polymer MRP8/14 is the more stable and common form. Interestingly, in a model of pneumococcal pneumonia, MRP8/14 was found to promote bacterial growth and lethality (Achouiti et al. 2014). However, there is no proof to date whether MRP8/14 participates in the inflammatory reaction and pathological process of SP meningitis. To inspect the role of MRP8/14 in bacterial meningitis, we established a murine animal model of bacterial meningitis by injection of SP into the cerebral ventricle of mice. Clinical disease status, degree of cerebral edema, meninges inflammatory response and expression of proinflammatory cytokines and chemokines in the brain and serum were measured in the presence or absence of MRP8/14 treatment. Finally, we further observed the phosphorylation of NF-κB p65 in the cerebral parenchyma. These results provided the first evidence for the role of MRP8/14 in the inflammatory response in the central nervous system (CNS) during bacterial meningitis.
Materials and Methods
Reagents and Bacteria
Recombinant mouse MRP8/14 was obtained from R&D Systems (Minneapolis, MN). Mouse TNF-α ELISA kits, mouse IL-6 ELISA kits and mouse MCP-1 ELISA kits were purchased from eBioscience (San Diego, CA). Mouse CRP ELISA kits were obtained from Abcam (Cambridge, MA). A rabbit anti-NF-κB p65 antibody was obtained from Cell Signalling Technology (Beverly, MA). 3,3N-diaminobenzidine tertrahydrochloride (DAB) and Power Vision ™ two step histochemical staining reagent were purchased from Gene Tech(Shanghai) Company Limited.
Gram-positive SP type 3 was purchased from the American Type Culture Collection (ATCC, Manassas, VA). Bacteria were cultured in trypticase soy broth at 37 °C, gathered at the mid-logarithmic growth period, washed twice and then resuspended in phosphate-buffer saline (PBS). The concentration of bacteria was determined by spectrophotometry at 550 nm.
Mice and Pneumococcal Meningitis
Pyrogen-free and sterile, 8-week-old male Balb/c mice were obtained from JOINN Laboratories (Suzhou, China). Under controlled conditions (12/12 h dark/light cycle, 55% ± 5% humidity, 22–24 °C), mice were housed at the Institute of Pediatric Research, Soochow University.
Weight- and age-matched mice (n = 48 in total) were anaesthetized by intraperitoneal injection of 0.5 ml chloral hydrate (10%). SP meningitis was induced by intracerebral injection of PBS containing SP as described previously (Chen et al. 2012, 2017a). Mice were randomly divided into the following groups (n = 12 per group): (1) mice in the control group injected with 45 μl PBS, (2) mice in the MRP8/14 group injected with 45 μl PBS containing 20 μg MRP8/14, (3) mice in the SP group injected with 45 μl PBS containing 0.5 × 108 CFU/ml SP and (4) mice in the SP plus MRP8/14 group injected with 5 μl PBS containing 4.5 × 108 CFU/ml SP and 40 μl PBS containing 20 μg MRP8/14. Mice were weighed and assessed at 6, 24 and 48 h after SP infection (n = 4 per point). We removed the brains, froze them immediately with liquid nitrogen and stored them at − 80 °C for PCR, ELISA and immunohistochemistry.
Assessment of Clinical Disease Status
Mice were weighed and assessed at 6, 24 and 48 h after injection. The clinical disease status was evaluated by spontaneous motor activity and body weight loss. The following scores were used to evaluate spontaneous motor activity as described previously (Leib et al. 2001): one indicated coma; two indicated the mouse did not turn upright when positioned on its back; three indicated the mouse turned upright in 30 s; four indicated the mouse turned upright in 5 s; and five indicated normal function.
Brain Oedema
At 6, 24 and 48 h after intervention, the brains were removed, and filter paper was used to absorb blood from the brain. Then, we took approximately one quarter of the brain tissue, put it in dry tin foil and weighed it on a precision electronic analytical balance. Then, we put the brain samples in a 110 °C electrothermal constant-temperature oven and baked them for 16 h to a constant weight (the difference between two dry weights should be less than 0.0002 g). By measuring wet weight and dry weight, the Elliot formula (Shahrokhi et al. 2012) could be used to calculate the degree of cerebral oedema by using the following equation: brain moisture content (%) = (wet weight − dry weight)/wet weight × 100%.
HE Staining
At 6, 24 and 48 h after intervention, we removed half of the brain tissue, fixed them in 4% formaldehyde overnight, embedded them in paraffin, sliced them into 5 μm sections and stained them with haematoxylin and eosin (HE). The brain sections were observed under a microscope at 400× magnification (Nikon, Japan) to indicate the degree of brain damage.
Quantitative Real-Time PCR
Total RNA was extracted from cerebral homogenate and reverse-transcribed into cDNA. cDNA was amplified by a LightCycler system (Roche Molecular Biochemicals, Indianapolis, IN). The following primers were used:
-
mouse TNF-α primers (sense-5′-TGTCTACTCCTCAGAGCCCC-3′ and antisense-5′-GACCCGTAGGGCGATTACAG-3′);
-
mouse CRP primers (sense-5′-CCTGAGGCTCCAACACACAT-3′ and antisense-5′-GTGTAGCCCTTGTGCAGACT-3′);
-
mouse IL-6 primers (sense-5′-CCAGTTGCCTTCTTGGGACT-3′ and antisense-5′-GTCTCCTCTCCGGACTTGTG-3′);
-
mouse MCP-1 primers (sense-5′-AGGTCCCTGTCATGCTTC-3′ and antisense-5′-GTGCTTGAGGTGGTTGTG-3′);
-
β-actin primers (sense-5′-GGTCATCACTATTGGCAACG-3′ and antisense-5′-ACGGATGTCAACGTCACACT-3′).
ELISA of Proinflammatory Cytokines
Brain and serum samples were collected at 6, 24 and 48 h after injection. The concentrations of TNF-α, IL-6 and MCP-1 were measured by ELISA kits (eBioscience, Hatfield, UK). CRP was detected with a kit (ABCAM, Cambridge, UK). The experimental procedures were performed according to the instructions.
Immunohistochemical Staining
Brain slices treated as above were dewaxed and washed. Endogenous peroxidase activity was suppressed by H2O2, and slices were incubated with primary antibody overnight at 4 °C and then incubated with secondary antibodies for 1 h at room temperature. All samples were stained with DAB and haematoxylin and observed under a microscope at 400× magnification. Brown particles appeared in positive cells but not in negative samples. Non-overlapping views in each sample were chosen randomly, and the cells were counted as the mean ± standard deviation (SD).
Statistical Analysis
SPSS 19.0 software was used for statistical analyses. All results were expressed as the mean ± SD. Multi-group comparisons were made with one-way analyses of variance (one-way ANOVAs), and group-group comparisons were performed with a t test. Differences were considered to be significant when P < 0.05.
Results
Assessment of Clinical Disease Status
At 24 and 48 h after SP injection, all infected mice showed signs of reduced spontaneous motor activity (Fig. 1a) and elevated body weight loss (Fig. 1b) compared with the PBS group. Additionally, the mice with SP plus MRP8/14 showed further aggravated the disease with largely increased body weight loss and decreased spontaneous motor activity (P < 0.05 vs. mice treated with SP alone; Fig. 1). MRP8/14 alone did not influence the clinical disease status (Fig. 1).
MRP8/14 a caused decreased spontaneous motor activity and b increased weight loss in SP-injected mice. Balb/c mice were treated with PBS, MRP8/14, live SP and live SP plus MRP8/14. The spontaneous motor activity and body weight were assessed at 6, 24 and 48 h after injection. Data are from 4 mice at all time points. aP < 0.05 vs. mice challenged with PBS, bP < 0.05 vs. mice challenged with SP alone. MRP8/14, myeloid-related protein 8/14; PBS, phosphate-buffered saline; SP, Streptococcus pneumoniae
Brain Oedema
The index of brain oedema was enhanced in the SP group at 24 and 48 h after injection compared with that in the PBS group (P < 0.05; Fig. 2). Moreover, a largely greater index of brain edema was observed in the SP plus MRP8/14 group than in the SP group at 24 and 48 h (P < 0.05; Fig. 2), reflecting that the administration of MRP8/14-aggravated SP-induced brain oedema.
MRP8/14 promoted brain oedema during SP meningitis. Balb/c mice were treated with PBS, MRP8/14, live SP and live SP plus MRP8/14. The index of brain oedema was assessed at 6, 24 and 48 h after injection. Data are from 4 mice at all time points. aP < 0.05 vs. mice challenged with PBS, bP < 0.05 vs. mice challenged with SP alone. MRP8/14, myeloid-related protein 8/14; PBS, phosphate-buffered saline; SP, Streptococcus pneumoniae
HE Staining
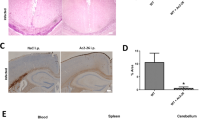
To confirm whether MRP8/14 aggravates the brain injury in SP-induced meningitis, brain slices were assessed using HE staining. The results displayed damage in brain tissues at 6, 24 and 48 h after SP infection. The majority of the damaged tissue showed soft meningeal vascular congestion, subarachnoid expansion and inflammatory cell infiltration (Fig. 3). In addition, compared with the SP group, the SP plus MRP8/14 group suffered a greater extent of brain tissue damage (Fig. 3).
Effect of MRP8/14 on the morphology and structure of brain tissue in Balb/c mice, HE 400×. Brains were classified as a–c PBS-injected, d–f MRP8/14-injected, g–i SP-injected and j–l SP plus MRP8/14-injected mice, and brain slices were subjected to HE staining. SP plus MRP8/14-infected mice showed significant injury in the brain and infiltration of inflammatory cells at j 6, k 24 and l 48 h after SP injection compared with those treated with SP alone (g–i, respectively). MRP8/14, myeloid-related protein 8/14; PBS, phosphate-buffered saline; SP, Streptococcus pneumoniae
MRP8/14 Augmented SP-Induced Production of Proinflammatory Cytokines
To further illustrate the effect of MRP8/14 on the SP-induced inflammatory response during pneumococcal meningitis, we assessed whether MRP8/14 was able to enhance proinflammatory cytokines in S. pneumoniae-infected mice.
While the release of TNF-α, IL-6 and MCP-1 at 6, 24 and 48 h after injection was significantly greater in the SP group than in the PBS group (P < 0.05; Fig. 4), mice challenged with SP plus MRP8/14 showed much higher levels of these cytokines (P < 0.05 vs. mice treated with SP alone; Fig. 4). In contrast, MRP8/14 alone did not induce the release of any of these cytokines. As a sensitive indicator of adverse reactions in the acute phase, CRP increased rapidly and significantly in the acute inflammatory reaction. As displayed in Fig. 4, there was very little CRP present in brain homogenates from PBS-treated mice. In contrast, intracerebral infection with SP caused substantial release of CRP in the brain and serum at 24 and 48 h after SP infection (P < 0.05). Moreover, increased levels of CRP were found in mice challenged with SP plus MRP8/14 (P < 0.05 vs. mice treated with SP alone; Fig. 4).
MRP8/14 amplifies the inflammatory response in SP-injected mice. Balb/c mice were treated with PBS, MRP8/14, live SP and live SP plus MRP8/14. The mRNA levels of TNF-α, IL-6, CRP and MCP-1 in the brain homogenates were evaluated by PCR (a), and the protein levels of those cytokines in brain homogenates (b) and in serum (c) were assessed by ELISA. Data are from 4 mice at all time points. aP < 0.05 vs. mice challenged with PBS, bP < 0.05 vs. mice challenged with SP alone
The amplifying effect of MRP8/14 on proinflammatory cytokine and chemokine release from SP-injected mice was further supported by the MRP8/14-induced increase in those cytokines at the mRNA level, where MRP8/14 extensively enhanced the mRNA expression of TNF-α, IL-6, CRP and MCP-1 at 24 and 48 h after SP infection (P < 0.05 vs. mice treated with SP alone) (Fig. 4).
Augmentation of SP-Induced Inflammatory Response by MRP8/14 Occur via the NF-κB Signalling Pathway
To clarify the underlying mechanism by which MRP8/14 amplified the SP-stimulated inflammatory response, we assessed whether MRP8/14 had a positive effect on the NF-κB signalling pathway. We assessed the protein expression of NF-κB p65 in the brain tissues by immunochemical staining. Intracerebral infection with SP increased protein expression of NF-κB p65 at 6, 24 and 48 h after injection (Fig. 5). Remarkably, a massive accumulation of NF-κB p65 in the brain was found in mice treated with SP plus MRP8/14 at all time points (P < 0.05 vs. mice treated with SP alone) (Fig. 5).
MRP8/14 upregulated NF-κB p65 expression in brain tissues of SP-injected mice. Balb/c mice were treated with PBS, MRP8/14, live SP and live SP plus MRP8/14. Brains were classified as a–c PBS, d–f MRP8/14, g–i SP and j–l SP plus MRP8/14, and the expression of NF-κB p65 in the brain was assessed by immunohistochemistry. The number of NF-κB p65-positive cells differed largely between the SP plus MRP8/14 group at j 6, k 24 and l 48 h, and the SP group at all time points (g–i, respectively); there was no significant difference in the number of positive cells between the PBS group at a 6, b 24 and c 48 h and the MRP8/14 group at these time points (d–f, respectively). The data are expressed as the mean ± SD. aP < 0.05 vs. mice challenged with PBS, bP < 0.05 vs. mice challenged with SP alone. MRP8/14, myeloid-related protein 8/14; PBS, phosphate-buffered saline; SP, Streptococcus pneumoniae
Discussion
Identifying new host factors that regulate innate immune responses during bacterial meningitis not only enhances our understanding of this complex disease but also provides approaches for designing new therapies that can minimise the sequelae rate and mortality. Our study explored whether MRP8/14 participated in the inflammatory reaction and pathological process of SP meningitis. First, the neurobehavioural score and body weight loss of the mice indicated that MRP8/14 protein alone had no effect on the mice, so MRP8/14 protein could not trigger an immune inflammatory reaction in uninfected mice. However, we found that challenge with SP plus MRP8/14 increased the severity of disease, shown by decreased neurobehavioural scores, and body weight from 6 to 48 h after SP infection, compared with SP-infection alone. These results showed that MRP8/14 protein promotes the aggravation process of clinical status during S. pneumoniae meningitis (SPM).
Cerebral oedema is a pathological phenomenon secondary to bacterial meningitis, and we assessed its severity accordingly. In compliance with this, the MRP8/14-induced inflammatory response in the CNS seen in this study is also related to the increased degree of cerebral oedema and aggravated clinical disease status. Challenge with SP plus MRP8/14 caused a further increase in cerebral oedema than challenge with SP-infection only, suggesting that MRP8/14 played a harmful role in the progress of pneumococcal meningitis.
Meninges inflammation is a sign of pathophysiological changes in bacterial meningitis. In this study, we confirmed that murine pneumococcal meningitis was distinguished by meningeal inflammation hours after intracerebral SP injection and that meningeal inflammation became more significant from 6 to 48 h after infection. Moreover, simultaneous treatment with MRP8/14 led to much stronger meningeal inflammation with expanded or opened subarachnoid space, ruptured or impaired integrity of the leptomeninges, dilated blood vessels, and more infiltrated inflammatory cells at 6, 24 and 48 h compared with SP-infection only. Long-term prognosis of meningitis is closely related to the excessive inflammatory response in the CNS. Therefore, our histopathologic data indicated that MRP8/14 may augment the SP-stimulated inflammatory response and thus, may result in the unfavourable outcome of pneumococcal meningitis.
In addition, we observed that the administration of exogenous MRP8/14 via intracerebroventricular injection in SP-infected Balb/c mice greatly amplified the SP-induced inflammatory response as shown by further accumulation of proinflammatory cytokines both in the brain and serum. SP infection resulted in significantly increased expression of TNF-α, IL-6 and MCP-1 at 6, 24 and 48 h after SP injection, and SP plus MRP8/14 injection further amplified the expression levels of these cytokines compared with those in mice challenged with SP alone. The expression of CRP was largely increased in SP-injected mice at 24, 48 h and was further increased in the mice injected with SP plus MRP8/14. These results demonstrate that SP infection contributes to the inflammation in the CNS and that the administration of exogenous MRP8/14 did not cause damage directly but deteriorated SP-induced brain inflammation.
A number of studies (Barichello et al. 2010a; Chen et al. 2012; Fassl et al. 2015; Chen et al. 2017b) have demonstrated that TNF-α and IL-6 are important proinflammatory cytokines in the immune inflammatory reaction of bacterial meningitis. Consistent with this, we observed elevated levels of TNF-α and IL-6 following SP infection at 6, 24 and 48 h in both brain homogenates and serum; moreover, MRP8/14 amplified the release of TNF-α and IL-6 protein in our study and was also associated with aggravated clinical disease status.
As a sensitive indicator of adverse reactions in the acute phase, CRP increased rapidly and significantly in the acute inflammatory reaction. CRP has obvious temporal characteristics. At the initial stage of infection, it can be normal or slightly elevated, rapidly elevate after 4–6 h and peak at 30–50 h. Extensive evidence (Coutinho et al. 2013; Grandgirard et al. 2013; McLoughlin et al. 2017) indicates that CRP at high levels may affect the severity of the inflammatory reaction during bacterial meningitis. In this study, the higher expression of CRP in both brain homogenates and serum in the MRP8/14 plus SP group compared with that in SP group from 24 h to 48 h after SP infected indicated that MRP8/14 aggravated the SP infection-induced immune inflammatory reaction not only in the brain but also in the whole body. The rise of CRP in serum is consistent with the increasing trend of the brain homogenates, which may have clinical significance as a convenient tool for the differential diagnosis and disease severity evaluation of bacterial meningitis.
MCP-1 is a kind of chemokine and is responsible for inducing leukocytes to enter the subarachnoid space during infection of the CNS. Chemokines have proved to play a critical role in the immune inflammatory reaction of SPM (Coutinho et al. 2013; Grandgirard et al. 2013; McLoughlin et al. 2017). In the results of this study, the increased expression of MCP-1 in brain tissues and serum of the mice challenged with SP plus MRP8/14 may reflect that MRP 8/14 participated in promoting the aggregation of chemotaxis and contributed to the enhanced immune inflammatory response during SPM.
NF-κB, an important transcriptional factor for a wide range of cells, is situated at the hub of the TLR signalling pathways downstream of the family. It regulates the transcription of a large number of genes, especially those related to the inflammatory immune response (Biragyn et al. 2002). The toll-like receptor (TLR) family in host cells identifies various microorganisms, activating NF-κB to enter the nucleus from the cytoplasm and regulate the expression of proinflammatory factors (Takeda and Akira 2004). Some studies have found that the mRNA expression of TLR2, TLR4 and TLR9 in the SP meningitis model is significantly increased (Böttcher et al. 2003), indicating that TLRs play an important role in inflammatory diseases. Furthermore, MRP8/14 is also the ligand of TLR4. After binding with TLR4, it can induce intracellular effects by means of signalling mechanisms such as NF-κB and p38 MAPKs (Lominadze et al. 2005; Foell et al. 2007a; Vogl et al. 2007). Therefore, the study of the NF-κB signalling pathway will be helpful to understand the pathological mechanism of the excessive immune inflammatory response in SPM. In the present study, we observed that the expression of NF-κB p65 increased in SP-infected mice and further increased in the mice from 6 to 48 h after treatment with SP plus MRP8/14. Together with the above reports, these results indicate that additional challenges with MRP 8/14 enhanced the immune inflammatory response in SPM mice and upregulated the expression of NF-κB p65. We speculated that MRP 8/14 aggravated the excessive immune inflammatory reaction of SPM and probably was related to the activation of the NF-κB signalling pathway.
In summary, the present preliminary results first documented a crucial role of MRP8/14 in the pathogenesis of the immune inflammatory reaction in a model of SPM, and the underlying molecular mechanism may be NF-κB signalling pathway-related. A subsequent inhibition study targeting MRP8/14 in SPM may help to further prove the effects of MRP8/14 in overwhelming the immune inflammatory reaction, which promotes the progress of SPM. All these results may promote further understanding of the immune mechanisms associated with the pathogenesis of bacterial meningitis and have further clinical implications for optimal comprehensive treatments of this disease.
References
Achouiti A, Vogl T, Endeman H, Mortensen BL, Laterre PF, Wittebole X, van Zoelen M, Zhang Y, Hoogerwerf JJ, Florquin S, Schultz MJ, Grutters JC, Biesma DH, Roth J, Skaar EP, van 't Veer C, de Vos AF, van der Poll T (2014) Myeloid-related protein-8/14 facilitates bacterial growth during pneumococcal pneumonia. Thorax. 69(11):1034–1042
Auburtin M, Wolff M, Charpentier J, Varon E, le Tulzo Y, Girault C, Mohammedi I, Renard B, Mourvillier B, Bruneel F, Ricard JD, Timsit JF (2006) Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med 34(11):2758–2765
Barichello T, dos SI, Savi GD et al (2010a) TNF-alpha, IL-1beta, IL-6, and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniae. J Neuroimmunol 221(1–2):42–45
Barichello T, Savi GD, Silva GZ, Generoso JS, Bellettini G, Vuolo F, Petronilho F, Feier G, Comim CM, Quevedo J, Dal-Pizzol F (2010b) Antibiotic therapy prevents, in part, the oxidative stress in the rat brain after meningitis induced by Streptococcus pneumoniae. Neurosci Lett 478(2):93–96
Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW (2002) Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 298(5595):1025–1029
Böttcher T, von MM, Ebert S et al (2003) Differential regulation of toll-like receptor mRNAs in experimental murine central nervous system infections. Neurosci Lett 344(1):17–20
Brouwer MC, Tunkel AR, van de Beek D (2010) Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 23(3):467–492
Chen X, Quinn EM, Ni H, Wang J, Blankson S, Redmond HP, Wang JH, Feng X (2012) B7-H3 participates in the development of experimental pneumococcal meningitis by augmentation of the inflammatory response via a TLR2-dependent mechanism. J Immunol 189(1):347–355
Chen X, Li Y, Blankson S, Liu M, Huang D, Redmond HP, Huang J, Wang JH, Wang J (2017a) B7-H3 augments inflammatory responses and exacerbates brain damage via amplifying NF-κB p65 and MAPK p38 activation during experimental pneumococcal meningitis. PLoS One 12(1):e0171146
Chen X, Meng X, Foley NM, Shi X, Liu M, Chai Y, Li Y, Redmond HP, Wang J, Wang JH (2017b) Activation of the TLR2-mediated downstream signaling pathways NF-κB and MAPK is responsible for B7-H3-augmented inflammatory response during S. Pneumoniae infection. J Neuroimmunol 310:82–90
Coutinho LG, Grandgirard D, Leib SL, Agnez-Lima LF (2013) Cerebrospinal-fluid cytokine and chemokine profile in patients with pneumococcal and meningococcal meningitis. BMC Infect Dis 13:326
Fassl SK, Austermann J, Papantonopoulou O et al (2015) Transcriptome assessment reveals a dominant role for TLR4 in the activation of human monocytes by the alarmin MRP8. J Immunol 194(2):575–583
Foell D, Wittkowski H, Roth J (2007a) Mechanisms of disease: a 'DAMP' view of inflammatory arthritis. Nat Clin Pract Rheumatol 3(7):382–390
Foell D, Wittkowski H, Vogl T, Roth J (2007b) S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 81(1):28–37
Grandgirard D, Gäumann R, Coulibaly B et al (2013) The causative pathogen determines the inflammatory profile in cerebrospinal fluid and outcome in patients with bacterial meningitis. Mediat Inflamm 2013:312476
Hoffmann O, Braun JS, Becker D, Halle A, Freyer D, Dagand E, Lehnardt S, Weber JR (2007) TLR2 mediates neuroinflammation and neuronal damage. J Immunol 178(10):6476–6481
Kastenbauer S, Pfister HW (2003) Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain. 126(Pt 5:1015–1025
Leib SL, Clements JM, Lindberg RL, Heimgartner C, Loeffler JM, Pfister LA, Täuber MG, Leppert D (2001) Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain. 124(Pt 9):1734–1742
Lominadze G, Rane MJ, Merchant M, Cai J, Ward RA, McLeish KR (2005) Myeloid-related protein-14 is a p38 MAPK substrate in human neutrophils. J Immunol 174(11):7257–7267
Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, Luger TA, Roth J, Beissert S (2010) The toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med 16(6):713–717
Malipiero U, Koedel U, Pfister HW et al (2006) TGFbeta receptor II gene deletion in leucocytes prevents cerebral vasculitis in bacterial meningitis. Brain. 129(Pt 9):2404–2415
McLoughlin A, Rochfort KD, McDonnell CJ, Kerrigan SW, Cummins PM. 2017. Staphylococcus aureus-mediated blood-brain barrier injury: an in vitro human brain microvascular endothelial cell model. Cell Microbiol 19(3)
Rauvala H, Rouhiainen A (2010) Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta 1799(1–2):164–170
Shahrokhi N, Haddad MK, Joukar S, Shabani M, Keshavarzi Z, Shahozehi B (2012) Neuroprotective antioxidant effect of sex steroid hormones in traumatic brain injury. Pak J Pharm Sci 25(1):219–225
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16(1):3–9
Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A, Emerging Infections Programs Network (2011) Bacterial meningitis in the United States, 1998-2007. N Engl J Med 364(21):2016–2025
Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen M, Nacken W, Foell D, van der Poll T, Sorg C, Roth J (2007) Mrp8 and Mrp14 are endogenous activators of toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13(9):1042–1049
Wache C, Klein M, Ostergaard C, Angele B, Häcker H, Pfister HW, Pruenster M, Sperandio M, Leanderson T, Roth J, Vogl T, Koedel U (2015) Myeloid-related protein 14 promotes inflammation and injury in meningitis. J Infect Dis 212(2):247–257
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301(5633):640–643
Funding
This work was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20161227), the Six One Projects of Jiangsu Province (LGY2017091), and Jiangsu Province Program of Key Research and Development Plan (BE2018661), Suzhou Clinical Key Disease Diagnosis and Treatment Technology Special Project (LCZX201810).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All studies were approved by the ethics committee for animal protection and use and were in compliance with the Animal Welfare Act.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, D., Liu, M., Zhou, Y. et al. Myeloid-Related Protein 8/14 Participates in the Progression of Experimental Pneumococcal Meningitis by Augmentation of Inflammation. J Mol Neurosci 68, 631–639 (2019). https://doi.org/10.1007/s12031-019-01314-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01314-y