Abstract
Disruption of apolipoprotein E (APOE) is responsible for age-dependent neurodegeneration and cognitive impairment. Elderly individuals are more sensitive than young individuals to the effects of ethanol (EtOH), particularly those affecting cognition. We investigated the role of APOE deficiency and EtOH exposure on age-dependent alterations in choline acetyltransferase (ChAT) and brain-derived neurotrophic factor (BDNF) mRNA and protein expression in the mouse hippocampus. Three-month-old (young) and 12-month-old (aged) ApoE-knockout (ApoE-KO) and wild-type (WT) mice were treated with saline or 2 g/kg EtOH, and the bilateral hippocampus was collected after 60 min for real-time PCR and western blotting analyses. ChAT (P < 0.01) and BDNF (P < 0.01) expression were significantly decreased in both young and aged saline- and EtOH-treated ApoE-KO mice versus young and aged saline- and EtOH-treated WT mice. Aged saline- and EtOH-treated ApoE-KO mice exhibited greater differences in ChAT and BDNF expression (P < 0.01) than young saline- and EtOH-treated ApoE-KO mice. Aged EtOH-treated WT mice also exhibited larger decreases in BDNF expression (P < 0.01)—but not in ChAT expression—than young EtOH-treated WT mice. EtOH decreased ChAT and BDNF expression in both young (P < 0.01) and aged (P < 0.01) ApoE-KO mice versus EtOH-free ApoE-KO mice of the same age. EtOH also decreased BDNF expression in aged (P < 0.01) WT mice versus EtOH-free aged WT mice. In summary, these results suggest that APOE deficiency and EtOH exposure cause age-dependent decreases in ChAT and BDNF in the hippocampus. Importantly, the decreases in ChAT and BDNF were greater in aged EtOH-treated mice, particularly those lacking APOE, raising the possibility that APOE-deficient individuals who consume alcohol may be at greater risk of memory deficit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apolipoprotein E (APOE) is a cholesterol carrier in the brain with roles in lipid transport and injury repair (Chen et al. 1997; Mahley and Rall 2000). This protein has recently been identified as a risk factor for several neurodegenerative diseases (Giau et al. 2015). Compared with healthy mice, ApoE-knockout (ApoE-KO) mice exhibit specific synapse loss in cholinergic (http://topics.sciencedirect.com/topics/page/Cholinergic), noradrenergic (http://topics.sciencedirect.com/topics/page/Norepinephrine), and serotinergic projections to relevant brain regions (Chapman and Michaelson 1998) and perform worse in memory tasks (Gordon et al. 1995; Dodart et al. 2000). APOE isoforms differentially regulate maturation and the secretion of brain-derived neurotrophic factor (BDNF) from primary human astrocytes (Sen et al. 2017). Disruption of BDNF contributes to age-related cognitive decline (Linnarsson et al. 1997). Both BDNF and APOE are involved in neuronal processes, such as cell growth, resilience to noxious stimuli, and synaptic plasticity, and their disruption is considered to be a risk factor for Alzheimer's disease (AD; Hashimoto et al. 2009; Richter-Schmidinger et al. 2011; Lim et al. 2015). Aging is the main risk factor for diseases, including cancer, diabetes, heart disease, and AD (Niccoli and Partridge 2012). Age-related cognitive deficits are partly explained by changes in neural plasticity and synaptic activity (Seidler et al. 2010). Therefore, we examined ApoE-KO mice to elucidate the age-dependent role of APOE in choline acetyltransferase (ChAT) and BDNF expression in the hippocampus.
Several investigations have studied the effects of ethanol (EtOH) on memory acquisition (White et al. 2000; Acheson et al. 2001). EtOH consumption increases the risk of AD in individuals with the ApoE e4 allele (Anttila et al. 2004; Kivipelto et al. 2008). Elderly individuals are more sensitive than young individuals to the effects of EtOH, particularly those on cognition (Swartzwelder et al. 1995). It has also been reported that spatial memory in aging adult rats is increasingly affected by EtOH (Rajendran and Spear 2004). Numerous studies have presented evidence of a link between the modulation of brain morphology and APOE expression (Cohen et al. 2001; den Heijer et al. 2002; Love et al. 2006; Espeseth et al. 2008), which suggests that APOE plays a pivotal role in aging. Disruption of APOE has an impact on longevity (Ang et al. 2008; Bonomini et al. 2010) and is involved in several age-related diseases (Bonomini et al. 2010). In addition, ApoE-KO mice exhibit an age-related decline in presynaptic terminals in the hippocampus (Buttini et al. 2002).
To date, no data have been published on the effects of APOE and EtOH on ChAT and BDNF expression in the hippocampus of aged mice. Thus, we evaluated the role of APOE deficiency and EtOH exposure in age-related changes in ChAT and BDNF expression in the mouse hippocampus. We compared 3-month-old (young) and 12-month-old (aged) ApoE-KO mice, with C57BL/6J (wild-type [WT]) mice to investigate ChAT and BDNF mRNA and protein expression in the hippocampus after saline or EtOH (2 g/kg) treatment using real-time PCR and western-blotting (WB) analyses.
Materials and Methods
Animals
All experimental mice had the C57BL/6J genetic background. Breeding pairs of ApoE-KO mice were used to generate the experimental groups. Two 8-week-old breeding pairs of ApoE-KO mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at the animal facility of Kagawa University (Takamatsu, Japan). WT C57BL/6J mice were also bred at our animal facility. All experiments were conducted with male mice. All mice were housed under a controlled environmental regimen of 21 ± 3 °C, 50–70% humidity, and 12:12-h light:dark cycle, and they all had access to food and water ad libitum. All animal experiments were approved by the Kagawa University Animal Investigation Committee.
Experimental Groups
ApoE-KO and WT mice were each divided into four experimental groups: (1) young saline; (2) young EtOH (2 g/kg); (3) aged saline; (4) aged EtOH (2 g/kg). EtOH was administered at a concentration chosen to produce physiologically relevant blood concentrations, reaching a peak of approximately 20 mM at 30 min and then gradually declining (Jamal et al. 2016). For the aged groups we used ApoE-KO mice aged 12 months due to their short life span (Moghadasian et al. 2001). In addition, ApoE-KO mice aged 12–15 months may exhibit an age-related decline in presynaptic terminals in the hippocampus (Buttini et al. 2002). EtOH was dissolved in 0.9% saline and administered via intraperitoneal (IP) injection in a volume of 10 ml/kg. The bilateral hippocampus was collected 60 min after the IP injection of EtOH (20%, w/v) or saline.
Brain Tissues
Mice were killed by cervical decapitation. The bilateral hippocampus (approx. 15 mg) was immediately dissected on ice, and one-half of the hippocampus was placed in a 2-ml tube for RNA extraction, and the other half was placed in a 2-ml tube for protein analysis. The tubes were stored at − 70 °C until use.
Quantitative Real-Time PCR Analysis
The hippocampus was homogenized using a Polytron® homogenizer (Kinematica AG, Lucerne, Switzerland) in 0.4 ml of Isogen (Nippon Gene Co., Ltd., Tokyo, Japan). An additional 0.4 ml of Isogen was added to yield a total sample volume of 0.8 ml. Total RNA was extracted using Isogen and a spin column (Nippon Gene Co., Ltd.). RNA was transcribed into cDNA using the PrimeScript™ RT Master Mix (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. Briefly, the reaction was conducted at 37 °C for 15 min in a total volume of 10 μl and then inactivated at 85 °C for 5 s. cDNA diluted five times was used as a template, and real-time PCR was performed using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus; Takara Bio Inc.). Specific primers were used to amplify ChAT (forward [F]: 5′-TGGATGAAACATACCTGATGAGCAA-3′; reverse [R]: 5′-CGTGAAAGCTGGAGATGCAGAA-3′), BDNF (F: 5′-GGTATCCAAAGGCCAACTGA-3′; R: 5′-CTTATGAATCGCCAGCCAAT-3′), and β-actin (F: 5′-CATCCGTAAAGACCTCTATGCCAAC-3′; R: 5′-ATGGAGCCACCGATCCACA-3′). Amplification was performed in 96-well plates using a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with an initial denaturation step at 95°C for 5 s followed by 40 cycles of 95°C for 5 s, 60°C for 40 s, and 60°C for 1 min. Melting curves were calculated at the end of cycling to ensure the amplification of a single PCR product. The data were normalized to the internal standard β-actin.
Western Blotting
The hippocampus was homogenized using a Polytron® homogenizer in 0.4 ml of RIPA lysis buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). An additional 0.4 ml of RIPA lysis buffer supplemented with phenylmethylsulfonyl fluoride, sodium orthovanadate, and protease inhibitor cocktail (8 μl each; Santa Cruz Biotechnology, Inc.) was added to yield a total sample volume of 0.8 ml. After centrifugation at 10,000 g at 4 °C for 15 min, the supernatant was used for WB analysis. The protein content of the supernatant was determined using the Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA), with bovine serum albumin (Sigma-Aldrich Corp., St. Louis, MO, USA) as the standard. Samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis with molecular weight markers (Bio-Rad Laboratories, Inc.) and then transferred to polyvinylidene difluoride membranes. The membranes were incubated first with primary antibodies against ChAT (1:500; Chemicon International, Inc., Temecula, CA, USA), BDNF (1:1000; Santa Cruz Biotechnology, Inc.), and β-actin (1:2000; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and then with corresponding horseradish peroxidase-linked secondary antibodies. Band intensities were evaluated using an LAS-1000plus Lumino-Imaging Analyzer (Fujix Ltd., Tokyo, Japan). The relative protein expressions were normalized to those of β-actin in each sample.
Statistics
All values were expressed as the mean ± standard error of the mean; P values of < 0.05 were considered to be significant. The data were analyzed with SigmaPlot (Systat Software Inc., San Jose, CA, USA) using three-way analysis of variance. Post-hoc tests were carried out where appropriate using the Tukey–Kramer’s test.
Results
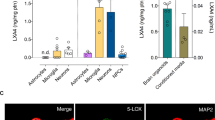
The effect of APOE deficiency, age, and EtOH on ChAT mRNA levels in the hippocampus is shown in Fig. 1. In saline-and EtOH-treated animals, the expression of ChAT mRNA was significantly decreased in APOE-deficient mice (young mice: P < 0.001; aged mice: P < 0.001) compared with WT mice of the same age. Also in both the saline and EtOH groups, aging had a significant effect on ChAT, as shown by the reduction in mRNA levels (all P levels, aged ApoE-KO mice: P < 0.001) compared with young ApoE-KO mice. EtOH administration caused a significant reduction in ChAT mRNA expression in both young and aged ApoE-KO mice (all P values, real-time PCR: <0.01) compared with saline-treated ApoE-KO animals of the same age.
The effects of apolipoprotein E (APOE), age, and alcohol (EtOH) on choline acetyltransferase (ChAT) mRNA expression in saline- (n = 5) and EtOH-treated mice (n = 5). †P < 0.01 vs. young (3 months old) saline wild-type (WT) mice; ‡P < 0.01 vs. old (12 months old) saline WT mice; §P < 0.001 vs. young EtOH WT mice; #P < 0.01 vs. old EtOH WT mice; ±P < 0.01 vs. young saline ApoE-knockout (ApoE-KO) mice; μP < 0.01 vs. young EtOH ApoE-KO mice; *P < 0.01 vs. young and old saline ApoE-KO mice. dF Degrees of freedom, ANOVA analysis of variance. Data are shown as the mean ± standard error of the mean (SEM)
The representative WB results for ChAT protein and the effect of APOE deficiency, age, and EtOH on ChAT protein levels in the hippocampus are shown in Fig. 2. In saline-and EtOH-treated animals, ChAT protein levels were significantly decreased in APOE-deficient mice (young mice: P < 0.01; aged mice: P < 0.01) compared with WT mice of the same age. Aging had a significant effect on ChAT levels, as evident by the reduction in protein (all P values, aged ApoE-KO mice: P <0.01) levels compared with young ApoE-KO mice in both the saline and EtOH groups. EtOH administration caused a significant reduction in ChAT protein in both young and aged ApoE-KO mice (all P values, protein: ≤ 0.01) compared with saline-treated ApoE-KO animals of the same age.
The effects of APOE, age and EtOH on ChAT protein expression in saline- (n = 5) and EtOH-treated mice (n = 5). †P < 0.001 vs. young saline WT mice; ‡P < 0.01 vs. old saline WT mice; §P < 0.01 vs. young EtOH WT mice; #P < 0.01 vs. old EtOH WT mice; ±P < 0.001 vs. young saline ApoE-KO mice; μP < 0.01 vs. young EtOH ApoE-KO mice; *P < 0.01 vs. young and old saline ApoE-KO mice. Data are shown as the mean ± SEM
The effect of APOE deficiency, age, and EtOH on BDNF mRNA levels in the hippocampus is shown in Fig. 3. In saline- and EtOH-treated animals, BDNF mRNA levels (young mice: P < 0.001; aged mice: P < 0.001) were significantly decreased in APOE-deficient mice compared with WT mice of the same age. Aging had a significant effect on BDNF, as evident by the reduction in mRNA levels (all P values, aged ApoE-KO mice: P < 0.001) compared with young ApoE-KO mice in both the saline and EtOH groups. BDNF also exhibited a significant decline with aging (aged WT mice: P < 0.001) compared with young WT mice in the EtOH group. EtOH administration caused a significant reduction in BDNF mRNA in young and aged ApoE-KO mice and aged WT mice (all P values, real-time PCR: < 0.01) compared with saline-treated ApoE-KO and WT animals of the same age.
The effects of APOE, age, and EtOH on brain-derived neurotrophic factor (BDNF) mRNA expression in saline- (n = 5) and EtOH-treated mice (n = 5). †P < 0.001 vs. young saline WT mice; ‡P < 0.001 vs. old saline WT mice; §P < 0.001 vs. young EtOH WT mice; #P < 0.001 vs. old EtOH WT mice; ±P < 0.001 vs. young saline ApoE-KO mice; μP < 0.001 vs. young EtOH ApoE-KO mice; ≠P < 0.001 vs. young EtOH WT mice; *P < 0.001 vs. young and old saline WT and ApoE-KO mice. Data are shown as the mean ± SEM
The representative WB results for BDNF protein and the effect of APOE deficiency, age, and EtOH on BDNF protein levels in the hippocampus are given in Fig. 4. In saline-and EtOH-treated animals, BDNF protein levels were significantly decreased in APOE-deficient mice (young mice: P < 0.01; aged mice: P < 0.01) compared with WT mice of the same age. Aging had a significant effect on BDNF, as evident by the reduction in protein levels (all P values, aged ApoE-KO mice: P < 0.01) compared with young ApoE-KO mice in both the saline and EtOH groups. BDNF also exhibited a significant decline with aging (aged WT mice: P <0.01) relative to young WT mice in the EtOH group. EtOH administration caused a significant reduction in BDNF protein in young and aged ApoE-KO and aged WT mice (all P values, protein: < 0.01) compared with saline-treated ApoE-KO and WT animals of the same age.
The effects of APOE, age, and EtOH on BDNF protein expression in saline- (n = 5) and EtOH-treated mice (n = 5). †P < 0.01 vs. young saline WT mice; ‡P < 0.01 vs. old saline WT mice; §P < 0.01 vs. young EtOH WT mice; #P < 0.01 vs. old EtOH WT mice; ≠P < 0.01 vs. young EtOH WT mice; μP < 0.001 vs. young EtOH ApoE-KO mice; *P < 0.01 vs. young and old saline WT and ApoE-KO mice. Data are shown as the mean ± SEM
Discussion
In this study, we first investigated whether APOE deficiency causes age-related changes in ChAT and BDNF expression in the hippocampus in young and aged ApoE-KO and WT mice. Second, we examined whether the administration of EtOH to these mice augments the effect of age on ChAT and BDNF expression. To our knowledge, this study is the first to investigate the role of APOE deficiency and EtOH exposure on ChAT and BDNF expression in the hippocampus of aged mice. We hypothesized that EtOH exposure would elicit greater cholinergic and trophic effects in ApoE-KO mice in an age-dependent manner. Our results indeed showed that APOE deficiency and EtOH exposure decreased ChAT and BDNF mRNA and protein levels in an age-dependent manner. Importantly, the decreases in ChAT and BDNF mRNA and protein levels were greater in aged EtOH-treated mice, particularly those lacking APOE, than in their younger counterparts. Our findings highlight the contribution of APOE and EtOH to the decreased ChAT and BDNF expression evident in the hippocampus of aged mice.
Different APOE isoforms have differing effects on mouse brain functions in vivo that are influenced by age and sex (Raber et al. 1998). The brain is second only to the liver with respect to the abundance of APOE expression. APOE-deficient mice thus represent an attractive model for studying the relationship between APOE and the cholinergic system and trophic factors of the brain. Cholinergic neurons are assumed to undergo moderate degenerative changes during aging, resulting in cholinergic hypofunction (Muller et al. 1991; Schliebs and Arendt 2011). Disrupted BDNF expression is also related to declining hippocampal volume and memory in late adulthood (Erickson et al. 2010). This decrease in BDNF may result in a lack of trophic support for cholinergic neurons (Hock et al. 2000).
Consistent with these observations, we noted significant decreases in both ChAT and BDNF mRNA and protein levels in the hippocampus of EtOH-free ApoE-KO mice (Figs. 1, 2, 3, and 4). These decreases were more pronounced in aged ApoE-KO mice than in young ApoE-KO mice, but absent in WT mice irrespective of age. These findings represent plausible evidence of a link between APOE and ChAT and BDNF expression in aged mice. APOE deficiency is associated with the dysfunction of basal forebrain cholinergic neurons (Fisher et al. 1998), but the mechanism by which its deficiency in aging decreases ChAT and BDNF expression remains unclear. Cognitive decline is a major concern of the APOE-deficient population, and aging is associated with heightened cognitive disturbance (de Chaves and Narayanaswami 2008). ApoE-KO mice have been demonstrated to be prone to an age-dependent decrease in synaptic density in distinct areas of the brain (Mashlia et al. 1995), with the result being reduced neurotransmitter release and poor performance in memory tasks (Raber et al. 1998; Buttini et al. 1999). Brain aging itself is linked to changes in the levels of neurotransmitters, such as acetylcholine (Baxter et al. 1999), neurotrophic factors, such as BDNF (Hwang et al. 2006), and dopamine receptor density (Roth and Joseph 1994). These findings lead us to believe that APOE deficiency causes significant decreases in ChAT and BDNF expression in the mouse hippocampus, especially in aged animals.
Studies examining cholinergic functions and trophic factors in relation to EtOH consumption in elderly individuals are rare. Our findings as well as those of other researchers (Rose et al. 2013; Jamal et al. 2016) demonstrate that blood EtOH values in adult WT mice are similar in various other strains of mice. Although we did not measure blood EtOH concentrations in ApoE-KO mice, it has been shown in rats that the peak blood EtOH concentrations for equivalent doses of EtOH increase with age (Seitz et al. 1989). Several studies have examined the effects of aging on EtOH metabolism and toxicity in rats, and the results reveal that the EtOH elimination rate decreases with age (Ritzmann and Springer 1980; Seitz et al. 1989; Kim et al. 2003). As such, the levels of EtOH in the blood and brain of adult and aged mice are higher than those of young mice (Ritzmann and Springer 1980). In this study, we investigated whether acute EtOH administration in ApoE-KO and WT mice reduces ChAT and BDNF expression to a greater degree with age. Using real-time PCR and WB, we found that EtOH exposure decreased ChAT and BDNF expression in ApoE-KO mice in an age dependent manner (Figs. 1, 2, 3, and 4). Likewise, EtOH also decreased BDNF expression—but not ChAT expression—in aged WT mice relative to young WT mice. Importantly, this heightened decline in ChAT and BDNF expression was evident in both young and aged ApoE-KO mice compared with EtOH-treated WT mice of the same age, suggesting that the effects of EtOH and APOE are additive.
Aging increases an individual’s sensitivity to and the effects of alcohol (Vestal et al. 1977; Spencer and Hutchison 1999). Some elderly people are impaired in many aspects of learning and memory (Bowles and Poon 1985; Eustache et al. 1995; Balota et al. 2000). Age-related cognitive decline is similar to the cognitive decline seen in chronic alcoholism (Bowles and Poon 1985; Eustache et al. 1995; Balota et al. 2000). Interestingly, our results show that the EtOH-related decline in ChAT and BDNF expression in aged mice of both genotypes was not parallel to that evident in young EtOH-treated mice, but exceeded it. Possible explanations for the age-related enhancement of the effects of EtOH include the increased rates of cellular deterioration that accompany normal aging (Yu 1996) and the pharmacokinetic induction of higher blood EtOH concentrations in older individuals compared with younger individuals (Vestal et al. 1977). Our data support the latter hypothesis by demonstrating that EtOH has a greater effect on ChAT and BDNF levels in aged mice than in young mice.
Cholinergic neurons and trophic factors appear to be particularly vulnerable to EtOH exposure (Davis 2008; Ehrlich et al. 2012). In support of this, we observed a significant effect of EtOH on ChAT and BDNF levels in both young and aged ApoE-KO mice compared with EtOH-free mice of the same age (Figs. 1, 2, 3, and 4). However, in WT mice, EtOH had similar to no effects, indicating that EtOH exerts some negative effects on cholinergic neurons and trophic factors. This is, in part, consistent with the lower spatial performance of aged rats with long-term moderate EtOH consumption compared with abstinent aged adult rats (Baird et al. 1998). In our study, EtOH failed to change the levels of ChAT and BDNF in young WT mice compared with saline-treated young WT mice, consistent with the findings of our previous study (Jamal et al. 2013).
EtOH is known to have effects on memory and other cognitive functions in humans (Brown et al. 2010) and animals (Hoffmann and Matthews 2001). ApoE-KO mice exhibit a greater EtOH (2 g/kg)-induced conditioned place preference than WT mice (Bechtholt et al. 2004). We recently showed that EtOH (2 g/kg) caused spatial memory deficits in ApoE-KO mice, suggesting a possible contribution of APOE to cognitive decline, although WT mice exhibited a similar decline in spatial task performance (Jamal et al. 2012). Our data of decreases in ChAT and BDNF levels after EtOH exposure in ApoE-KO mice further support the notion that APOE and EtOH act synergistically to reduce ChAT and BDNF expression in the hippocampus. These findings raise the possibility that individuals with APOE deficiency who consume alcohol may be at greater risk of memory deficit. APOE deficiency is associated with many diseases, such as dyslipidemia, atherosclerosis, AD, and Parkinson’s disease (Giau et al. 2015). Our data indicate that APOE deficiency, aging, and EtOH administration decrease cholinergic and trophic factor expression in the hippocampus of mice, which may be similar to APOE deficiency in humans.
In conclusion, our findings suggest that APOE deficiency, aging, and EtOH administration decrease ChAT and BDNF mRNA and protein levels in the mouse hippocampus. Importantly, heightened decreases in ChAT and BDNF levels were most evident in aged EtOH-treated mice, particularly those lacking APOE. Our results also suggest that APOE deficiency and EtOH exposure act in concert to decrease ChAT and BDNF levels in the mouse hippocampus, thereby providing support for the involvement of these two markers in age-related cognitive deficits.
References
Acheson SK, Ross EL, Swartzwelder HS (2001) Age-independent and dose-response effects of ethanol on spatial memory in rats. Alcohol 23:167–175
Ang LS, Cruz RP, Hendel A, Granville DJ (2008) Apolipoprotein E, an important player in longevity and age-related diseases. Exp Gerontol 43:615–622
Anttila T, Helkala EL, Viitane M, Kåreholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissine A, Kivipelto M (2004) Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ 329(7465):1–6
Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Carl KL, Gauvin DV (1998) Moderate, long-term, alcohol consumption potentiates normal, age-related spatial memory deficits in rats. Alcohol Clin Exp Res 22:628–636
Balota DA, Dolan PO, Duchek JM (2000) Memory changes in healthy young and older adults. In: Tulving E (ed) The Oxford Handbook of Memory. Oxford University Press, Oxford
Baxter MG, Frick KM, Price DL, Brekler SJ, Markowska AL, Gorman LK (1999) Presynaptic markers of cholinergic function in the rat brain: relationship with age and cognitive status. Neuroscience 89:771–779
Bechtholt AJ, Smith R, Raber J, Cunningham CL (2004) Enhanced ethanol-, but not cocaine-induced, conditioned place preference in Apoe (-/-) mice. Pharmacol Biochem Behav 77:783–792
Bonomini F, Filippini F, Hayek T, Aviram M, Keidar S, Rodella LF, Coleman R, Rezzani R (2010) Apolipoprotein E and its role in aging and survival. Exp Gerontol 45:149–157
Bowles NL, Poon LW (1985) Aging and retrieval of words in semantic memory. J Gerontol 40:71–77
Brown J, Brignell CM, Dhiman SK, Curran HV, Kamboj SK (2010) Acute effects of alcohol on memory: impact of emotional context and serial position. Neurobiol Learn Mem 93:428–434
Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahle RW (1999) Expression of human apolipoprotein E3 or E4 in the brains of Apoe-/- mice: isoform-specific effects on neurodegeneration. J Neurosci 19:4867–4880
Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L (2002) Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci 22:10539–10548
Chapman S, Michaelson DM (1998) Specific neurochemical derangements of brain projecting neurons in apolipoprotein E-deficient mice. J Neurochem 70:708–714
Chen Y, Lomnitski L, Michaelson DM, Shohami E (1997) Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience 80:1255–1262
Cohen RM, Small C, Lalonde F, Friz J, Sunderland T (2001) Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology 57:2223–2228
Davis MI (2008) Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther 118:36–57
de Chaves EP, Narayanaswam V (2008) Apolipoprotein E and cholesterol in aging and disease in the brain. Futur Lipidol 3:505–530
den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hoffman A, Breteler MM (2002) Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology 59:746–748
Dodart JC, Mathis C, Bales KR, Paul SM, Ungerer A (2000) Behavioral deficits in APP(V717F) transgenic mice deficient for the apolipoprotein E gene. Neuroreport 11:603–607
Ehrlich D, Pirchl M, Humpel C (2012) Ethanol transiently suppresses choline acetyltransferase in basal nucleus of Meynert slices. Brain Res 1459:35–42
Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF (2010) Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci 30:5368–5375
Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I (2008) Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E ε4. Neurobiol Aging 29:329–340
Eustache F, Rioux P, Desgranges B, Marchal G, Petit-Taboué MC, Dary M, Lechevalier B, Baron JC (1995) Healthy aging, memory subsystems and regional cerebral oxygen consumption. Neuropsychologia 33:867–887
Fisher A, Brandeis R, Chapman S, Pittel Z, Michaelson DM (1998) M1 muscarinic agonist treatment reverses cognitive and cholinergic impairments of apolipoprotein E-deficient mice. J Neurochem 70:1991–1997
Giau VV, Bagyinszky E, An SS, Kim SY (2015) Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat 11:1723–1737
Gordon I, Grauer E, Genis I, Sehayek E, Michaelson DM (1995) Memory deficits and cholinergic impairments in apolipoprotein E-deficient mice. Neurosci Lett 199:1–4
Hashimoto R, Hirata Y, Asada T, Yamashita F, Nemoto K, Mori T, Moriguchi Y, Kunugi H, Arima K, Ohnishi T (2009) Effect of the brain-derived neurotrophic factor and the apolipoprotein E polymorphisms on disease progression in preclinical Alzheimer's disease. Genes Brain Behav 8:43–52
Hock C, Heese K, Hulette C, Rosenberg C, Otten U (2000) Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol 57:846–851
Hoffmann SE, Matthews DB (2001) Ethanol-induced impairments in spatial working memory are not due to deficits in learning. Alcohol Clin Exp Res 25:856–861
Hwang IK, Yoo KY, Jung BK, Cho JH, Kim DH, Kang TC, Kwon YG, Kim YS, Won MH (2006) Correlations between neuronal loss, decrease of memory, and decrease expression of brain-derived neurotrophic factor in the gerbil hippocampus during normal aging. Exp Neurol 201:75–83
Jamal M, Ameno K, Miki T, Tanaka N, Ono J, Shirakami G, Sultana R, Yu N, Kinoshita H (2012) High ethanol and acetaldehyde impair spatial memory in mouse models: opposite effects of aldehyde dehydrogenase 2 and apolipoprotein E on memory. Pharmacol Biochem Behav 101:443–449
Jamal M, Ameno K, Ruby M, Miki T, Tanaka N, Nakamura Y, Kinoshita H (2013) Ethanol- and acetaldehyde-induced cholinergic imbalance in the hippocampus of Aldh2-knockout mice does not affect nerve growth factor or brain-derived neurotrophic factor. Brain Res 1539:41–47
Jamal M, Ameno K, Tanaka N, Ito A, Takakura A, Kumihashi M, Kinoshita H (2016) Ethanol and acetaldehyde after intraperitoneal administration to Aldh2-knockout mice—reflection in blood and brain levels. Neurochem Res 41:1029–1034
Kim YC, Kim SY, Sohn YR (2003) Effect of age increase on metabolism and toxicity of ethanol in female rats. Life Sci 74:509–519
Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, Hachinski V, Cedazo-Minguez A, Soininen H, Tuomilehto J, Nissinen A (2008) Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med 12:2762–2771
Lim YY, Villemagne VL, Laws SM, Pietrzak RH, Snyder PJ, Ames D, Ellis KA, Harrington K, Rembach A, Martins RN, Rowe CC, Masters CL, Maruff P (2015) APOE and BDNF polymorphisms moderate amyloid β-related cognitive decline in preclinical Alzheimer's disease. Mol Psychiatry 20:1322–1328
Linnarsson S, Björklund A, Ernfors P (1997) Learning deficit in BDNF mutant mice. Eur J Neurosci 9:2581–2587
Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ (2006) Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging 27:797–803
Mahley RW, Rall SC Jr (2000) Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1:507–537
Masliah M, Mallory N, Ge M, Alford I, Veinberg AD, Roses D (1995) Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol 136:107–122
Moghadasian MH, McManus BM, Nguyen LB, Shefer S, Nadji M, Godin DV, Green TJ, Hill J, Yang Y, Scudamore CH, Frohlich JJ (2001) Pathophysiology of apolipoprotein E deficiency in mice: relevance to ApoE-related disorders in humans. FASEB J 15:2623–2630
Müller WE, Stoll L, Schubert T, Gelbmann CM (1991) Central cholinergic functioning and aging. Acta Psychiatr Scand Suppl 366:34–39
Niccoli T, Partridge L (2012) Ageing as a risk factor for disease. Curr Biol 22:R741–R752
Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L (1998) Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci USA 95:10914–10919
Rajendran P, Spear LP (2004) The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci 1021:441–444
Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, Lewczuk P, Sidiropoulos C, Kneib T, Perneczky R, Doerfler A, Kornhuber J (2011) Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm (Vienna) 118:249–257
Rose JH, Calipari ES, Mathews TA, Jones SR (2013) Greater ethanol-induced locomotor activation in DBA/2J versus C57BL/6J mice is not predicted by presynaptic striatal dopamine dynamics. PLoS One 8:e83852
Roth GS, Joseph JA (1994) Cellular and molecular mechanisms of impaired dopaminergic function during aging. Ann N Y Acad Sci 719:129–135
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav. Brain Res 221:555–563 Review
Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733
Seitz HK, Meydani M, Ferschke I, Simanowski UA, Boesche J, Bogusz M, Hoepker WW, Blumberg JB, Russell RM (1989) Effect of aging on in vivo and in vitro ethanol metabolism and its toxicity in F344 rats. Gastroenterology 97:446–456
Sen A, Nelson TJ, Alkon DL (2017) ApoE isoforms differentially regulates cleavage and secretion of BDNF. Mol Brain 10:19
Spencer RL, Hutchison KE (1999) Alcohol, aging, and the stress response. Alcohol Res Health 23:272–283
Ritzmann RF, Springer A (1980) Age-differences in brain sensitivity and tolerance to ethanol in mice. Age 3:15–17
Swartzwelder HS, Wilson WA, Tayyeb MI (1995) Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res 19:1480–1485
Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, Mezey E (1977) Aging and ethanol metabolism. Clin Pharmacol Ther 21:343–354
White AM, Matthews DB, Best PJ (2000) Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus 10:88–93
Yu BP (1996) Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 21:651–668
Acknowledgements
This work was supported in part by the Grant-in-Aid for Scientific Research [Grand No (c) 22590636, 20590681] from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to declare
Rights and permissions
About this article
Cite this article
Jamal, M., Ito, A., Tanaka, N. et al. The Role of Apolipoprotein E and Ethanol Exposure in Age-Related Changes in Choline Acetyltransferase and Brain-Derived Neurotrophic Factor Expression in the Mouse Hippocampus. J Mol Neurosci 65, 84–92 (2018). https://doi.org/10.1007/s12031-018-1074-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1074-6