Abstract
In a group of older adults with very mild dementia, we aimed to characterize the nature and magnitude of cognitive decline as measured by the Cogstate Brief Battery, in relation to Aβ levels and hippocampal volume. Participants were characterized according to their status on the Clinical Dementia Rating (CDR) scale. A total of 308 individuals who were CDR 0 and had low cerebral Aβ levels (Aβ−), 32 individuals who were Aβ− and CDR 0.5, and 43 individuals who were Aβ+ and CDR 0.5 were included in this study. Participants completed the CogState brief battery at baseline, and at 18-, 36-, 54- and 72-month follow-up. Linear mixed model analyses indicated that relative to the Aβ− CDR 0 group, the Aβ+ CDR 0.5 group showed increased rates of memory decline and hippocampal volume loss. However, compared to the Aβ− CDR 0 group, the Aβ− CDR 0.5 group showed no changes in cognitive function or hippocampal volume over 72 months. The results of this study confirm that in individuals with very mild dementia, who also have biomarker confirmation of Aβ+, changes in cognitive function manifest primarily as deterioration in memory processing, and this is associated with hippocampal volume loss. Conversely, the absence of any cognitive decline or loss in hippocampal volume in individuals with very mild dementia but who are Aβ− suggests that some other non-AD disease process may underlie any static impairment in cognitive function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is now agreement that in non-demented older adults, an elevated level of beta-amyloid (Aβ), determined from positron emission tomography (PET) or cerebral spinal fluid (CSF) sampling, is associated with cognitive decline particularly in episodic memory (Doraiswamy et al. 2014, Fagan et al. 2007, Lim et al. 2014). Such data is consistent with the amyloid cascade hypothesis of Alzheimer’s disease (AD) which proposes that AD begins with Aβ accumulation, which leads to the expression and aggregation of tau, both of which then combine to cause synaptic and neuronal loss (Hardy and Selkoe 2002, Masters and Selkoe 2012). The progressive loss of neurons and neuronal function manifests behaviourally as cognitive deterioration, reduced functional activities of daily living and ultimately dementia.
Older adults, for whom they, or their caregivers, report a noticeable decline in cognitive abilities are now investigated for early dementia. If reported difficulties are corroborated by objective evidence of cognitive impairment (e.g., performance between 1 and 2 standard deviations below the mean of an appropriate control group) on formal cognitive assessment, as well there being no medical or situational information to account for this impairment, individuals can be considered to be in the early stages of dementia (Morris et al. 2001, Petersen 2004). This early stage of dementia is classified clinically as 0.5 on the Clinical Dementia Rating (CDR) scale, and has been termed “very mild dementia” (Morris et al. 2001) or “mild cognitive impairment” (MCI) (Petersen 2004). It has been estimated that individuals with a CDR score of 0.5 have an approximately 35 % risk of progressing to CDR 1 (mild dementia) over 5 years (Morris et al. 2001), and this risk is increased to approximately 80 % in individuals who also have high Aβ (Aβ+) (Rowe et al. 2013). Recent consensus criteria that integrate risk from biomarkers and the amyloid cascade hypothesis stipulate that individuals with a CDR score of 0.5 and who are also Aβ+ can be considered as being in the prodromal stage of AD (Albert et al. 2011). Such criteria also raises the hypothesis that the pathological processes underlying cognitive impairment in individuals who have a CDR score of 0.5 but who have low levels of cerebral Aβ (Aβ−) may reflect neurodegenerative or psychiatric processes other than AD (Albert et al. 2011, Dubois and Albert 2004). However, the nature and magnitude of cognitive decline associated with known biomarkers of AD (i.e., cerebral Aβ and hippocampal volume) in individuals with a CDR score of 0.5 remain poorly defined. In a previous study of MCI, we observed that Aβ+ was associated with a moderate decline in memory (i.e., d ~ 0.5) but a much greater loss of hippocampal volume (d ~ 1.5) over 3 years (Lim et al. 2015a). However, given the short follow-up interval, it is possible that these relative estimates are unreliable and warrant replication over longer periods of assessment.
The aim of this study was to follow prospectively changes in cognition and hippocampal volume over 72 months in Aβ− and Aβ+ adults with a CDR score of 0.5. The first hypothesis was that at baseline, compared to CDR 0 Aβ− older adults and CDR 0.5 Aβ- older adults, CDR 0.5 Aβ+ older adults would show large impairments in memory and working memory, and that this would be accompanied by smaller hippocampal volumes. The second hypothesis was that compared to CDR 0 Aβ− older adults, CDR 0.5 Aβ+ older adults would show faster decline in memory and working memory over 72 months and that this would also be accompanied by increased loss of hippocampal volume. The third hypothesis was that any cognitive decline observed in CDR 0.5 Aβ− older adults would be less than that observed in CDR 0.5 Aβ+ older adults.
Methods
Participants
Participants were recruited from among individuals enrolled in the Australian Imaging, Biomarkers and Lifestyle (AIBL) Flagship Study of Ageing. The process of recruitment and classification of the AIBL cohort has been described in detail previously (Ellis et al. 2009, Rowe et al. 2010). Briefly, individuals who volunteered were excluded from the AIBL study if they had any of the following: schizophrenia; depression (Geriatric Depression Score [GDS] of 6 or greater); Parkinson’s disease; cancer (other than basal cell skin carcinoma) within the last 2 years; symptomatic stroke; uncontrolled diabetes; or current regular alcohol use exceeding two standard drinks per day for women or four per day for men.
All participants enrolled in AIBL underwent a thorough medical and neuropsychological evaluation at each assessment and this information was used to classify the clinical disease stage. The neuropsychological test battery used to measure cognition in the AIBL study has been described in detail previously (Ellis et al. 2009). A clinical review panel chaired by DA reviewed all available medical, psychiatric and neuropsychological information to confirm the cognitive health of individuals enrolled in the CN group. In this clinical review panel, participants’ cognitive and functional status was rated using the CDR scale. For this study, individuals classified as CDR = 0.5 were considered to have very mild dementia or MCI, while those with CDR = 0 were classified as cognitively normal (CN) (Morris 1983). The CDR rating of participants was made without reference to Aβ imaging, and magnetic resonance imaging (MRI) data. The study was approved by and complied with the regulations of the institutional research and ethics committees of Austin Health, St. Vincent’s Health, Hollywood Private Hospital, and Edith Cowan University (Ellis et al. 2009). All participants provided written informed consent prior to participating in the study.
Measures
Amyloid Imaging
Aβ imaging with positron emission tomography (PET) was conducted using either 11C-Pittsburgh Compound B (PiB), 18F-florbetapir or 18F-flutemetamol. PET methodology has been described in detail previously (Clark et al. 2011, Rowe et al. 2010, Vandenberghe et al. 2010). A 30-min acquisition was started 40 min after injection of PiB and 20-min acquisitions were performed 50 min after injection of florbetapir and 90 min after injection of flutemetamol (Clark et al. 2011, Rowe et al. 2010, Vandenberghe et al. 2010). For PiB and flutemetamol, PET standardized uptake value (SUV) data were summed and normalized to the cerebellar cortex SUV, resulting in a region-to-cerebellar ratio termed SUV ratio (SUVr). For florbetapir, SUVr was generated using the whole cerebellum as the reference region. In accord with previous studies, SUVr was classified dichotomously as either low or high. For PiB and flutemetamol, an SUVr threshold ≥1.5 was used. For florbetapir, an SUVr threshold of ≥1.1 was employed. Hippocampal volume (HV) was normalized for head size using total intracranial volume (sum of grey matter, white matter and cerebral spinal fluid volumes) (Dore et al. 2013).
Cognitive Assessments
All participants were assessed with standard clinical rating scales and a comprehensive neuropsychological battery from the AIBL study. All of these measures have been described in detail elsewhere and were administered according to standard protocols by trained research assistants (Ellis et al. 2009, Lim et al. 2012a). In addition to the tests used to inform the rating on the CDR, premorbid intelligence was estimated using the Wechsler Test of Adult Reading (WTAR) and levels of depressive and anxiety symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS). Cognition was measured using the Cogstate brief battery (CBB) (Lim et al. 2012a). This has been described in detail previously but briefly. This battery consists of four computerized tests: the Detection (DET) task is a simple reaction time test shown to measure psychomotor function; the Identification (IDN) task is a choice reaction time test shown to measure visual attention; the One Card Learning (OCL) task is a continuous visual recognition learning task that assesses visual learning within a pattern separation model and the One-Back (OBK) task is a task of working memory and attention (Lim et al. 2012a, b). From these four tests, two composite scores were computed. The Attention/Psychomotor Function composite was computed by standardizing the speed of performance on the Detection and Identification tests and averaging these scores (Maruff et al. 2013). The Learning/Working Memory composite was computed by standardizing the accuracy of performance on the One Card Learning and One Back tests (Maruff et al. 2013). Performance on the CBB tests was not used to determine participants’ clinical status.
Data Analysis
To characterize the nature and magnitude of cognitive impairment in the CDR 0.5 groups, a series of analyses of covariance (ANCOVA) were used to compare the cognitive composite scores and HV between the Aβ− CDR 0, Aβ− CDR 0.5 and Aβ+ CDR 0.5 groups at baseline. In these analyses, the cognitive composite score or HV was entered as the dependent variable and age, premorbid intelligence and GDS scores were added as covariates.
To characterize the nature of magnitude of change in cognition over time, a series of linear mixed effects models (LMM) using an unstructured covariance matrix and maximum likelihood estimation were conducted to examine the relation between group (Aβ− CDR 0, Aβ− CDR 0.5, Aβ+ CDR 0.5) and time (baseline, 18-, 36-, 54- and 72-month assessments) on each cognitive composite score and HV. For each LMM, the cognitive composite score or HV was treated as the dependent variable. Group, time and the interaction between group and time were also specified as fixed factors, and participant was specified as a random factor. Age, premorbid intelligence and depressive symptoms at the first assessment were specified as covariates. Group mean slopes were computed for each cognitive composite score to reflect estimates of the rate of cognitive change over time; and estimates of slope in the Aβ− CDR 0.5 and Aβ+ CDR 0.5 groups were compared to that in the Aβ− CDR 0 group. Differences in slopes were expressed using Cohen’s d.
Results
Demographic and Clinical Characteristics
Statistically significant differences between groups were observed for age, premorbid intelligence and depressive symptoms at baseline (Table 1). The Aβ+ CDR 0.5 group also had a significantly higher number of APOE ε4 carriers when compared to the Aβ− CDR 0.5 and Aβ− CDR 0 groups.
Effect of Aβ on Cognitive Function and Hippocampal Volume at Baseline
At the baseline assessment, there was a significant effect of group membership on both cognitive composites and for hippocampal volume (Table 2). Mean and standard deviation for each cognitive composite and for hippocampal volume for each group are summarized in Table 2. Compared to the Aβ− CDR 0 group, the Aβ− CDR 0.5 group showed statistically significant impairment of a moderate magnitude on both the Psychomotor/Attention composite and the Learning/Working Memory composite (Fig. 1). Compared to the Aβ− CDR 0 group, the Aβ+ CDR 0.5 group also showed statistically significant impairment of a moderate magnitude on both the Psychomotor/Attention composite and the Learning/Working Memory composite (Fig. 1). The Aβ+ CDR 0.5 group also performed significantly worser than the Aβ− CDR 0.5 group on the Learning/Working Memory composite (Fig. 1), despite there being no statistically significant difference in their hippocampal volumes at baseline, d (95 %CI) = 0.06 (−0.62, 0.74) (Table 2). In the overall CDR 0.5 group, no association with HV was observed for the Learning/Working Memory composite (r = 0.20, p = .24) or for the Psychomotor/Attention composite (r = −0.10, p = .58).
Effect of Aβ on Cognitive Function and Hippocampal Volume over 72 Months
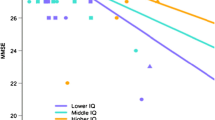
Significant group x time interactions were observed only for the Cogstate Learning/Working Memory composite and hippocampal volume (Table 3). Table 3 also provides a summary of the group mean slopes for each cognitive composite and for hippocampal volume for each Aβ group. When compared to the Aβ− CDR 0 group, the Aβ+ CDR 0.5 group showed a significantly increased rate of decline on the Learning/Working Memory composite (Fig. 2b), and hippocampal volume (Fig. 2c) and the magnitude of these differences were moderate-to-large (Fig. 3). The Aβ+ CDR 0.5 group did not differ significantly from the Aβ− CDR 0 group on their rate of change on the Psychomotor/Attention composite (Fig. 2a, Fig. 3). Compared to the Aβ− CDR 0 group, the Aβ− CDR 0.5 group did not differ in their rate of change on any cognitive composite, nor did they differ on the rate of change in hippocampal volume (Fig. 2c).
Discussion
The first hypothesis that at baseline, compared to CDR 0 Aβ− older adults and CDR 0.5 Aβ− older adults, CDR 0.5 Aβ+ older adults would show a large impairment in learning and working memory, and that this would be accompanied by smaller hippocampal volume was supported. Older adults who had met criteria for very mild dementia or MCI, based on their AIBL assessment showed impairment in learning and working memory of a magnitude that was by convention large (~0.8), which is accompanied by smaller hippocampal volume, of a large magnitude (Fig. 1). In contrast, this same group showed only a subtle impairment (~0.4) in attention and psychomotor function. Interestingly, the pattern of impairment in older adults who had a CDR score of 0.5 but who were Aβ− was qualitatively opposite to this, with moderate and statistically significant impairment in attention and psychomotor function and only a small and non-significant impairment in learning and working memory Fig. 1). This difference persisted despite this group meeting the AIBL consensus clinical criteria for MCI based on medical and neuropsychological workup (Ellis et al. 2009). Similarly, no differences in hippocampal volume were observed between CDR 0 Aβ−older adults and CDR 0.5 Aβ− older adults (Fig. 1). Thus, at baseline the Aβ+ CDR 0.5 group showed the cognitive profile characteristic of early AD with impairments in memory and lower HV. In contrast, the Aβ− CDR 0.5 group showed a qualitatively different cognitive profile, and no evidence of hippocampal volume loss when compared to the CDR 0 Aβ− group (Fig. 1).
The second hypothesis that compared to CDR 0 Aβ− older adults, CDR 0.5 Aβ+ older adults would show increased cognitive decline in learning and working memory over 72 months, and that this would also be accompanied by increased loss of HV support. In the Aβ+ CDR 0.5 group, performance on measures of learning and working memory deteriorated substantially across the 6 years, however, in the Aβ− CDR 0 group, there was no deterioration in memory function at all (Fig. 2b). The difference in the rate at which learning and working memory performance over time worsened in the Aβ+ CDR 0.5 group compared to the Aβ− CDR 0 group was, by convention, moderate (d ~ 0.5). Further, this decline in learning and working memory was accompanied by a substantial loss of HV over the same time (Fig. 2c). Hippocampal volume, in the Aβ+ CDR 0.5 group continued to deteriorate over the 72 months, while individuals in the Aβ− CDR 0 group showed very subtle, and non-significant loss of HV over the same period. The magnitude of the difference between these two groups in the rate at which HV deteriorated over time was very large (d ~ 1.7). In contrast to the decline observed in learning and working memory and in hippocampal volume, no decline was observed for the measure of psychomotor function and attention in the Aβ+ CDR 0.5 group (Fig. 2a). This suggests strongly that in the Aβ+ CDR 0.5 group, the memory decline observed was not an indirect result of any impairment in attentional function.
The third hypothesis that any cognitive decline observed in CDR 0.5 Aβ− older adults would be less than that observed in CDR 0.5 Aβ+ older adults was supported. Although the CDR 0.5 Aβ− group showed impairment in attention and psychomotor function at their baseline assessment, this group showed no further decline in these functions over the subsequent 72 months (Fig. 2a). Similarly, there was no decline in learning and working memory over the same period (Fig. 2b). Instead, there was some indication that over 72 months, the Aβ− CDR 0.5 group showed some improvement in learning and working memory. In fact, at the 72-month assessment, performance on the Learning/Working Memory composite was better than that in the Aβ− CDR 0 group (Fig. 2b). Finally, the Aβ− CDR 0.5 group also showed no further reduction in HV over time (Fig. 2c). Consequently, differences between the CDR 0 Aβ− and CDR 0.5 Aβ− groups in the rate of change in cognitive function over 72 months and in the rate of HV loss over the same time were small and not statistically significant (Fig. 2c). The absence of any progressive loss in cognitive function or HV suggests that the cognitive impairment that accorded with the blinded clinical diagnosis of MCI by the AIBL clinical review panel was not the consequence of a neurodegenerative disease process. One hypothesis is that the cognitive deficit in the CDR 0.5 Aβ−group may reflect a static injury or disruption that has occurred in the past. Alternatively, the cognitive impairment observed in the CDR 0.5 Aβ− group may reflect some neurodegenerative disease process that is much slower than that related to Aβ (Dubois and Albert 2004). If this is the case, then, it is likely that individuals will gradually accumulate Aβ to a level that would be considered abnormal (e.g., PiB-PET SUVR >1.5) and once that threshold has reached, cognitive decline and faster loss of HV will become evident.
When considered together, the results of the current study show that in individuals with very mild dementia, there is substantial loss of higher cognitive functions such as memory and working memory. This loss of cognition is accompanied by a loss of brain volume, which in the current study was determined from the hippocampus. It is important to note that while individuals in the current study were classified as having very mild dementia, their average score on the MMSE was 27, indicating that despite being symptomatic, the severity of clinical symptoms was very mild. Despite this, the rate of loss of memory and working memory functions were substantial. The results presented here are consistent with, and extend, those reported over 36 months (Lim et al. 2015a), suggesting that even with shorter assessment intervals, the estimates of memory decline and hippocampal volume loss remained reliable. When taken together, these data also show that very mild dementia is a suitable disease stage for interventions designed to halt or slow disease progression prior to the development of dementia (Golde et al. 2011). The data reflecting the loss of higher cognitive function and loss of HV in the CDR 0.5 Aβ+ older adults can be considered to reflect the nature of disease progression in untreated very mild dementia and therefore could be utilized in statistical models aimed at determining sample sizes for clinical trials of licenced or novel drugs for AD.
When interpreting the results of this study, there are several important caveats to note. First, participants enrolled in the AIBL study were not recruited through epidemiological sampling methods, and participants with memory complaints were recruited primarily through memory clinics and referrals from their primary care physicians. As such, it will be important for the estimates of cognitive decline reported here to be replicated and extended in more population-based samples, such as the Mayo Clinic Study of Ageing (Roberts et al. 2008). Second, we were unable to investigate the effect of APOE ε4 carriage on Aβ related cognitive decline in this population of older adults. This was primarily due to the low number of APOE ε4 carriers in the CDR 0.5 Aβ− group (n = 6). The observation that the number of APOE ε4 carriers was low in the CDR 0.5 Aβ− group is consistent with clinical studies that have shown that APOE ε4 is associated with increased Aβ levels (Morris et al. 2010, Rowe et al. 2010). However, as a number of recent studies have now shown that APOE ε4 can increase the rate of memory decline and the rate of clinical disease classification in Aβ+ cognitively normal older adults (Lim et al. 2015b, Mormino et al. 2014, Petersen et al. 2016), it will be important for future studies with larger samples of older adults with MCI or very mild dementia to determine whether this exacerbation of memory decline in Aβ+ cognitively normal older adults similarly extends to individuals who have evidence of some cognitive impairment.
These caveats notwithstanding, the results of this study, along with those from previous studies, confirm that in individuals who have been classified clinically as being in the very early stages of AD and who have biomarker confirmation of Aβ, changes in cognitive function manifest primarily as deterioration in memory processing (Braak and Braak 1991, Knopman et al. 2003). Further, the gradual loss of hippocampal volume observed in this study is also consistent with neurobiological studies showing the predilection of early AD pathological processes for medial temporal lobe structures (Dickerson and Sperling 2008, Dore et al. 2013, Jack et al. 2009). Finally, this study provides additional evidence that the combination of AD biomarkers and sensitive cognitive assessments are important in the identification of individuals in the very early stage of AD who may be appropriate targets of clinical trials of anti-amyloid therapies seeking to modify or alter the course of cognitive deterioration in AD.
References
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging and Alzheimer's Association workgroup. Alzheimers Dement 7(3):270–279
Braak H, Braak E (1991) Neuropathological staging of Alzheimer–related changes. Neuropathologica 82(4):239–259
Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman EP, Zehntner SP, Skovronsky DM, Group A-AS (2011) Use of florbetapir–PET for imaging beta–amyloid pathology. J Am Med Assoc 305:275–283
Dickerson BC, Sperling RA (2008) Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia 46(6):1624–1635
Doraiswamy PM, Sperling RA, Johnson K, Reiman EM, Wong TZ, Sabbagh MN, Sadowsky CH, Fleisher AS, Carpenter A, Joshi AD, Lu M, Grundman M, Mintun MA, Skovronsky DM, Pontecorvo MJ, Group A-AS (2014) Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry 19(9):1044–1051
Dore V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetelat G, Zhou L, Martins R, Ellis KA, Masters CL, Ames D, Salvado O, Rowe CC (2013) Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer's disease. JAMA. Neurology 70(7):903–911
Dubois B, Albert ML (2004) Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurol 3(4):246–248
Ellis KA, Bush AI, Darby D, De Fazio D, Foster J, Hudson P, Lautenschlager NT, Lenzo N, Martins RN, Maruff P, Masters C, Milner A, Pike K, Rowe C, Savage G, Szoeke C, Taddei K, Villemagne V, Woodward M, Ames D, Group TAR (2009) The Australian imaging, biomarkers and lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr 21:672–687
Fagan AM, Roe CM, Xiong CJ, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64:343–349
Golde TE, Schneider LS, Koo EH (2011) Anti-Aβ therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron 69(2):203–213
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner MW, Petersen RC, Initiative, T.A.s.D.N (2009) Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 132:1355–1365
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62(11):1087–1095
Lim YY, Ellis KA, Harrington K, Ames D, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Darby D, Maruff P, AIBL RG (2012a) Use of the CogState brief battery in the assessment of Alzheimer's disease related cognitive impairment in the Australian imaging, biomarker and lifestyle (AIBL) study. J Clin Exp Neuropsychol 34(4):345–358
Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Villemagne VL, Maruff P, AIBL RG (2012b) Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology 79:1645–1652
Lim, Y.Y., Maruff, P., Pietrzak, R.H., Ames, D., Ellis, K.A., Harrington, K., Lautenschlager, N.T., Szoeke, C., Martins, R.N., Masters, C.L., Villemagne, V.L., Rowe, C.C., AIBL, R.G. 2014. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease. Brain 137, 221–231. doi:doi:10.1093/brain/awt286.
Lim YY, Pietrzak RH, Bourgeat P, Ames D, Ellis KA, Rembach A, Harrington K, Salvado O, Martins RN, Snyder PJ, Masters CL, Rowe CC, Villemagne VL, Maruff P (2015a) Relationships between performance on the Cogstate brief battery, neurodegeneration, and Aβ accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol 30(1):49–58
Lim YY, Villemagne VL, Pietrzak RH, Ames D, Ellis KA, Harrington K, Snyder PJ, Martins RN, Masters CL, Rowe CC, Maruff P (2015b) APOE ε4 moderates amyloid-related memory decline in preclinical Alzheimer's disease. Neurobiol Aging 36(3):1239–1244 javascript:void(0)
Maruff P, Lim YY, Darby D, Ellis KA, Pietrzak RH, Snyder PJ, Bush AI, Szoeke C, Schembri A, Ames D, Masters CL, AIBL RG (2013) Clinical utility of the Cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer's disease. BMC Pharmacol Toxicol 1(30):1–11
Masters CL, Selkoe DJ (2012) Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. In: Selkoe DJ, Mandelkow E, Holtzman DM (eds) Biology of Alzheimer disease. Cold Spring Harbor Laboratory Press, New York, pp. 181–204
Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, Rentz DM, Johnson KA, Sperling RA (2014) Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer's disease. Neurology 82(20):1760–1767
Morris JC (1983) The clinical dementia rating (CDR): current version and scoring rules. Neurology 43:2412–2414
Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L (2001) Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 58:397–405
Morris JC, Roe CM, Xiong CJ, Fagan AM, Goate AM, Holtzman DM, Mintun MA (2010) APOE predicts Ab but not tau Alzheimer's pathology in cognitively normal aging. Ann Neurol 67(1):122–131
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194
Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, Lowe VJ, Knopman DS, Pankratz VS, Machulda MM, Geda YE, Jack CRJ (2016) Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA. Neurology 73(1):85–92
Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA (2008) The Mayo Clinic study of aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30(1):58–69
Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O'Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins RM, C.L., Ames, D., Villemagne, V (2010) Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging 31:1275–1283
Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R, Jones G, Maruff P, Woodward M, Price R, Robins P, Tochon-Danguy H, O'Keefe G, Pike KE, Szoeke C, Salvado O, Macaulay SL, O'Meara T, Head R, Cobiac L, Martins R, Masters CL, Ames D, Villemagne VL, AIBL RG (2013) Predicting Alzheimer disease with β-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol 74(6):905–913
Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, Minthon L, Garraux G, Nelissen N, Bormans G, Buckley C, Owenius R, Thurfjell L, Farrar G, Brooks DJ (2010) 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 68:319–329
Acknowledgments
Alzheimer’s Australia (Victoria and Western Australia) assisted with promotion of the study and the screening of telephone calls from volunteers. The AIBL team wishes to thank the clinicians who referred patients with MCI or AD to the study: Associate Professor Brian Chambers, Professor Edmond Chiu, Dr. Roger Clarnette, Associate Professor David Darby, Dr. Mary Davison, Dr. John Drago, Dr. Peter Drysdale, Dr. Jacqueline Gilbert, Dr. Kwang Lim, Professor Nicola Lautenschlager, Dr. Dina LoGiudice, Dr. Peter McCardle, Dr. Steve McFarlane, Dr. Alastair Mander, Dr. John Merory, Professor Daniel O’Connor, Dr. Ron Scholes, Dr. Mathew Samuel, Dr. Darshan Trivedi and Associate Professor Michael Woodward. We thank all those who participated in the study for their commitment and dedication to helping advance research into the early detection and causation of AD.
Funding
Funding for the study was provided in part by the study partners [Commonwealth Scientific Industrial and research Organization (CSIRO), Edith Cowan University (ECU), Mental Health Research institute (MHRI), National Ageing Research Institute (NARI) and Austin Health, CogState Ltd.]. The study also received support from the National Health and Medical Research Council (NHMRC) and the Dementia Collaborative Research Centres program (DCRC2), as well as funding from the Science and Industry Endowment Fund (SIEF) and the Cooperative Research Centre (CRC) for Mental Health, an Australian Government Initiative. YYL is currently funded by the National Health & Medical Research Council-Australian Research Council (NHMRC-ARC) Dementia Research Development Fellowship (APP1111603).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
YYL, SML, CF, SRS and OS report no disclosures. CLM is an advisor to Prana Biotechnology Ltd. and a consultant to Eli Lilly. PM is a full-time employee of Cogstate Ltd. RHP and PJS are scientific consultants to Cogstate Ltd. DA has served on scientific advisory boards for Novartis, Eli Lilly, Janssen and Pfizer Inc. RNM is a consultant to Alzhyme. C.C.R. has served on scientific advisory boards for Bayer Pharma, Elan Corporation, GE Healthcare and AstraZeneca; has received speaker honoraria from Bayer Pharma and GE Healthcare and has received research support from Bayer Pharma, GE Healthcare, Piramal Lifesciences and Avid Radiopharmaceuticals. VLV served as a consultant for Bayer Pharma and received research support from a NEDO grant from Japan.
Rights and permissions
About this article
Cite this article
Lim, Y.Y., Villemagne, V.L., Laws, S.M. et al. Performance on the Cogstate Brief Battery Is Related to Amyloid Levels and Hippocampal Volume in Very Mild Dementia. J Mol Neurosci 60, 362–370 (2016). https://doi.org/10.1007/s12031-016-0822-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-016-0822-8