Abstract
Microglia, the major immune cells of the central nervous system (CNS), can be driven to adopt M1 and M2 phenotypes. Recently, the distinct functions of M1 and M2 microglia have been intensively studied. M1-activation microglia are pro-inflammatory and may contribute to the development of several CSN disorders, while M2-activation microglia are anti-inflammatory and may promote tissue reconstruction. TRAM1 is a protein involved in translocation of nascent polypeptides and functions as a sorting adaptor of TLR4. Here, we found that TRAM1 plays an important role in microglia M1 polarization. Our results showed that the expression of TRAM1 is highly induced in LPS/interferon (IFN)-γ-stimulated BV2 cells and primary microglia cells. Flag-TRAM1 transfection, but not Flag-GFP used as a control, significantly enhanced M1 polarization by strongly increasing expression of M1 makers, such as IL-6, IL-1β, iNOS, and so on. Silence of TRAM1 effectively inhibited LPS/IFN-γ-induced expression of M1-related genes in BV2 cells. In addition, TRAM1 was found to cooperate with TLR4 to induce an M1 genetic program in Flag-TRAM1-transfected and LPS/IFN-γ-induced BV2 cells. TRAM1 is essential for LPS/IFN-γ induced expressions of adapter molecule (IRAK1, phosphorylation of TBK1, and IRF3) of TLR4. TRAM1 is also essential for phosphorylation of IκB and P65 and for P65-NF-kB translocation to nucleus. Overall, our findings showed that TRAM1 could promote microglia M1 polarization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia are resident immune cells of the central nervous system (CNS) which possess the ability to react to diverse physiological and pathological stimuli (Hanisch and Kettenmann 2007; David and Kroner 2011). In normal brains, microglia exist as a “resting” phenotype and play the role of immune supervision (Tremblay et al. 2011). However, activated microglia have different functions in several CNS neurodegenerative pathologies covering Alzheimer’s disease (AD) (Colton et al. 2006; Shimizu et al. 2008; Colton and Wilcock 2010), Parkinson’s disease (PD) (Ponomarev et al. 2007, 2011), and multiple sclerosis (MS) (Smith 2008; Rojo et al. 2010). Because of different properties of microglia, there is increasing debate on whether the response of microglia is beneficial or detrimental for tissue repair (David and Kroner 2011). Previous study has found that like peripheral macrophage, microglia exhibit a high morphological and functional plasticity (Hanisch and Kettenmann 2007; Ajmone-Cat et al. 2013). Under various stimulations, microglia can be polarized into different phenotypes, the M1 and M2 (Lawrence and Natoli 2011). Stimulated by lipopolysaccharides (LPS) accompanied by interferon (IFN)-γ, M1-polarized microglia over-express lots of inflammatory cytokines including interferon (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, and inducible nitric oxide synthase (iNOS), which are involved in cytotoxicity and microbial killing (Verreck et al. 2004; Gwack et al. 2007). However, under the treatment with IL-4, M2-polarized microglia can upregulate chitinase 3-like 3 (Chi3l3, Ym1), arginase-1 (Arg1), and IL-10 which are related to neuroprotection (Mantovani et al. 2002; Martinez et al. 2009). The difference of the functions of M1 and M2 microglia has offered an important tool for studying the mechanism of the inflammatory process in the central neural system. However, till now, little is known about molecular mechanisms that regulate microglia polarization.
Increasing study have shown that Toll-like receptors (TLRs) are a family of pathogen recognition receptors (PRRs) that play an important role in the innate immune system by triggering pro-inflammatory pathways in response to microbial pathogens such as LPS (Wang et al. 2009). TLR4 especially has been reported to play an important role in initiating the M1 polarization of microglia and is necessary for activated microglia to induce neuronal injury in vivo (Verstak et al. 2009). When activated, TLR4 recruits distinct of intracellular adaptor proteins including translocation associated membrane protein 1 (TRAM1) and then initiates diverse downstream signaling, resulting in activation of nuclear factor-κB (NF-κB), and then promotes the release of downstream pro-inflammatory cytokines (Akira and Takeda 2004). TRAM-1 belongs to steroid receptor coactivator-3 (SRC-3) subtype. Previous study showed that TRAM1 is involved in translocation of nascent polypeptides and the dislocation of a type I membrane protein under viral influences (Ng et al. 2010). However, its role in microglia polarization is still with limited acquaintance.
Here, our results confirm that TRAM1 plays an essential role in the M1 polarization of microglia. In particular, TRAM1 increases M1-related gene expression. Mechanistically, we demonstrated for the first time that TRAM1 regulates microglia M1 polarization through TLR4-mediated NF-κB activation via a TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent mechanism.
Materials and Methods
Reagents and Antibodies
LPS was obtained from Sigma-Aldrich (St. Louis, MO, USA). Cytokines IL-4 and IFN-γ were obtained from Stemcell Technologies (Vancouver, BC, Canada). Antibodies anti-TLR4, anti-TRAM1, anti-iNOS, anti-CD86, anti-Rab5, anti-TRIF, anti-TBK1, anti-phospho-TBK1, anti-IRF3, anti-phospho-IRF3, anti-IκB, anti-phosphor-IκB, anti-NF-kB-P65, and anti-phospho-NF-kB-P65 were purchased from Santa Cruz Biotechnology (1:500; Santa Cruz, CA). Anti-GAPDH antibody (1:1000) was purchased from Sigma.
Cell Culture and Treatments
Microglia secondary cultures were prepared from 10 to 14 day mixed primary glial cultures. Zero to 24-h-old C57BL/6 mice pups, which were purchased from the Experimental Animal Center of Nantong University, were rendered hypothermic and decapitated and cerebral cortex dissected out, as previously described. Briefly, microglia cells were detached by mild shaking, plated on uncoated well plates or coverslides at a density of 1.25 × 105 cells/cm2, allowed to adhere for 20 min, and washed to remove nonadhering cells. After incubation for 24 h, the medium was replaced with fresh medium, containing the stimulating agents. All animal procedures in this study were admitted by the Department of Animal Center, Medical College of Nantong University. All animal protocols were approved by the Institutional Animal Care and Use Committee according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and guidelines of the International Association for the Study of Pain (Zimmermann 1983).
The murine microglia cell line BV2 was cultured in DMEM that was supplemented with 10 % heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and 0.1 % antibiotics (penicillin and streptomycin). Cultures were kept at 37 °C in 5 % CO2/95 % humidified air atmosphere. Prior to treatment, cells were washed with phosphate-buffered saline (PBS) and serum-free medium was added.
For mRNA and protein analysis, primary microglia were cultured with DMEM/F12 media to generate M0 microglia, or cultured with DMEM/F12 including 100 ng/ml LPS and 10 ng/ml INF-γ for 12 h to generate M1 microglia, or cultured in DMEM/F12 media including 10 ng/ml IL-4 for 36 h to generate M2 microglia. BV2 cells were cultured with DMEM media to generate M0 microglia, or including 100 ng/ml LPS and 10 ng/ml INF-γ for 12 h to generate M1 microglia, or cultured in DMEM media including 10 ng/ml IL-4 for 36 h to generate M2 microglia.
Quantitative Reverse Transcription-PCR
Total RNA was isolated from the frozen specimens. The cDNA synthesis was carried out with RevertAidTM RT Kit (Fermentas) in a 25-μl reaction volume according to the manufacturer’s protocol. A specific cDNA fragment was amplified by Taq DNA polymerase (Life Technologies) using specific primers for Tram1 and GAPDH. The primer sequences used were as follows: TRAM1 (F) 5′-CTGGTCAGCTTAGTGCGTTC-3′and (R) 3′-CTCCAGCAATGTGGAAGAGG-5′ and GAPDH (F) 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and (R) 5′-TCCTTGGAGGCCATGTGGGCCAT-3′. PCR (25 cycles) was performed as follows: denaturation for 30 s, annealing at 58 °C for 40 s, and elongation at 72 °C for 40 s. PCR products were electrophoresed through 1.5 % agarose gel containing ethidium bromide (0.5 μg/ml). Gels were visualized under UV light and photographed, and optical densities of the bands were analyzed with Molecular Dynamics densitometer (Scion).
Double Immunofluorescence Staining
For in vitro immunocytochemistry, microglia were plated in 24-well plates. After 2 days, the cultures were switched to serum-free DMEM (HyClone) for 36 h, or exposed to 100 ng/ml LPS and 10 ng/ml INF-γ for 12 h, or exposed to 10 ng/ml IL-4 for 36 h. Washed three times for 5 min each in cold PBS, cells were fixed at 4 °C for 20 min with precooled PBS that contained 4 % formaldehyde, permeabilized with 0.1 % Triton X-100 for 10 min, and then blocked by 1 % BSA for 2 h. After washing in PBS, the cells were incubated at 4 °C overnight with anti-TRAM1and anti-Rab5 antibody at 1:200 dilutions, followed by incubation with corresponding TRAM1 and Rab5 secondary antibodies at 1:1000. Nuclei were counterstained using 4′,6-diamidino-2-phenylindole (DAPI, Roche). Phase contrast and fluorescence images were captured using the Leica AF6000 imaging system.
Enzyme-Linked Immunosorbent Assay
The BV2 cells were cultured with DMEM media to generate M0 microglia, or including 100 ng/ml LPS and 10 ng/ml INF-γ for 12 h to generate M1 microglia, or cultured in DMEM media including 10 ng/ml IL-4 for 36 h to generate M2 microglia. The concentrations of IL-6, IL-1β, and TNF-α in different culture supernatants were determined using enzyme-linked immunosorbent assay (ELISA) kit (BioSource Europe, SA). The assays were performed according to the manufacturer’s protocol.
Small Interfering RNA, Flag-TRAM1, and Transfection
For over-expression and knockout of TRAM1, a custom-designed Flag-TRAM1 and siRNA was chemically synthesized and annealed (GeneChem, Shanghai, China). TRAM1 siRNA, control small interfering RNA (siRNA) and Flag-TRAM1 control expression vector which were transfected by Lipofectamine 2000 (Invitrogen, Shanghai, China) and Plus reagents in OptiMEM (Invitrogen, Shanghai, China) as suggested by the manufacturer. Twenty hours after transfection of siRNA and Flag-TRMA1, LPS/INF-γ was added to treat the BV2 cells for 12 h or IL-4 was added to treat for 36 h.
Coimmunoprecipitation
The physical interaction of TRAM1 with TLR4 was assessed in coimmunoprecipitation assays. BV2 cells were transfected with plasmids encoding Flag-TRAM1. Twenty-four hours after transfection, cells were stimulated with LPS/IFN (10 ng/ml) for 12 h. TRAM1S containing complexes were immunoprecipitated using the Flag antibody (Sigma), resolved on a denaturing gel and immunoblotted for TLR4 (1:500; Santa Cruz, CA).
Preparation of Cytosolic and Nuclear Extracts
Cells were lysed on ice for 20 min in cytosolic buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 2 mM dithiothreitol), after which NP-40 was added to a final concentration of 1 %, and the cells were then vortexed for 10 s. Nuclei were sedimented by 30 s of centrifugation at 13,000×g, and the supernatant containing the cytoplasmic fraction was snapfrozen. The pellet containing the nuclei was resuspended in cytosolic buffer with 400 mM NaCl-1 % NP-40 and rotated at maximum speed on a wheel at 4 °C for 15 min. The samples were then centrifuged at 4 °C for 5 min at 13,000×g. The supernatant containing the nuclear fraction was snapfrozen and kept at −70 °C.
Western Blot Analysis
After the indicated treatments, cells were washed three times with cold PBS and lysed in a cell lysis buffer for 30 min on ice. The lysates were then centrifuged at 12,000 rpm, at 4 °C for 15 min. The protein concentration of the supernatant obtained was measured by the Bradford assay (Bio-Rad, Hercules, CA, USA), and equal amounts of supernatant from each sample were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membrane was blocked in 20 mM Tris HCl, 137 mM NaCl, and 0.1 % Tween 20 (pH 7.4) containing 5 % nonfat milk for 2 h at room temperature. Then, the membranes were immunoblotted with primary antibodies for 6–8 h followed by incubation with a horseradish peroxidase secondary antibody (1:2000; SouthernBiotech) for 1–2 h. They were then visualized using an enhanced chemiluminescence system (ECL; Pierce Company, Woburn, MA, USA). ImageJ (NIH) was used to analyze the densities of the bands.
Statistical Analyses
All data were analyzed with Stata 7.0 statistical software. All values were expressed as mean ± SEM. One-way ANOVA followed by Tukey’s post hoc multiple comparison tests was used for statistical analysis. P values less than 0.05were considered statistically significant. Each experiment consisted of at least three replicates per condition.
Results
The Expression of TRAM1 Is Highly Increased in LPS/IFN-γ-Induced Microglia Cells
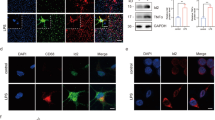
Previous reports showed that TLR4 agonist LPS, IFN-γ, and TNF-a can induce microglia M1 polarization, while IL-4 and IL-13 can lead to microglia M2 polarization (Chhor et al. 2013). Our results showed that the expressions of iNOS, TNF-α, and IL-6, which are markers of the M1 phenotype, were highly increased by LPS/IFN-γ but not by IL-4 stimulation in microglia (Fig. 1a). TRAM1 reportedly function as a sorting adaptor to recruit TRIF to TLR4 that controls the initiation of the TRIF-dependent signaling pathway from an endosomal compartment (Kagan et al. 2008). To determine whether the expression of TRAM1 is related to microglia polarization, we performed real-time PCR and Western blot analysis for TRAM1 in BV2 cells and primary microglia cells after stimulation with the well-established M1-polarizing agent LPS/IFN-γ and well-established M2-polarizing agent IL-4. In BV2 cells, LPS/IFN-γ increased the expression of TRAM1 at both messenger RNA (mRNA) and protein levels (Fig. 1b). Consistent results were found in primary microglial cells (Fig. 1c). To further determine the expression and subcellular localization of TRAM1, we performed double immunofluorescence staining analysis. Our results revealed that LPS/IFN-γ highly increased the expression of TRAM1 in cultured BV2 cells and TRAM1 was present at both the plasma membrane and in Rab5+ early endosomes (Fig. 1d). Altogether, the expression of TRAM1 is highly increased by well-established M1-polarizing agent LPS/IFN-γ.
The expression of TRAM1 is highly increased in LPS/IFN-γ-induced microglia cells. a BV2 cells were treated with LPS (100 ng/ml) plus IFN-γ (10 ng/ml)for 12 h and IL-4 (10 ng/ml) for 36 h. The protein levels of iNOS were detected by Western blot and the expression of selected M1 genes (TNF-α, IL-6) were assessed by ELISA (*P < 0.01 vs. untreated control or IL-4, n = 5). b BV2 cells were treated with LPS (100 ng/ml) plus IFN-γ (10 ng/ml) for 12 h and IL-4 (10 ng/ml) for 36 h. The mRNA and protein levels of TRAM1 were determined by real-time PCR or Western blot analysis (*P < 0.05 vs. untreated control or IL-4, n = 4). c Murine primary microglia cells were treated with LPS (100 ng/ml) plus IFN-γ (10 ng/ml) for 12 h and IL-4 (10 ng/ml) for 36 h. The mRNA and protein levels of TRAM1 were determined by real-time PCR or Western blot analysis (*P < 0.05 vs. untreated control or IL-4, n = 4). d BV2 cells were treated with LPS (100 ng/ml) plus IFN-γ (10 ng/ml) for 12 h and IL-4 (10 ng/ml) for 36 h. Immunofluorescence was used to detect the subcellular localization of TRAM1 (green) and Rab5+ (red). The data were the representative images from three independent experiments. Scale bars, 20 μm
TRAM1 over-Expression Increases the Expression of iNOS and CD86 as well as the Production of Cytokine IL-6, IL-1β and TNF-α
As the TRAM1 expression level was significantly higher in LPS/IFN-γ-induced microglia cells, we further explored whether TRAM1 influences the M1 microglia polarization. Over-expression of TRAM1 in BV2 cells was conducted through Flag-TRAM1 plasmid transfection with Flag-GFP as a control. Briefly, BV2 cells were infected with either Flag-GFP or Flag-TRAM1 for 24 h prior to treatment with LPS/IFN-γ for 12 h. The protein and mRNA levels of TRAM1 were detected by Western blot and real-time PCR analysis. Compared with those in Flag-GFP BV2 cells, TRAM1 mRNA and protein levels in Flag-TRAM1 BV2 cells were markedly upregulated before LPS/IFN-γ stimulation (Fig. 2a). The increased expression of TRAM1 may further regulate microglial polarization. To test this hypothesis, we next determined how TRAM1 influences M1-related gene expression in Flag-TRAM1 BV2 cells. Compared with those in Flag-GFP BV2 cells, iNOS protein and mRNA levels in Flag-TRAM1 BV2 cells were markedly upregulated after LPS/IFN-γ stimulation (Fig. 2b). Moreover, TRAM1 over-expression significantly elevated the expression of several other important M1 markers, such as IL-6, IL-1β, and TNF-α, which were analyzed through ELISA (Fig. 2c, d, e). Consistently, the expression level of CD86 was also highly increased in Flag-TRAM1 BV2 cells than in Flag-GFP BV2 cells (Fig. 2f). Altogether, TRAM1 increases the expression of iNOS and CD86 as well as the production of M1-related cytokine IL-6, IL-1β, and TNF-α.
TRAM1 increases the expression of iNOS and CD86 as well as the production of cytokine IL-6, IL-1β, and TNF-α. BV2 cells were infected with either Flag-GFP or Flag-TRAM1 for 24 h prior to treatment with LPS/IFN for 12 h. a The protein levels of TRAM1 in BV2 cells transfected with either Flag-GFP or Flag-TRAM1 were detected by Western blot and quantification of TRAM1 levels in the indicated groups. b, f Protein levels of iNOS and CD86 were determined by Western blot analysis and quantification of iNOS and CD86 levels in the indicated groups. c–e the expression of selected M1-related genes (TNF-α, IL-6, and IL-1β) were assessed by ELISA (*P < 0.05 between groups; #P < 0.05 vs. within groups; n = 3–5)
Silence of TRAM1 Inhibits the Expression of M1-Related Markers in LPS/IFN-γ-Induced BV2 Cells
To further characterize the role of TRAM1 in microglia M1 and M2 polarization, we synthesized siRNA specifically against TRAM1 (si-TRAM1) and examined the effect of TRAM1 silence on M1 microglia polarization. BV2 cells were transfected with scrambled control siRNA (si-Ctrl) or si-TRAM1 for 24 h prior to treatment with LPS/IFN-γ for 12 h. We found that siRNA-TRAM1c effectively decreased the protein level of TRAM1 in BV2 cells compared with other si-TRAM1s and scrambled control siRNA (si-Ctrl) (Fig. 3a). Thus, we employed si-TRAM1 in the following experiments. Interestingly, TRAM1 silence effectively blocked the upregulation of iNOS triggered by LPS/IFN-γ in BV2 cells (Fig. 3b). Moreover, siRNA-TRAM1 largely decreased the LPS/IFN-γ-induced expression of M1-related markers mentioned above, including IL-6, IL-1β, and TNF-α (Fig. 3c, d, e). In addition, siRNA-TRAM1 also effectively blocked the upregulation of CD86 triggered by LPS/IFN-γ in BV2 cells (Fig. 3f). All these results show that silencing TRAM1 can hamper LPS/IFN-stimulated M1 microglia activation.
Silence of TRAM1 inhibits the expression of M1-related markers in LPS/IFN-γ-induced BV2 cells. BV2 cells were transected with scrambled control si-RNA (si-Ctrl) or si-TRAM1 for 24 h prior to treatment with LPS/IFN for12h. a The protein levels of TRAM1 in BV2 cells transfected with nonspecific si-RNA or TRAM1 si-RNA were detected by Western blot and quantification of TRAM1 levels in the indicated groups. b, f Protein levels of iNOS and CD86 were determined by Western blot analysis and quantification of iNOS and CD86 levels in the indicated groups. c–e The expression of selected M1 genes (TNF-α, IL-6, and IL-1β) were assessed by ELISA (*P < 0.05 between groups; #P < 0.05 vs. within groups; n = 3–5)
Effect of TRAM1 on Activation of TLR4-Dependent Pathway and NF-κB Nuclear Translocation in LPS/IFN-γ-Induced BV2 Cells
TLR4 has been shown to play an important role in initiating the inflammatory response, and myeloid differentiation factor 88 (MyD88), an adapter protein for TLR4, leads to the activation of downstream NF-κB and the subsequent production of proinflammatory cytokines implicated in neurotoxicity (Wang et al. 2009) TRAM is targeted to the plasma membrane by myristoylation, and the present model of TRAM function is that it recruits TRIF to the plasma membrane, where TLR4 is located (Oshiumi et al. 2003; Rowe et al. 2006). Therefore, we investigated whether TRAM1 affected the polarization of microglia through the TLR4-dependent pathway in LPS/IFN-γ-induced BV2 cells. BV2 cells were infected with either Flag-GFP or Flag-TRAM1 for 24 h prior to treatment with LPS/IFN-γ for 12 h. Then, the cell lysate was immunoprecipitated with anti-Flag antibody and the pellet was used for Western blot analysis with anti-TLR4 antibody. As predicted, coimmunoprecipitation assays revealed the interaction of Flag-TRAM1 and TLR4 in BV2 cells, which was significantly increased by LPS/IFN-γ-stimulation (Fig. 4a). Furthermore, LPS/IFN-γ significantly increased the expression of TRIF and phosphorylation of TBK1 and IRF3 protein in BV2 cells, indicating the TLR4-independent signaling activation (Fig. 4b), which was strongly downregulated by silence of TRAM1 in BV2 cells. TLR4 stimulation reportedly leads to NF-κB activation and the subsequent target M1-related gene transcription. In consistence with this notion, we found that LPS/IFN-γ significantly upregulated IκB and P65 phosphorylation (Fig. 4c) and increased P65-NF-κB nuclear transportation (Fig. 4d). Interestingly, inhibiting TRAM1 expression by RNAi significantly aborted the LPS/IFNγ-triggered NF-κB activation events (Fig. 4c, d). All results showed that TRAM1 promotes microglia M1 polarization through a TLR4-NF-κB-dependent pathway.
Effect of Tram1 on activation of the TLR4-dependent pathway and NF-κB nuclear translocation in LPS/IFN-γ-induced BV2 cells. a Coimmunoprecipitation of TRAM1 and TLR4. Cells were transfected with Flag-TRAM1, and cell lysate was immunoprecipitated with anti-flag antibody, and the pellet was used for Western blot analysis with anti-TLR4 antibody. The experiment was repeated three times. b BV2 cells were transfected with si-TRAM1 for 24 h prior to treatment with LPS/IFN for 12 h. Protein levels of TRIF, p-TBK1, and p-IRF3 were evaluated by Western blot analysis and quantification of protein levels in the indicated groups (*P < 0.05 between groups; #P < 0.05 vs. within groups; n = 3–5). c The levels of P65-NF-κB in both cytosol and nuclear fractions were measured by Western blot assay. Quantification of protein levels in the indicated groups (*P < 0.05 between groups; #P < 0.05 vs. within groups; n = 3–5). d Protein levels of p-IκB and p-P65 were evaluated by Western blot analysis and quantification of protein levels in the indicated groups (*P < 0.05 between groups; #P < 0.05 vs. within groups; n = 3–5)
Discussion
Microglia exhibit various activation states in response to different stimulus (LPS/IFN-γ, IL-4) (Crain et al. 2013). Previous studies have demonstrated that pro-inflammatory stimuli (e.g., LPS, IFN-γ, TNF-α, and IL-1β) induce microglia to produce pro-inflammatory cytokines and to contribute to tissue destruction, which are referred to as M1-like microglia (David and Kroner 2011; Chhor et al. 2013; Ellert-Miklaszewska et al. 2013). M1-mediated neuroinflammation is a major component enhancing neurodegeneration and causing neuronal death and brain injury, most likely due to the production of high levels of cytotoxic and pro-inflammatory factors (Minghetti and Levi 1998; Liu and Hong 2003; Rivest 2011; Kim et al. 2015). Thus, downregulation of these inflammatory molecules could be beneficial for alleviating the progression of neurodegeneration caused by microglial activation (Kim et al. 2015). However, the mechanisms that regulate the polarization of microglia remain unclear (Shimizu et al. 2008; Hu et al. 2012; Tanaka et al. 2015). Our results demonstrated that TRAM1 promotes microglia M1 polarization. The mechanisms underlying this polarized effect might be related to an increase in TLR4-induced NF-kB activation via regulating TRIF-dependent pathways.
TRAM1 is a multi-pass membrane protein that is part of the mammalian endoplasmic reticulum (Kagan et al. 2008). TRAM1 is a sorting adaptor used by TRL4 signals through TRIF (Kagan and Medzhitov 2006; Kagan et al. 2008). TRAM1 seems to be necessary for coupling of the TLR4 located on the cell surface to the endosomal activation of TRIF–TRAF3 (Kagan et al. 2008). In addition, previous studies have defined that IL-6 release were predominately mediated by a TRAM–TRIF-dependent pathway (Hirotani et al. 2005; Kagan et al. 2008). Notably, previous studies have demonstrated that TRAM1 did not induce TRIF-dependent signaling from the plasma membrane. Instead, delivery of TLR4 and TRAM to endosomes was necessary for activation of the IRF3 signaling pathway (Kagan et al. 2008). However, little information on the roles of the TRAM1 on microglia polarization has been reported before. Our results showed that the level of protein and mRNA of TRAM1 is much highly increased in M1-polarized BV2 cells and primary microglia. TRAM1 over-expression increases the expression of iNOS and CD86 as well as the production of cytokines IL-6, IL-1β, and TNF-α. TRAM1 silence restrained the expression of M1-related molecules (CD86, iNOS, IL-6, IL-1β, and TNF-α). Furthermore, we performed immunofluorescent staining to determine the location of TRAM1. As expected, the total expression level of TRAM1 was highly increased in LPS/IFN-γ-stimulated BV2 cells compared with WT or IL-4-activated cells and TRAM1 was present at both the plasma membrane and in Rab5+ early endosomes. These demonstrate that TRAM1 plays an essential role in microglia M1 polarization in vitro.
Increasing studies have showed that TLRs are a family of pathogen-recognition receptors that play an important role in the innate immune system by triggering pro-inflammatory pathways in response to microbial pathogens such as LPS (Wang et al. 2009). TLR4 especially has been reported to play an important role in initiating the polarization of microglia and is necessary for activated microglia to induce neuronal injury in vivo (Verstak et al. 2009). When activated, TLR4 recruits distinct of intracellular adaptor proteins MyD88, TIRAP, TRAM, and TRIF. To date, the control of microglia polarization has largely been attributed to the function of a small group of factors including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB, the activator protein 1 (AP-1, hypoxia-inducible factors (HIFs), signal transducers and activators of transcription (STATs), and peroxisome proliferator-activated receptors (PPARs) (Ajmone-Cat et al. 2013). TRAM1 plays an important role of promoting inflammation through the TLR4-TRAM-NF-ΚB pathway (Ghosh et al. 2015). TRIF is recruited by TRIF-related adaptor molecule (TBK1) and has a critical role for the activation of IRF3 and the late activation of NF-kB (Plociennikowska et al. 2015). However, little is known about whether TRAM1 promotes microglia M1 polarization via regulating TRIF-dependent pathways. In the present study, our data indicated that LPS/IFN-γ caused a greater activation of the TRIF-dependent (upregulation of TRIF, p-TBK1, and p-IRF3 protein levels) pathway. Silence of TRAM1 exhibited a statistically significant inhibitory effect on the increased protein levels of TRIF, p-TBK1, and p-IRF3. In addition, TRAM1 silence also decreased the formation of the complexes of TLR4 with TRAM1.
In summary, the expression of TRAM1 is highly upregulated in LPS/IFN-γ-induced BV2 and primary microglia cells, and TRAM1 promotes LPS/IFN-γ-induced M1 microglia polarization. The mechanisms underlying this polarized effect might be related to an increase in TLR4-induced NF-kB activation via regulating TRIF-dependent pathways. Given the critical role of M1 microglia in the regulation of immune responses and the pathogenesis of diseases (Minghetti and Levi 1998; Liu and Hong 2003), a better understanding of the role and molecular mechanisms by which TRAM1 regulates M1 microglia will be helpful to develop a novel therapeutic strategy for diseases such as protozoan infection, cancer, and allergic and metabolic diseases.
References
Ajmone-Cat MA, Mancini M, et al. (2013) Microglial polarization and plasticity: evidence from organotypic hippocampal slice cultures. Glia 61(10):1698–1711
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7):499–511
Chhor V, Le Charpentier T, et al. (2013) Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun 32:70–85
Colton C, Wilcock DM (2010) Assessing activation states in microglia. CNS Neurol Disord Drug Targets 9(2):174–191
Colton CA, Mott RT, et al. (2006) Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation 3:27
Crain JM, Nikodemova M, et al. (2013) Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res 91(9):1143–1151
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12(7):388–399
Ellert-Miklaszewska A, Dabrowski M, et al. (2013) Molecular definition of the pro-tumorigenic phenotype of glioma-activated microglia. Glia 61(7):1178–1190
Ghosh M, Subramani J, et al. (2015) CD13 restricts TLR4 endocytic signal transduction in inflammation. J Immunol 194(9):4466–4476
Gwack Y, Srikanth S, et al. (2007) Biochemical and functional characterization of orai proteins. J Biol Chem 282(22):16232–16243
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394
Hirotani T, Yamamoto M, et al. (2005) Regulation of lipopolysaccharide-inducible genes by MyD88 and toll/IL-1 domain containing adaptor inducing IFN-beta. Biochem Biophys Res Commun 328(2):383–392
Hu X, Li P, et al. (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43(11):3063–3070
Kagan JC, Medzhitov R (2006) Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell 125(5):943–955
Kagan JC, Su T, et al. (2008) TRAM couples endocytosis of toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9(4):361–368
Kim M, Choi SY, et al. (2015) Neochlorogenic acid inhibits lipopolysaccharide-induced activation and pro-inflammatory responses in BV2 microglial cells. Neurochem Res 40(9):1792–1798
Lawrence T, Natoli G (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11(11):750–761
Liu B, Hong JS (2003) Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 304(1):1–7
Mantovani A, Sozzani S, et al. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Martinez FO, Helming L, et al. (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483
Minghetti L, Levi G (1998) Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol 54(1):99–125
Ng CL, Oresic K, et al. (2010) TRAM1 is involved in disposal of ER membrane degradation substrates. Exp Cell Res 316(13):2113–2122
Oshiumi H, Sasai M, et al. (2003) TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem 278(50):49751–49762
Plociennikowska A, Hromada-Judycka A, et al. (2015) Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 72(3):557–581
Ponomarev ED, Maresz K, et al. (2007) CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci 27(40):10714–10721
Ponomarev ED, Veremeyko T, et al. (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med 17(1):64–70
Rivest S (2011) The promise of anti-inflammatory therapies for CNS injuries and diseases. Expert Rev Neurother 11(6):783–786
Rojo AI, Innamorato NG, et al. (2010) Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia 58(5):588–598
Rowe DC, McGettrick AF, et al. (2006) The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci U S A 103(16):6299–6304
Shimizu E, Kawahara K, et al. (2008) IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1–42) by rat primary type 2 microglia. J Immunol 181(9):6503–6513
Smith PF (2008) Inflammation in Parkinson’s disease: an update. Curr Opin Investig Drugs 9(5):478–484
Tanaka T, Murakami K, et al. (2015) Interferon regulatory factor 7 participates in the M1-like microglial polarization switch. Glia 63(4):595–610
Tremblay ME, Stevens B, et al. (2011) The role of microglia in the healthy brain. J Neurosci 31(45):16064–16069
Verreck FA, de Boer T, et al. (2004) Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A 101(13):4560–4565
Verstak B, Nagpal K, et al. (2009) MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem 284(36):24192–24203
Wang X, Stridh L, et al. (2009) Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol 183(11):7471–7477
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, No. 2012CB822104); the National Natural Science Foundation of China (Nos. 31500647, 31440037, and 31270802); the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (15KJA310003); the Natural Science Foundation of Jiangsu Provience (BK20150408); and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Hanxiang Wang and Chun Liu contribute equally to this work.
Rights and permissions
About this article
Cite this article
Wang, H., Liu, C., Han, M. et al. TRAM1 Promotes Microglia M1 Polarization. J Mol Neurosci 58, 287–296 (2016). https://doi.org/10.1007/s12031-015-0678-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0678-3