Abstract
The contribution of endothelial progenitor cells (EPCs) to new vessel formation has been studied in different physiological and pathological conditions for decades. As previously suggested, insulin-like growth factor binding protein-2 (IGFBP-2) may interact with integrins and promote cell migration. However, the role of IGFBP-2 in regulation of EPC functions remains largely unknown. In this present study, we found that overexpression of IGFBP-2 in human umbilical vein endothelial cells (HUVECs) promoted EPC-endothelial adhesion. Conversely, siRNA-mediated depletion of IGFBP-2 inhibited oxygen-glucose deprivation (OGD)-induced EPC-endothelial adhesion. Further, we demonstrated that the arginine-glycine-aspartic acid (RGD) motif in its C-domain is required for interaction with integrin α5β1. In addition, treatment with IGFBP-2 significantly enhanced incorporation of EPCs into tubule networks formed by HUVECs. Thus, our findings suggest that exogenous administration of IGFBP-2 may facilitate neovascularization and improve treatment of ischemic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is burdened with a high morbidity and mortality. Approximately, it accounts for 5.5 million deaths annually, with 44 million disabilities worldwide (Mukherjee and Patil 2011). Of all strokes, 87 % are ischemic and 10 % are intracerebral hemorrhagic strokes (Strong et al. 2007). In response to tissue hypoxia and injury, endothelial progenitor cells (EPCs) are activated and mobilize to the injury site where they differentiate into endothelial cells (ECs) (Hristov et al. 2003). This process is essential for neovascularization and recovery from stroke (Krupinski et al. 1994; Font et al. 2010).
Integrins are a large family of non-covalently associated heterodimeric transmembrane glycoprotein which mediates cell-extracellular matrix (ECM) and cell-cell interactions (Barczyk et al. 2010; Humphries 2006). Each integrin is composed of two subunits: one α-subunit and one β-subunit (Barczyk et al. 2010). EPCs express multiple integrin subunits, including α1, α2, α3, α4, α5, α6, α9, αv, β1, β2, β3, β5 and β7 (Caiado and Dias 2012). In the last few decades, integrins have been associated with mobilization of EPCs from the bone marrow, homing to sites of vascular injury, and differentiation into ECs during the process of neovascularization (Caiado and Dias 2012).
Insulin-like growth factor binding protein (IGFBP) regulates the bioavailability, transportation and localization of insulin-like growth factor-I (IGF-I) (Hwa et al. 1999). Specific IGFBPs have been shown to either stimulate or inhibit IGF1 action (Firth and Baxter 2002), while the IGF1-independent role of IGFBPs is then discovered (McCarthy et al. 2009; Sokolovic et al. 2010). IGFBPs are present in both human and rats and are highly conserved among mammalian species (Hwa et al. 1999). Among all the IGFBPs, IGFBP-2 is the most abundant IGFBP in the central nervous system (Russo et al. 2005). Previous studies suggest that IGFBP-2 promotes cell movement via its interaction with integrin α5 (Wang et al. 2006). In addition, it is reported that IGFBP-2 level was significantly up-regulated by oxygen-glucose deprivation in brain microvascular endothelial cells (Wang et al. 2013). An increase in IGFBP-2 expression was also found in the stroke penumbra and core, or hippocampus in animal models (Jin et al. 2001; Fletcher et al. 2013). Thus, we hypothesize that IGFBP-2 may contribute to the interaction and adhesion of EPCs to ECs, namely EPC-endothelial adhesion.
In the present study, we found overexpression of IGFBP-2 in human umbilical vein endothelial cells (HUVECs) promoted EPC-endothelial adhesion, while depletion of IGFBP-2 significantly suppressed oxygen-glucose deprivation (OGD)-induced EPC-endothelial adhesion. Further, we showed that the role of IGFBP-2 was mediated by the interaction with integrin α5β1 through arginine-glycine-aspartic acid (RGD) motif. Finally, treatment with IGFBP-2 promoted EPC incorporation into HUVEC tubule networks.
Material and Methods
Cell Culture and Characterization of EPCs
HUVECs were purchased and grown in Medium 200PRF, supplemented with low serum growth supplement (all from Life Technologies, Carlsbad, CA, USA). EPCs were obtained according to previously described techniques (Kalka et al. 2000). Briefly, total human peripheral blood mononuclear cells (MNCs) were isolated from blood of healthy human volunteers using a density gradient centrifugation method with Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA). After washing three times with phosphate-buffered saline (PBS), cells were plated on fibronectin (Sigma-Aldrich)-pre-coated dish and maintained in EC basal medium-2 (EBM-2) (Clonetics, San Diego, CA, USA) supplemented with EGM-2 MV Single Quots containing 5 % FBS, human vascular endothelial growth factors, human fibroblast growth factor-2, human epidermal growth factor, insulin-like growth factor-1 and ascorbic acid (all from Sigma-Aldrich). After 4 days, non-adherent cells were removed, and the culture was maintained until 7 days. Fluorescence staining was performed to detect the binding of Ulexeuropaeus agglutinin I (UEA-1) and the uptake of acetylated LDL (acLDL) of adherent cells. Dual-staining cells which were positive were considered as EPCs. This study was approved by the Medical Ethics and Human Clinical Trial Committee of the First Clinical Hospital of Harbin Medical University. Written informed consent was obtained from all volunteers.
Flow Cytometric Analysis
Cells were digested with trypsin to obtain single cell suspension. Then, the cells (3 × 105) were incubated with primary antibodies or isotype controls for 1 h on ice in PBS containing 1 % bovine serum albumin (BSA). After several rinses in PBS, cells were incubated with Alexa Fluor 594-conjugated secondary antibody on ice for 30 min in the dark. The stained cells were acquired on a LSR II flow cytometer (BD Biosciences), and FlowJo software is used for the analysis of flow cytometry data. The antibodies used in this assay are as follows: anti-CD31 antibody (BD Biosciences, Franklin Lakes, New Jersey, USA), anti-KDR antibody (Sigma-Aldrich) and anti-vascular endothelium (VE) cadherin antibody (Millipore, Billerica, MA, USA).
Plasmid, Transfection and Mutagenesis
For overexpression of IGFBP-2, the protein coding region of IGFBP-2 was cloned into pcDNA3.1 expression vector and transfected into HUVECs using Xtremegene reagent (Roche Diagnostics Corporation, Indianapolis, IN, USA) according to the manufacturer’s instructions. Cells transfected with empty pcDNA3.1 vector were used as control. The mutation of RGD into arginine-glycine-glutamate (RGE) sequences in wild-type IGFBP-2 plasmid was introduced using the Quik Change II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) with the primer sequences as previously described (Wang et al. 2006). To knockdown expression of IGFBP-2, a scrambled siRNA and a siRNA targeting IGFBP-2 were purchased from Santa Cruz Biotechnologies (Dallas, TX, USA) and transfected into cells using siRNA transfection reagent (Santa Cruz Biotechnologies) according to manufacturer’s protocol.
Western Blot Assay
Cells were lysed with RIPA buffer (Pierce, Rockford, IL, USA), supplemented with proteinase inhibitor (YTHX Biotechnology, Beijing, China). Protein concentrations were determined using the BCA method (Pierce). Eluted proteins were resolved by 10 % SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membrane. Then, membranes were incubated with primary antibodies followed by washing and incubation with HRP-conjugated secondary antibody. The antibody/antigen complex was visualized by enhanced chemiluminescence (Pierce). The antibodies used in this assay are as follows: anti-IGFBP-2, anti-integrin α5, anti-integrin β1 and anti-GAPDH antibodies (all from Santa Cruz Biotechnologies)
Cell Adhesion Assay
Monolayer of HUVECs was seeded on 4-well Millicell EZ slides (EMD Millipore) at 2 × 105 cells/well for 24 h. EPCs were starved in EBM-2 overnight and labeled with acLDL. EPCs were then incubated with HUVECs in EBM-2 medium at 37 °C for 0, 10, 30, 60, 120 and 240 min. The wells were washed twice with PBS to remove non-attached cells. Adhered EPCs were fixed with 4 % paraformaldehyde for 10 min and counted. To assess the role of integrins in EPC adhesion, EPCs were pre-treated either with anti-integrin antibodies (20 μg/ml) of IgG isotype control. The antibodies used are as follows: anti-integrin β1 (Abcam, Cambridge, England), anti-integrin β2 (EMD Millipore), anti-integrin α4 (Abcam), anti-integrin α5 (Abcam), anti-integrin αv (Abcam) or anti-integrin α1β5 (EMD Millipore), or 1 μg/ml synthetic RGD and RGE (Gly-Arg-Glu) peptides (Sigma-Aldrich) for 4 h. Adherent cells were quantified by inverted-phase contrast microscope (Olympus TH4–200, Tokyo, Japan) on multiple ×10 fields.
Oxygen-Glucose Deprivation
To induce OGD condition, HUVECs in DMEM medium (without l-glucose, Life Technologies) was placed in a sealed chamber (Billups-Rothenberg, Del Mar, CA) at 37 °C, which has been flushed with 95 % N2/5 % CO2 gas. The concentration of oxygen in the atmosphere was maintained at 0 % oxygen and the PO2 in the medium was below 25 mmHg. After 16 h, the cells were returned back to normal growth condition.
Immunofluorescence Staining
EPCs (1 × 105 cells) were seeded onto 4-well Millicell EZ slides and starved in EBM-2 medium overnight. Cell were treated with or without IGFBP-2 (100 ng/ml) for 24 h and then fixed in 4 % paraformaldehyde at room temperature for 10 min, followed by permeabilization with PBS containing 0.5 % Triton X-100 for 30 min. The cells were then blocked in blocking solution (PBS containing 1 % BSA and 0.05 % TritonX-100) for 2 h. After washing with PBS, the cells were incubated with anti-human active integrin β1 antibody (EMD Millipore) at 4 °C overnight. To visualize integrin β1, an Alexa Fluor 594-conjugated secondary antibody (Life Technologies) was incubated with the cells at room temperature for 1 h. Images were captured by phase-contrast fluorescence microscopy (Olypmus, Tokyo, Japan).
Tubule Formation Assay
Six-well plates were coated with Matrigel (BD Biosciences) at room temperature for 30 min to allow solidification. AcLDL-labeled EPCs (2 × 104) were co-plated with 4 × 104 HUVECs and incubated at 37 °C for 16 h. The proportion of EPCs in tubules was determined by counting 10 random fields.
Statistical Analysis
All experiments were repeated at least three times. Results are shown as the mean ± SEM. One-way ANOVA was used to for statistical comparisons. A p value <0.05 was considered significantly different. All statistical analyses were carried out with SPSS 19.0.
Results
Overexpression of IGFBP-2 Enhances EPC-Endothelial Adhesion
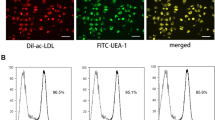
After isolation from human blood, we confirmed the endothelial phenotype of the EPCs by flow cytometric analysis of representative markers including CD31 (79.9 ± 4.9 %), KDR (86.1 ± 5.7 %) and VE-cadherin (81.7 ± 6.3 %) (Fig. 1a). In addition, cultured EPCs were found to bind UEA-1 lectin and endocytose acLDL (Fig. 1b). Next, to assess the effect of IGFBP-2 on EPC adhesion to ECs, IGFBP-2 was overexpressed in HUVECs (Fig. 1c). In cell adhesion assay, adherent acLDL-labeled EPCs with IGFBP-2 overexpressing HUVECs are significantly higher than control group at 30, 60, 120 and 240 min (Fig. 1d). Four hours after plating, approximate 1.5-fold more adherent EPCs were found in IGFBP-2 overexpression group (Fig. 1e). These results suggest that overexpression of IGFBP-2 in endothelial cells enhances the EPC-endothelial adhesion.
Overexpression of IGFBP-2 in HUVECs enhances EPC-endothelial adhesion. a Representative histograms of flow cytometry analysis of CD31, KDR and VE-cadherin markers on cultured EPCs after 1 week. Plot diagram shows isotype controls (black) and specific antibody staining (grey). b EPCs simultaneously bound UEA-1 lectin (green) and endocytosed acLDL (red) after cultured for 1 week. c Western blot analysis of IGFBP-2 expression in vector control or IGFBP-2-overexpressing HUVECs after 2 days from transfection. GAPDH was used as loading control. d Numbers of adherent EPCs to control or IGFBP-2-overexpressing HUVEC monolayer at 0, 10, 30, 60, 120 and 240 min. n = 4. e Representative images of acLDL-labelled EPCs adhered to control or IGFBP-2-overexpressing HUVEC monolayer after co-incubation for 4 h. **p < 0.01
Knockdown of IGFBP-2 Reduces EPC-Endothelial Adhesion Induced by OGD
Ischemic injury induces IGFBP-2 expression in brain microvascular endothelial cells and stimulates EPC adhesion to brain endothelial cells (Wang et al. 2013; Urbich and Dimmeler 2004). Next, we used OGD condition to investigate the role of IGFBP-2 in EPC-endothelial adhesion. HUVECs were subjected to 16-h OGD followed by co-culture with EPCs for additional 4 h. IGFBP-2 expression was significantly up-regulated by OGD, while this increase was abolished by siRNA-mediated depletion of IGFBP-2 (Fig. 2a, b). Further, knockdown of IGFBP-2 suppressed the adhesion of endothelial progenitor cells to endothelial cells stimulated by OGD (Fig. 2c, d). These data provide supportive evidence that IGFBP-2 is required for OGD-induced EPC-endothelial adhesion.
Knockdown of IGFBP-2 in HUVEC cells reduces EPC adhesion induced by oxygen-glucose deprivation (OGD). a Western blot analysis of IGFBP-2 expression in untreated control, pre-OGD control or pre-OGD IGFBP-2-knockdown HUVEC. GAPDH was used as loading control. b Relative intensity of western blot in a. c Representative images of acLDL-labelled EPCs adhered to untreated control, pre-OGD control or pre-OGD IGFBP-2-knockdown HUVEC monolayer after co-incubation for 4 h. d Numbers of EPCs attached to untreated control, pre-OGD control or pre-OGD IGFBP-2-knockdown HUVEC monolayer after co-incubation for 4 h. n = 4. **p < 0.01
IGFBP-2 Enhances EPC Adhesion to HUVECs via Integrin α5β1
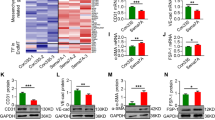
Since integrins mediate EPC mobilization to sites of vascular remodeling, repair and angiogenesis (Caiado and Dias 2012), and IGFBP-2 was found to interact with integrins in many cell types (Hamidouche et al. 2010; Schutt et al. 2004a; Holmes et al. 2012), we sought to see if IGFBP-2 stimulates EPC adhesion to HUVEC via binding to integrins. Functional blocking antibodies against monomeric or heterodimeric integrins β1, β2, α4, α5, αv and α5β1 were added to block the potential integrin-binding sites of IGFBP-2. As shown in Fig. 3a, a significant decrease of EPC-endothelial adhesion was observed when the cells were pre-treated with anti-integrin β1, anti-integrin β2 and anti-integrin α5β1 antibodies. Furthermore, Anti-integrin β1 and anti-integrin α5 and anti-integrin α5β1 antibodies reduced the EPC-endothelial adhesion stimulated by IGFBP-2 overexpression (Fig. 3b). Consistent with previous studies (Wang et al. 2006), we found IGFBP-2 up-regulated the expression of integrin α5β1, as determined by western blot (Fig. 3c). We next performed flow cytometry experiments and immunofluorescence analysis to determine if IGFBP-2 activates integrin β1. As shown in Fig. 3d, higher proportion of cells were recognized by active-integrin β1 antibody in IGFBP-2-treated EPCs than PBS-treated EPCs. Moreover, IGFBP-2 treatment resulted in stronger staining of active-integrin β1 along cell membrane in EPCs detected by immunofluorescence staining (Fig. 3e). Taken together, these results indicate that IGFBP-2 enhances EPC adhesion to HUVECs via integrin α5β1.
Overexpression of IGFBP-2 in HUVECs enhances EPC-endothelial adhesion via integrin α5β1. a Numbers of attached EPCs to HUVEC monolayer after co-incubation for 4 h in presence of different integrin subunit functional blocking antibodies. *p < 0.05, **p < 0.01 compared to PBS-treated cells. n = 4. b Numbers of attached EPCs to control or IGFBP-2-overexpressing HUVEC monolayer after co-incubation for 4 h in presence of different integrin subunit functional blocking antibodies. *p < 0.05, **p < 0.01 compared to PBS-treated control cells. #p < 0.05, ##p < 0.01 compared to PBS-treated IGFBP-2-overexpressing cells. n = 4. c Western blot analysis of integrin α5 and integrin β1 expression in EPCs treated with PBS or IGFBP-2 (100 ng/ml) for 24 h. EPCs were starved overnight with EBM-2 medium before the treatment. d Flow cytometric analysis of active conformation of integrin β1 in PBS or IGFBP-2 (100 ng/ml)-treated EPCs. n = 4. EPCs were starved overnight with EBM-2 medium before the treatment. e Immunocytochemistry showed accumulation of active integrin β1 (red) on EPC membrane after PBS or IGFBP-2 (100 ng/ml) treatment for 24 h. EPCs were starved overnight with EBM-2 medium before the treatment. Nuclei were stained with DAPI
IGFBP-2 Enhances EPC Adhesion to HUVECs via RGD Motif
It was reported that IGFBP-2 binds to integrin α5β1 through its RGD motif (Schutt et al. 2004a); we next examined the role of the RGD motif in IGFBP-2-induced EPC-endothelial adhesion. A point mutation of Asp306 to Glu306 (RGD to RGE) was generated in IGFBP-2 expression vector. We then performed the EPC adhesion assay using HUVECs transfected with control (wild type) or IGFBP-2 mutant vector. As expected, the result showed no significant difference in EPC-endothelial adhesion (Fig. 4a). Further, RGD-containing peptide, but not RGE-containing peptide, inhibited the IGFBP-2-induced EPC-endothelial adhesion (Fig. 4b). These results suggest that IGFBP-2 enhances EPC adhesion to HUVEC via binding to integrin α5β1 through its RGD motif.
IGFBP-2 enhances EPC adhesion to endothelial cells via RGD motif and promotes EPCs incorporation into HUVEC tubule networks. a Numbers of attached EPCs to control or IGFBP-2-mut-overexpressing HUVEC monolayer at 0, 10, 30, 60, 120 and 240 min of co-incubation. b Numbers of attached EPCs to control or IGFBP-2-overexpressing HUVEC monolayer at 4 h of co-incubation in presence of PBS, RGD (1 μg/ml) or RGE peptide (1 μg/ml). **p < 0.01 compared to PBS-treated control cells. #p < 0.05 compared to PBS-treated IGFBP-2-overexpressing cells. c Representative images of acLDL-labelled EPCs co-cultured with control or IGFBP-2-overexpressing HUVEC cells (transparent) to form tubule structures within Matrigel (left) at 37 °C for 16 h. d Quantification of % EPC tubulization into control or IGFBP-2 overexpressing HUVECs (right). n = 4. **p < 0.01
IGFBP-2 Promotes EPC Incorporation into HUVECs Tubule Networks
We next investigated the ability of EPC to integrate into vascular structures in vitro. As shown in Fig. 4c, d, overexpression of IGFBP-2 in HUVECs significantly enhanced the incorporation of EPCs into HUVEC tubule networks (25.5 vs 42.8 %, p < 0.01).
Discussion
EPC-mediated neovascularization involves four steps as previously defined: (1) mobilization from the bone marrow to the peripheral blood, (2) home in on sites of new vessel formation, (3) invade and migrate at the same sites, and (4) incorporation into the endothelium of the injured or newly formed blood vessels (Caiado and Dias 2012). Studies have shown that vascularization may be a potential therapeutic target in ischemic stroke (Font et al. 2010).
In this study, we investigated the role of IGFBP-2 in regulation of EPC-endothelial adhesion and the mechanism by which ECs interacts with EPCs. To achieve this, primary EPCs were isolated from human blood and identified by binding to UEA-1 lectin and uptake of acLDL. Most EPCs are positive with CD31, KDR and VE-cadherin, as determined by flow cytometric analysis. Flow cytometric analysis of cell surface markers is one of the most commonly used approach to isolate EPCs from human peripheral blood. Currently, there is no specific and unique cell surface determinant that has been identified as EPC marker. Different combinations of stem cell markers and endothelial cell markers are being used by different groups. Besides CD31, KDR and VE-cadherin, co-expression of CD34, CD133 and VEGFR-2 are also widely accepted to identify EPCs (Friedrich et al. 2006).
Our findings demonstrated that IGFBP-2 enhanced adhesion of EPCs to HUVECs in vitro. When IGFBP-2 was overexpressed in HUVECs, the adhesion of EPCs to HUVECs was strongly enhanced. In addition, neovascularization is a complicated and highly regulated process triggered by tissue hypoxia and injury. In our study, OGD was used to stimulate adhesion of EPCs to HUVECs. We found that depletion of IGFBP-2 in HUVECs significantly inhibited the OGD-induced EPC-endothelial adhesion.
It is previously reported that the integrin-mediated action of IGFBP-2 is associated with cell migration and invasion in tumor cells (Schutt et al. 2004b). To assess if the interaction is a result of the direct occupation of integrins, the EPCs were pre-treated with function-blocking antibodies against monomeric or heterodimeric integrins β1, β2, α4, α5, αv and α5β. We found that block of integrins β1, α5 and α5β1 inhibited the EPC-endothelial adhesion induced by IGFBP-2 overexpression, which suggests the role of IGFBP-2 in EPC-endothelial adhesion is mediated by heterodimeric integrin α5β1. Interestingly, expression of integrin α5 and integrin β1 is up-regulated by IGFBP-2 treatment. Specifically, IGFBP-2 has an RGD sequence in its C-domain. To determine whether the RGD motif of IGFBP-2 mediates cell surface binding, we introduced a point mutation (RGD to RGE) into IGFBP-2 overexpression vector. The mutated IGFBP-2 failed to promote EPC-endothelial adhesion. Thus, RGD motif is necessary for interaction of IGFBP-2 and integrin α5β1, and EPC-endothelial adhesion. However, we did not investigate the role of heparin-binding domain (HBD) in IGFBP-2 in our study. IGFBP-2 contains two HBDs, localized in the linker (HBD1) and C-terminal regions (HBD2), which are important for its binding to receptor protein tyrosine phosphatase β (RPTPβ) in regulating IGF-1 signalling (Shen et al. 2012). In addition, integrin-mediated action of IGFBP-2 may contribute to the process of EPC homing to the injury site, which may be addressed through in vivo studies in the future. Both IGFBP-2 knockout mice and transgenic mice have been generated (Schneider et al. 2000). IGFBP-2 knockout mice exhibited minimal difference, except a decreased spleen size during the early postnatal stages and increased circulating levels of several other IGFBPs in adults (Pintar et al. 1995). The IGFBP-2 transgenic mice showed weight reductions in the pancreas, spleen and liver and have been widely used in studies on metabolism (Hoeflich et al. 1999; Hedbacker et al. 2010).
We also examined the incorporation of EPCs into tubule networks formed by HUVECs. Neovascularization occurs through vasculogenesis, angiogenesis and/or arteriogenesis. Angiogenesis refers to the growth of new vessels from pre-existing ones, and vasculogenesis is the de novo formation of new blood vessels from EPCs (Peplow 2014). EPC incorporation into the endothelium of the injured or newly formed blood vessels is essential for the vessel remodeling and blood circulation (Yoder 2012). In our study, we found incorporation of EPCs into tubule networks was enhanced by treatment of IGFBP-2, indicating exogenous administration of IGFBP-2 might be helpful for neovascularization in clinic therapies.
The human IGFBP superfamily consists of at least seven proteins (IGFBP-1–7) (Hwa et al. 1999). The studies on IGFBP-2 knockout mice revealed the redundancy in the IGFBP family (Pintar et al. 1995). Chang et al. reported that IGFBP-3 promoted EPC migration, differentiation and capillary formation (Chang et al. 2007). More importantly, overexpression of IGFBP-3 in proliferating ECs enhanced proper vascular repair after hyperoxic insult in the oxygen-induced retinopathy model (Chang et al. 2007). Consistently, we showed that overexpression of IGFBP-2 enhanced EPC-endothelial adhesion, and IGFBP-2 treatment promoted incorporation of EPCs into HUVEC tubule networks. In contrast, two other IGFBP superfamily members, IGFBP-5 and IGFBP-6, inhibit VEGF-induced tube formation of HUVECs (Zhang et al. 2012; Rho et al. 2008). The opposite activities among different IGFBP family members need to be further investigated. Since endothelial cell phenotype can be influenced by the local microenvironment, the in vivo function of IGFBP-2 on endothelial cells might be different with our observation from in vitro grown cells.
In summary, we found IGFBP-2 promoted adhesion of EPCs to HUVECs via integrin α5β1, during which the RGD motif of IGFBP-2 is required for the interaction with integrin α5β1. Further, IGFBP-2 treatment enhanced incorporation of EPCs into tubule networks formed by HUVECs. Our findings indicate that exogenous administration of IGFBP-2 may facilitate the neovascularization and recovery from stroke.
References
Barczyk M, Carracedo S, Gullberg D (2010) Integrins. Cell Tissue Res 339:269–280
Caiado F, Dias S (2012) Endothelial progenitor cells and integrins: adhesive needs. Fibrogenesis Tissue Repair 5:4
Chang KH, Chan-Ling T, McFarland EL, Afzal A, Pan H, Baxter LC, Shaw LC, Caballero S, Sengupta N, Li Calzi S, Sullivan SM, Grant MB (2007) IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci U S A 104:10595–10600
Firth SM, Baxter RC (2002) Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23:824–854
Fletcher L, Isgor E, Sprague S, Williams LH, Alajajian BB, Jimenez DF, Digicaylioglu M (2013) Spatial distribution of insulin-like growth factor binding protein-2 following hypoxic-ischemic injury. BMC Neurosci 14:158
Font MA, Arboix A, Krupinski J (2010) Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev 6
Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N (2006) CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res 98:e20–e25
Hamidouche Z, Fromigue O, Ringe J, Haupl T, Marie PJ (2010) Crosstalks between integrin alpha 5 and IGF2/IGFBP2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol 11:44
Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM (2010) Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab 11:11–22
Hoeflich A, Wu M, Mohan S, Föll J, Wanke R, Froehlich T, Arnold GJ, Lahm H, Kolb HJ, Wolf E (1999) Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology 140:9
Holmes KM, Annala M, Chua CY, Dunlap SM, Liu Y, Hugen N, Moore LM, Cogdell D, Hu L, Nykter M, Hess K, Fuller GN, Zhang W (2012) Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-kappaB network. Proc Natl Acad Sci U S A 109:3475–3480
Hristov M, Erl W, Weber PC (2003) Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 23:1185–1189
Humphries JD, Byron A, Humphries MJ (2006) Integrin ligands at a glance. J Cell Sci 119:3
Hwa V, Oh Y, Rosenfeld RG (1999) The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 20:27
Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, Greenberg DA (2001) Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol 50:11
Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T (2000) Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 86:1198–1202
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (1994) 25, no. 9 1794–1798., Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25:5
McCarthy K, Laban C, McVittie CJ, Ogunkolade W, Khalaf S, Bustin S, Carpenter R, Jenkins PJ (2009) The expression and function of IGFBP-3 in normal and malignant breast tissue. Anticancer Res 29:6
Mukherjee D, Patil CG (2011) Epidemiology and the global burden of stroke. World Neurosurg 76:S85–S90
Peplow PV (2014) Growth factor- and cytokine-stimulated endothelial progenitor cells in post-ischemic cerebral neovascularization. Neur Reg Res 9
Pintar JE, Schuller A, Cerro JA, Czick M, Grewal A, Green B (1995) Genetic ablation of IGFBP-2 suggests functional redundancy in the IGFBP family. Prog Growth Factor Res 6:9
Rho SB, Dong SM, Kang S, Seo SS, Yoo CW, Lee DO, Woo JS, Park SY (2008) Insulin-like growth factor-binding protein-5 (IGFBP-5) acts as a tumor suppressor by inhibiting angiogenesis. Carcinogenesis 29:2106–2111
Russo VC, Gluckman PD, Feldman EL, Werther GA (2005) The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev 26:916–943
Schneider MR, Lahm H, Wu M, Hoeflich A, Wolf E (2000) Transgenic mouse models for studying the functions of insulin-like growth factor-binding proteins. FASEB J 14:12
Schutt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW (2004a) Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol 32:859–868
Schutt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW (2004b) Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol 32:10
Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR (2012) Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase beta and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol 32:4116–4130
Sokolovic A, Sokolovic M, Boers W, Elferink RP, Bosma PJ (2010) Insulin-like growth factor binding protein 5 enhances survival of LX2 human hepatic stellate cells. Fibrogenesis Tissue Repair 3:3
Strong K, Mathers C, Bonita R (2007) Preventing stroke: saving lives around the world. Lancet Neurol 6:182–187
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353
Wang GK, Hu L, Fuller GN, Zhang W (2006) An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell mobility. J Biol Chem 281:14085–14091
Wang J, Tang Y, Zhang W, Zhao H, Wang R, Yan Y, Xu L, Li P (2013) Insulin-like growth factor-1 secreted by brain microvascular endothelial cells attenuates neuron injury upon ischemia. FEBS J 280:3658–3668
Yoder MC (2012) Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2:a006692
Zhang C, Lu L, Li Y, Wang X, Zhou J, Liu Y, Fu P, Gallicchio MA, Bach LA, Duan C (2012) IGF binding protein-6 expression in vascular endothelial cells is induced by hypoxia and plays a negative role in tumor angiogenesis. Int J Cancer 130:2003–2012
Acknowledgments
This work was supported by the grants from science and technology project of the Education Department of Heilongjiang Province (12521336), science project of the Health Department of Heilongjiang Province (2011–089) and the Foundation of the Second Clinical Hospital of Harbin Medical University QN2011-18.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Nianping Feng and Zhuo Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Feng, N., Zhang, Z., Wang, Z. et al. Insulin-Like Growth Factor Binding Protein-2 Promotes Adhesion of Endothelial Progenitor Cells to Endothelial Cells via Integrin α5β1. J Mol Neurosci 57, 426–434 (2015). https://doi.org/10.1007/s12031-015-0589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0589-3