Abstract
Neuropeptide W (NPW), which was originally isolated from the porcine hypothalamus, has been identified as the endogenous ligand for both the NPBWR1 (GPR7) and NPBWR2 (GPR8) receptors. These receptors, which belong to the orphan G protein-coupled receptor (GPCR) family, share a high sequence homology with the opioid and somatostatin receptor families. NPW and NPBWR1 are widely distributed in the rat central nervous system (CNS). While the intracerebroventricular (i.c.v.) injection of NPW elevates plasma corticosterone levels, the intravenous administration of NPW in conjunction with a corticotropin-releasing hormone (CRH) antagonist blocks NPW-induced corticosterone secretion. It has been reported that NPW is involved in regulating the hypothalamus-pituitary-adrenal cortex (HPA) axis and that i.c.v. administration of NPW decreases feeding behavior. The aim of the present study was to ascertain if NPW’s role in feeding regulation is mediated (or not) through corticotropin-releasing hormone (CRH)-containing neurons. We found that NPW-containing axon terminals make synapses with CRH-immunoreactive cell bodies and dendritic processes in the hypothalamic paraventricular nucleus (PVN). The central infusion of NPW significantly induced c-Fos expression in CRH-immunoreactive neurons in the mouse PVN, but not in vasopressin- or oxytocin-immunoreactive neurons. To determine if NPW regulates feeding behavior through CRH neurons, the feeding behavior of mice was studied following the i.c.v. administration NPW in the presence or absence of pretreatment with a CRH antagonist. While NPW administration decreased feeding activity, the CRH antagonist inhibited this effect. These results strongly suggest that NPW regulates feeding behavior through CRH neurons in the mouse brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
O’Dowd et al. (1995) used oligonucleotides based on the opioid receptor and the structurally related somatostatin receptor to identify two genes—GPR7 and GPR8—which were predicted to encode two G protein-coupled receptors (GPCRs) in the human brain. GPR7 NPBWR1 is expressed in the brain and peripheral organs of humans and rodents, whereas NPBWR2 is not found in rodent genomes (Lee et al. 1999). In 2002, neuropeptide W (NPW) was isolated from the porcine hypothalamus (Shimomura et al. 2002), and its endogenous ligand receptors were considered to be neuropeptide B/W receptor 1 (NPBWR1, former name GPR7) and neuropeptide B/W receptor 2 (NPBWR2, former name GPR8). NPW has two isoforms, designated as neuropeptide W-23 and neuropeptide W-30, and these two peptides are thought to be derived from the precursor NPW peptide by proteolytic processing at two pairs of arginine residues (position 24 and 25 and position 31 and 32; Shimomura et al. 2002).
Given NPBWRs receptor gene expression patterns in rodents, NPW and NPB most likely bind to NPBWR1, which mediates their physiological functions (O’Dowd et al. 1995; Lee et al. 1999; Brezillon et al. 2003; Tanaka et al. 2003). It has been shown that NPBWR1 knockout mice are hyperphagic and exhibit decreased energy expenditure, suggesting that NPB and NPW play an important role in feeding behavior (Shimomura et al. 2002). The intracerebroventricular (i.c.v) infusion of NPW in the light phase in animals exposed to a 12:12-h light–dark schedule increased food intake during the first 2 h in male rats (Shimomura et al. 2002), while Levine et al. (2005) also reported that injection of NPW into the rat paraventricular nucleus (PVN) increased food intake. These results suggest that NPW may have an orexigenic function in the acute feeding phase (Shimomura et al. 2002). However, a previous report demonstrated that both forms of NPW suppress fasting-induced food intake in the dark phase, indicating that the effect of NPW on feeding differs according to whether animals are maintained in a light or dark phase (Mondal et al. 2003). The physiological significance and mechanisms underlying these effects are still not clear.
It should be noted that NPBWR1 is expressed in the hypothalamus, including the PVN and supraoptic nuclei (SON), and the arcuate nucleus (ARC) and that these areas contain neurons which regulate anterior pituitary hormone release (Singh et al. 2004; Kitamura et al. 2006; Skrzypski et al. 2012). Moreover, NPBWR1 is abundantly expressed in the central nucleus of the amygdala and bed nucleus of the stria terminalis, which is involved in the regulation of stress and emotion (Lee et al. 1999; Tanaka et al. 2003). From these observations, NPBWR1 may be involved in responding to stress via the hypothalamus-pituitary-adrenal cortex (HPA) axis (Mazzocchi et al. 2005; Niimi and Murao 2005). In addition, the intracerebroventricular (i.c.v.) infusion of NPW slightly elevates plasma levels of growth hormone and significantly increases plasma levels of corticosterone (Baker et al. 2003; Samson et al. 2004). The intraperitoneal (i.p.) injection of NPW also increases plasma levels of corticosterone and adrenocorticotropic hormone (ACTH; Hochol et al. 2007). These observations suggest that NPW’s action is related to the activation of the HPA axis (Baker et al. 2003; Brezillon et al. 2003; Samson et al. 2004; Taylor et al. 2005; Seki et al. 2008).
Immunohistochemistry experiments have shown that NPW-containing neurons are widely distributed in the rat brain (Dun et al. 2003; Takenoya et al. 2010b). We have recently shown that NPW messenger RNA (mRNA) is expressed in the rat PVN, ventromedial nucleus, ventromedial hypothalamus (VMH), arcuate nucleus (ARC), and lateral hypothalamus (LH) (Takenoya et al. 2010a). Many NPW-containing axon terminals have also been observed in the PVN (Dun et al. 2003; Takenoya et al. 2010a). In addition, corticotropin-releasing hormone (CRH)-immunoreactive neurons are abundantly expressed in the PVN. Based on these observations, we hypothesized that NPW-containing neurons in the PVN may inhibit food intake in a manner mediated by CRH-containing neurons. To test this hypothesis, we studied interactions between NPW- and CRH-containing neurons by using morphological, physiological, and molecular biology methods to determine whether NPW directly affects food intake via an action on CRH neurons in the PVN.
Materials and Methods
Animals
Adult male C57BL/6NCr mice were purchased from Sankyo Lab Service (Tokyo, Japan). Animals had ad libitum access to standard chow and water and were housed on a 12:12-h light–dark schedule (lights on at 8 a.m. and off at 8 p.m.) with the ambient temperature maintained at 23 ± 2 °C. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Showa University.
Cannulation
Animals were anesthetized with an i.p. injection of sodium pentobarbital (Somnopentyl, 50 mg/kg body weight; Kyoritsu Seiyaku, Tokyo, Japan), placed in a stereotaxic apparatus and implanted with a guide cannula (external diameter 0.5 mm, length 7.2 mm) through the skull that reached the lateral ventricle of the brain. Stereotactic coordinates were 0.2 mm posterior to bregma, 1.1 mm lateral from the midline, and 2.2 mm below the outer surface of the skull, according to the atlas of Franklin and Paxinos (16). For all cannulations, the incisor bar was set at 2.0 mm below the ear bars. The guide cannula was secured by dental cement on the dorsal surface of the skull. After surgery, a stylus was inserted into the guide cannula to prevent occlusion. An injector cannula (external diameter 0.2 mm) was designed to fit flush with the end of the guide cannula. The animals were housed in individual cages with free access to food and water. Following surgery, they were allowed to recover for 7 days and were handled daily during that time to minimize nonspecific stress related to the injection procedure. Verification of cannula placement was performed 7 days after surgery by monitoring the water intake of mice in response to an i.c.v. infusion of angiotensin II (50 ng/2 μl; Sigma Chemicals, St Louis, MO, USA). Those mice that did not drink water within 5 min of the angiotensin II injection were excluded from the study. Animals were allowed to recover for 7 days after this injection to rule out the action of any bioavailable angiotensin II on subsequent experimental procedures.
Surgical Procedures and Administration of NPW
Animals were anesthetized with pentobarbital sodium (50 mg/kg body wt. i.p. injection) and then placed in a stereotaxic frame. Two stainless steel anchoring screws were fixed to the skull, and the cannula was secured in place by acrylic dental cement. The animals were then returned to their cages and allowed to recover for at least 7 days. They were then handled every day and housed in cages before the start of the experiments. For i.c.v. administration of NPW or vehicle, a stainless steel injector was introduced through the cannula at a depth of 1.0 mm beyond the end of the guide. The total volume of injected solution of NPW and saline into the lateral ventricle was 2 μl. NPW was purchased from the Peptide Institute (Minoh, Japan) and dissolved in pyrogen-free sterile 0.9 % saline solution (Otsuka Phar, Japan). We administered NPW in the animals at the time of onset of dark phase at 6 p.m.
The animals used for in situ hybridization histochemistry for CRH mRNA were decapitated 6 h after the i.c.v. administration of NPW-30 (2.8 nmol/mouse) or vehicle (n = 5 in each group) to conscious mice. Brains were rapidly removed and placed on powdered dry ice for in situ hybridization histochemistry for CRH mRNA.
In Situ Hybridization Histochemistry for CRH mRNA
In situ hybridization histochemistry was performed on frozen 12-μm-thick coronal brain sections cut on a cryostat at −20 °C, thawed, and mounted on gelatin/chrome alum-coated slides. Brain tissue was stored at −80 °C before cutting. Sections containing the PVN were chosen from plate 18 in the atlas. Ten sets of two sections containing the PVN were used from each mouse to measure the density of autoradiography. Slides were warmed to room temperature, allowed to dry for 10 min, and then fixed in 4 % formaldehyde in phosphate-buffered saline (PBS) for 5 min. They were then washed two times in PBS and incubated in 0.9 % NaCl containing 0.25 % acetic anhydride (vol/vol) and 0.1 M triethanolamine at room temperature for 10 min. The sections were dehydrated using a series of 70 % (1 min), 80 % (1 min), 95 % (2 min), and 100 % (1 min) ethanol solutions consecutively and delipidated in 100 % chloroform for 5 min. The slides were then partially rehydrated first in 100 % (1 min) and then in 95 % (1 min) ethanol and allowed briefly to air-dry.
Hybridization was performed at 37 °C overnight in 45-μl of buffer solution consisting of 50 % formamide and 4 × saline sodium citrate (SSC; 1 × SSC = 150 mM NaCl and 15 mM sodium citrate), containing 500 μg/ml sheared salmon sperm DNA (Sigma, St. Louis, MO, USA), 250 μg/ml baker’s yeast total RNA (Roche Molecular Biochemicals, Mannheim, Germany), 1 × Denhardt’s solution, and 10 % dextran sulfate (500,000 MW; Sigma). The hybridization was performed under a Nescofilm (Bando Chemical IMD, Osaka, Japan) coverslip. A 35S-3ʹ-end-labeled deoxyoligonucleotide that was complementary to transcripts coding for CRH (5ʹ-CAGTTTCCTGTTGCTGTGAGCTTGCTGAGCTAACTGCTCTGCCCTGGC-3ʹ) was used as a specific probe. The specificity of the probe has been described previously (Harbuz et al. 1993). A total of 1 × 106 cpm/slide for CRH anti-sense transcripts were used. After hybridization, the sections were washed for 1 h in four separate 1 × SSC rinses at 55 °C and for another hour in two changes of 1 × SSC at room temperature. All independent experimental sections were treated simultaneously to minimize the variable effects of hybridization and wash stringency. Hybridized sections containing the PVN were opposed to autoradiography film (Hyperfilm; Amersham, Buckinghamshire, UK) for 5 days to detect CRH transcripts. The autoradiographic images were quantified using a MCID imaging analyzer (Imaging Research, St. Catherines, Ontario, Canada). The images were captured by a charge-coupled device camera (DAGE-MTI, Michigan City, IN, USA) with 40× magnification. The mean optical density (OD) of autoradiographs was measured by comparison with simultaneously exposed 14C microscale samples (Amersham). The standard curve was fitted by the OD of the 14C microscale on the same film.
c-Fos Experiments
Two nmol of NPW30 (Peptide Institute) or saline was i.c.v. administered to ad libitum fed mice (n = 4/group) before onset of the dark phase (1945 h). Ninety minutes after the injection, mice were perfused and fixed with 4 % paraformaldehyde/0.01 M PBS under anesthesia. Brains were removed and 20-μm-thick free-floating sections were prepared. Sections were blocked for 60 min in phosphate-buffered saline (PBS) containing 10 % normal horse serum (Vector Laboratories, Burlingame, CA, USA). Then in order to detect c-Fos immunoreactivity, the sections were incubated overnight with anti-c-Fos antiserum (1:20000, Ab-5; Oncogene Research Products, Cambridge, MA, USA) at 4 °C, washed three times with PBS for 10 min, and incubated with biotinylated anti-rabbit IgG (1:400; DAKO, Carpinteria, CA, USA) for 2 h at room temperature. The signals were amplified by ABC kit (Vector Laboratories), and visualized using a peroxidase substrate (diaminobenzidine, DAB) kit (Vector Laboratories) following washes with PBS. After dehydration, the mounted sections were coverslipped with malinol (Muto Pure Chemicals, Tokyo, Japan). c-Fos-like immunoreactivity (c-Fos-LI) was detected with an optical microscope (PROVIS, Olympus, Tokyo, Japan). As a control, the procedure described above was performed with the primary antibody step omitted. No specific immunoreactivity was observed in these control sections.
Double Immunofluorescence Staining Using Anti-cFos and Anti-CRH and Anti-Vasopressin and Anti-Oxytocin Antibodies
NPW30 (2 nmol) or saline were administered i.c.v. to ad libitum fed mice (n = 4/group) before the onset of dark phase (1900–2000 h). Ninety minutes after the injection, anesthetized mice were perfused and fixed with 4 % paraformaldehyde/0.01 M PBS. Brains were removed and 5-μm-thick paraffin sections were prepared. Brain sections from saline- and NPW-treated animals were carefully matched under a microscope according to the shape of brain structures in immunohistochemically stained sections. After being deparaffinized and rehydrated, the sections were blocked in PBS containing 10 % normal horse serum (Vector Laboratories) and then incubated with goat anti-c-Fos antibody (1:1000, Santa Cruz Biotechnology) overnight at 4 °C, followed by incubation with Alexa Fluor 488-conjugated donkey anti-goat IgG (1:400, Invitrogen, Carlsbad, CA, USA) for 1.5 h at room temperature. Next, the sections were incubated with rabbit anti-corticotropin-releasing factor (CRF) antibody (1:500, Advanced Targeting Systems, San Diego, CA, USA), guinea pig anti-vasopressin antibody (1:500, Peninsula Laboratories, San Carlos, CA, USA), or rabbit anti-oxytocin antibody (1:400, Incstar, Stillwater, MN, USA) overnight at 4 °C. After washing with PBS, the sections were incubated with Alexa Fluor 568-labeled goat anti-rabbit IgG or Alexa Fluor 546-labeled goat anti-guinea pig IgG (1:400, Invitrogen) for 1.5 h at room temperature. Double-immunostained sections were observed with the aid of a confocal laser microscope (A1si, Nikon, Tokyo, Japan).
Quantification of c-Fos-Like Immunoreactivity and/or CRH-Like Immunoreactivity
Brain sections from saline- and NPW-treated animals were carefully matched under a microscope according to the appearance of brain structures in immunohistochemically stained sections. Sections were selected at around 0.50 mm posterior to the bregma. The area in which CRH immunoreactivity was present was defined as the PVN. Images from the selected two sections per mouse (n = 4/group) were captured using a confocal laser microscope (A1Si, Nikon). In addition, the number of c-Fos-positive or CRH-positive cells in the PVN was counted in two sections from each group.
Electron Microscopy Observations
For electron microscopy experiments, vibratome-cut sections (40- to 50-m thickness) that had been fixed as described above were incubated in normal horse serum (1:20) for 20 min and then in mouse anti-NPW antiserum (diluted to 1:2000) for 2 h at room temperature, followed by overnight incubation at 4 °C. The following day, sections were incubated with biotinylated anti-mouse IgG (Lam et al. 2011) for 1 h and then with ABC for 45 min at room temperature (Vectastain Elite ABC kit, Vector Laboratories). The sections were then treated for about 3 min in the dark with diaminobenzidine (DAB) in 0.05 M Tris–HCl (pH 7.6) buffer containing 0.005 % hydrogen peroxide. After the DAB reaction, some of the sections were further treated with silver–gold intensification (Guan et al. 2001). These sections were post-fixed with 1 % OsO4 in 0.1 M PB (pH 7.4) for 1 h at 4 °C, dehydrated in a graded ethanol series, and then embedded in a mixture of Epon-Araldite. Ultrathin sections were then cut and examined by using a Hitachi H-7000 electron microscope. The specificity of the NPW antiserum used in this study has been reported elsewhere (Date et al. 2010; Takenoya et al. 2010a).
Feeding Behavior
Cannulated mice were individually housed in cages (dimensions: 240 mm wide, 290 mm long, and 300 mm high) and habituated to feeding behavior measuring equipment (ACTIMO-100, Shinfactory, Fukuoka, Japan) for approximately 1 week. Food intake and water intake were recorded at 1-min intervals. Food intake was calculated according to the weight change of the chow box. Water intake was measured using a drop sensor. Saline (2 μl) was i.c.v. administered to ad libitum fed mice (n = 3/group) before onset of dark the phase (1945 h), and food intake, water intake, and locomotor activity were simultaneously recorded for 24 h. Body weight was measured 24 h after the injection. One day later, the same animals were i.c.v. infused with NPW30 at a dose of 2 nmol (2 μl of 1 nmol/μl). Similar to procedures followed after the saline injection, food intake, water intake, and locomotor activity were recorded for 24 h. Body weight was measured 24 h after the NPW30 injection.
Effects of Pretreatment with CRH Antagonist (α-Helical-CRH) on Food Intake and Body Weight of NPW-Infused Mice
Animals (11-week-old male C57BL/6J mice) that passed the angiotensin II drinking water test (n = 6) were pretreated with the CRH antagonist α-helical-CRH (1 nmol) in saline injected i.c.v. into each lateral ventricle at 1830 h. Thirty minutes later (1900 h), NPW (1 nmol) was infused i.c.v into each mouse. Cumulative food intake, body weight, and blood glucose levels were measured after 24 h.
Statistical Analyses
Differences between groups (means ± SEM) were analyzed by analysis of variance (ANOVA) and post hoc Fisher’s test. P values less than 0.05 were considered to indicate significance (two-tailed tests).
Results
NPW-Containing Neurons and NPW mRNA Expression in the PVN
At the light microscopic level, NPW-containing nerve processes and their terminals could be identified in the mouse PVN (Fig. 1a). In situ hybridization histochemistry on brains excised 90 min after the i.c.v. administration of saline or NPW showed increased CRH mRNA expression in NPW-infused mice compared to control (Fig. 1b, c). Average CRH probe-binding activity (arbitrary units) was also increased in NPW-treated animals compared to control (saline-treated) animals (n = 5, P < 0.01; Fig. 1d).
a NPW-containing nerve processes are evident in the hypothalamic PVN of a control (saline-treated) mouse brain. b, c Effects of i.c.v. administration of NPW (2 nmol) or saline on CRH mRNA expression as measured by in situ hybridization histochemistry. Compared with saline-treated animals, CRH mRNA is expressed more strongly in the PVN after infusion of NPW. d Semiquantitative analysis of CRH mRNA expression induced by infusion of NPW vs. saline; NPW has a significant effect on expression
Interaction Between NPW- and CRH-Containing Neurons in the PVN
Light microscope images of NPW- and CRH-containing neurons in the PVN clearly showed NPW-containing axon terminals in close contact with CRH-containing cell bodies and dendritic process (Fig. 2a, b). At the electron microscope level, double immunolabeling showed NPW-containing axon terminals in the PVN making synaptic contacts with CRH-containing dendritic processes (Fig. 2c, d). These synapses were found to be of both the symmetrical (Fig. 2c) and asymmetrical (Fig. 2b) type.
Light and electron microscopic images of interaction between NPW- and CRH-containing neurons in the PVN. a, b NPW-containing axon terminals making direct contact with CRH-containing neuronal cell bodies and dendritic processes (arrows). Scale bar = 20 μm (a) and 10 μm (b). c, d Synaptic (arrow heads) interaction between NPW-containing axon terminals (N) with CRH-containing dendritic processes (C)
c-Fos Expression in the PVN and SON After Administration of NPW
The i.c.v. administration of NPW induced an increase in the level of c-Fos expression in the PVN. We examined the number of c-Fos-immunoreactive neurons in the PVN 90 min after the i.c.v. infusion of saline or NPW and found that compared to control (saline) group (Fig. 3a) NPW infusion led to a significant increase in c-Fos expression in the PVN (Fig. 3b). c-Fos-immunoreactive neurons were both found in the magnocellular and parvocellular divisions of the PVN (Fig. 3b). Small numbers of c-Fos-immunoreactive neurons were also observed in the SON of both control (Fig. 3c) and NPW-treated (Fig. 3d) animals. Overall, an average of 46.3 ± 7.4 and 163.3 ± 24.3 c-Fos-immunopositive neurons were seen in the PVN in control and NPW-injected mice, respectively, whereas in the SON, an average of 12.7 ± 4.4 and 46.7 ± 17.6, respectively, were observed (Fig. 3e).
Effects of i.c.v. infusion of NPW (2 nmol) or saline on c-Fos expression in the PVN (a, b) and SON (c, d). c-Fos immunoreactivity is strongly detected in the PVN after infusion of NPW but less in the SON. a, c Saline infusion. b, d NPW infusion. e NPW infusion significantly increased the number of c-Fos-immunopositive cells in the PVN and SON, but more so in the PVN. Data are expressed as the mean ± SEM. Number of c-Fos was counted by number of cell in section (cells/section)
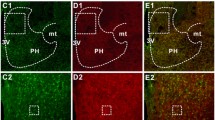
Dual Immunofluorescence for c-Fos and CRH-, Oxytocin-, or Vasopressin-Like Immunoreactivities and Quantification of c-Fos-Like and/or CRH-Like Immunoreactivities
To identify the target neurons of NPW, we subsequently performed double immunofluorescence experiments for c-Fos and CRH, oxytocin or vasopressin immunoreactivity in the mouse brain (Fig. 4). As shown above, compared to saline-injected brains, the i.c.v. infusion of NPW led to a significant increase in the number of c-Fos-like immunoreactive neurons in the PVN (Fig. 3). We found that the i.c.v. injection of NPW significantly elevated the number of neurons co-expressing c-Fos and CRH in the PVN compared to control (Fig. 4a, b) In contrast, double immunofluorescence studies employing antibodies against c-Fos and oxytocin (Fig. 4c, d) or c-Fos and vasopressin (Fig. 4e, f) showed that a few number of neurons in the PVN displayed overlapping immunoreactivity.
a, b Representative immunofluorescence photomicrographs showing c-Fos expression in CRH-like immunopositive neurons within the PVN following i.c.v. injection of NPW. Dual immunostaining of c-Fos (green) and CRH (red) in neurons of mice infused with vehicle (a) or NPW (b) infusion. c-Fos-expressing CRH neurons in the PVN of NPW-injected (2 nmol) mice. Scale bar is 20 μm. c, d, e, f Representative immunofluorescence photomicrographs showing c-Fos expression in oxytocin- (OXT; c, d) and vasopressin (AVP; e, f)-like immunopositive neurons within the PVN following i.c.v. injection of NPW
Effect of I.c.v. Infusion of NPW on Food Intake and Body Weight
We examined the effect of NPW on food intake in the dark phase. Here, NPW administered i.c.v. significantly reduced food intake, body weight, and water intake compared to saline-treated animals (*P < 0.05 vehicle vs. NPW icv; Student’s t test; Fig. 5).
Effect of a CRH Antagonist on NPW-Modulated Food Intake, Body Weight, and Blood Glucose Level
We next examined whether the anorexigenic effect of NPW was mediated via an action on CRH neurons. Body weight and food intake was compared to the previous day’s level. Animals were pretreated with the CRH antagonist α-helical-CRH (1 nmol) or saline injected into each lateral ventricle in the dark phase. Thirty minutes later, NPW (2 nmol) was infused (see “Materials and methods”). Food intake and body weight in the group pretreated with the CRH antagonist were increased compared with the saline-pretreated group (*P < 0.05 saline + NPW vs. α-h-CRH+NPW; Student’s t test). In contrast, blood glucose levels were not significantly different between the two groups (Fig. 6).
Effect of α-h-CRH on NPW-induced food intake, body weight and blood glucose glucose levels in the dark phase. Animals were pretreated with the CRH antagonist α-h-CRH (1 nmol) or saline, and 30 min later i.c.v. infused with NPW (2 nmol) (n = 6). Data are expressed as the mean ± SEM. *P < 0.05 vs. saline + NPW group
Discussion
NPW, which was identified as an endogenous ligand for the GPR7 and GPR8 receptors, is expressed in the hypothalamic region of the mouse brain. As GPR7 knockout mice have been shown to exhibit hyperphagic behavior and decreased energy expenditure, this could suggest that NPW acts as a modulator of feeding activity. Previous reports have shown that the central administration of NPW increases food intake during the first 2 h in the light phase (Shimomura et al. 2002), and Levine et al. (2005) similarly reported that injection of NPW into the PVN of rats also increased food intake. In contrast, Mondal et al. (2003) reported that NPW suppresses food intake in the dark phase (feeding phase), reduces fasting-induced food intake, and suppresses body weight gain. In the present study, we confirmed that the i.c.v. injection of NPW decreases food intake in the dark or feeding phase (Fig. 5), which perhaps indicates that endogenous NPW exerts both catabolic and anabolic functions depending on whether the animal is treated in the dark or light phase.
NPW-like immunoreactivity was particularly abundant in the PVN and SON of the hypothalamus (Takenoya et al. 2010a). In addition, NPW mRNA is expressed in the hypothalamus, pituitary gland, and adrenal gland (Tanaka et al. 2003; Kitamura et al. 2006; Seki et al. 2008; Takenoya 2010b). Reports have showed that i.c.v. infusion of NPW elevated prolactin and corticosterone in the rat (Shimomura et al. 2002; Baker et al. 2003), while NPW significantly altered prolactin, growth hormone, and ACTH release from dispersed rat anterior pituitary cells in vitro (Baker et al. 2003). In addition, Yogo et al. (2012) have reported that i.c.v. infusion of NPW stimulates ACTH secretion from the pituitary gland and that pretreatment with a vasopressin receptor antagonist does not inhibit this effect. These data suggest that AVP is not involved in the NPW-mediated increase in plasma ACTH. Rather; it has been suggested that NPW activates the HPA axis and plays an important role in the hypothalamic response to stress (Niimi and Murao 2005; Taylor et al. 2005; Beck et al. 2010). To this end, hypothalamic CRH-containing neurons are localized in the medial parvocellular part of the PVN and modulate the HPA axis response to the stress (Vale et al. 1981). GPR7 mRNA has been previously detected in the parvocellular part of the PVN (Lee et al. 1999), and it has been shown that NPW-immunoreactive neuronal cell bodies and fibers are present in that brain region (Dun et al. 2003; Takenoya et al. 2010b). Moreover, both NPW-immunoreactive cell bodies and NPBWR1 have been detected in the parvocellular part of the PVN (Kitamura et al. 2006).
Further to the above, CRH plays an important role in the stress response and affects feeding behavior (Drescher et al. 1994). Several studies have assessed the inhibitory role of CRH on food intake (Arase et al. 1989; Krahn et al. 1990). It has been proposed that CRH is released from the nerve terminals in the ARC, thereby inhibiting NPY/AGRP neurons; on this basis, it was suggested that CRH is normally responsible for stimulating feeding behavior and suppressing energy expenditure after acute stress (Richard et al. 2002). Further to this, pretreatment with α-h-CRH, administered intravenously, attenuated the ability of NPW to increase the plasma concentration of corticosterone (Taylor et al. 2005), a steroid hormone intimately involved in the stress response.
In the present study, we found that infusion of NPW into the mouse brain induced a significant increase in c-Fos expression in the PVN and SON. Other investigators have reported similar results (Levine et al. 2005; Niimi and Murao 2005; Kawasaki et al. 2006). While c-Fos-immunoreactive neurons were previously reported to be distributed in the parvocellular and magnocellular parts of the PVN (Mouri et al. 1993), the present study confirmed this and clearly showed that many c-Fos-immunoreactive neurons were co-labeled with CRH neurons. In this way, double-immunostaining experiments showed co-labeling of neurons with anti-c-Fos and anti-CRH antibodies in the parvocellular part of the PVN, but less so with anti-AVP and anti-OXT antibodies.
In addition to NPW, NPB also binds to GPR7 and GPR8. NPB-regulated food intake is mediated by CRF activity (Aikawa et al. 2008), implying that feeding regulation by the NPW/NPB systems is closely related to CRF activity. These results suggest that the role of NPW in feeding regulation could be partly through the action of CRH and that the main target of NPW could be CRH-containing neurons. Kawasaki et al. (2006) reported that i.c.v. infusion of NPW in the light phase demonstrated the coexistence of c-Fos and magnocellular AVP/OXT neurons in the PVN and SON in the rat. In addition, they reported that central administration of NPW30 causes a significant increase in plasma AVP and OXT levels in conscious rats (Kawasaki et al. 2006). However, in the present study, we found that a small number of these neurons did not respond to NPW administration in mice in the dark phase. The discrepancy in these results may due to the different animal strains used and whether the NPW infusion takes place in the dark or light phase.
At an ultrastructural level, our immunohistochemical study enabled us to observe that many NPW-immunoreactive fibers were in close apposition with CRH-immunoreactive cell bodies and dendritic processes in the PVN. Moreover, NPW-positive nerve terminals were found to make axo-somatic and axo-dendritic synaptic contacts with CRH-containing neurons in this brain region. We previously reported the relationship between NPW-containing neurons and other peptide-containing neurons in the lateral hypothalamus of the brain, along with NPW-containing nerve fibers being in direct contact with orexin- or melanin-concentrating, hormone-containing neurons in the rat (Takenoya et al. 2005). NPW-containing neurons have also been shown to project to POMC- and NPY-containing neurons in the ARC; in this way, loose patch extracellular recordings showed that treatment with NPW inhibits the firing of NPY-containing neurons and decreases the frequency of spontaneous inhibitory postsynaptic currents in POMC-containing neurons in the ARC (Date et al. 2010). Thus, NPW may inhibit food intake and body weight gain through at least two pathways: one that acts via inhibition of NPY-containing neurons and stimulation of POMC-containing neurons, and the other that acts by stimulating a CRH-mediated pathway.
In contrast to this, Yogo et al. (2012) reported that pretreatment with a CRF receptor antagonist inhibited the increase in plasma ACTH levels induced by the i.c.v. administration of NPW, suggesting that centrally applied NPW activates the HPA axis by activating hypothalamic CRF.
We also demonstrated here that the CRH antagonist α-h-CRH affects NPW-induced food intake and body weight, but not blood glucose levels. Taken together, these results indicate that NPW may, via the HPA axis, mediate stress responses and control feeding regulation, albeit in a complex manner given that blood glucose levels remain unaffected. This fits with numerous reports showing that stress affects food intake in different ways, with both increased and decreased feeding behavior described in humans and animals and in response to acute or chronic stress (Marti et al. 1994; Pecoraro et al. 2004; Schulz and Laessle 2012).
To summarize, we have shown that NPW regulates feeding behavior via an action on CRH neurons by direct synaptic innervation that results in decreased food intake and reduced weight. It remains unknown, however, why NPW does not affect glucose metabolism, thus leaving this issue as an important topic for future research.
References
Aikawa S, Ishii M, Yanagisawa M, Sakakibara Y, Sakurai T (2008) Effect of neuropeptide B on feeding behavior is influenced by endogenous corticotropin-releasing factor activities. Regul Pept 151:147–152
Arase K, Shargill NS, Bray GA (1989) Effects of corticotropin releasing factor on genetically obese (fatty) rats. Physiol Behav 45:565–570
Baker JR, Cardinal K, Bober C, Taylor MM, Samson WK (2003) Neuropeptide W acts in brain to control prolactin, corticosterone, and growth hormone release. Endocrinology 144:2816–2821
Beck B, Bossenmeyer-Pourie C, Pourie G (2010) Association of neuropeptide W, but not obestatin, with energy intake and endocrine status in Zucker rats. A new player in long-term stress-feeding interactions. Appetite 55(2):319–324
Brezillon S, Lannoy V, Franssen JD, Le Poul E, Dupriez V, Lucchetti J, Detheux M, Parmentier M (2003) Identification of natural ligands for the orphan G protein-coupled receptors GPR7 and GPR8. J Biol Chem 278:776–783
Date Y, Mondal MS, Kageyama H, Ghamari-Langroudi M, Takenoya F, Yamaguchi H, Shimomura Y, Mori M, Murakami N, Shioda S, Cone RD, Nakazato M (2010) Neuropeptide W: an anorectic peptide regulated by leptin and metabolic state. Endocrinology 151:2200–2210
Drescher VS, Chen HL, Romsos DR (1994) Corticotropin-releasing hormone decreases feeding, oxygen consumption and activity of genetically obese (ob/ob) and lean mice. J Nutr 124:524–530
Dun SL, Brailoiu GC, Yang J, Chang JK, Dun NJ (2003) Neuropeptide W-immunoreactivity in the hypothalamus and pituitary of the rat. Neurosci Lett 349:71–74
Guan JL, Saotome T, Wang QP, Funahashi H, Hori T, Tanaka S, Shioda S (2001) Orexinergic innervation of POMC-containing neurons in the rat arcuate nucleus. Neuroreport 12(3):547–551
Harbuz MS, Chalmers J, De Souza L, Lightman SL (1993) Stress-induced activation of CRF and c-fos mRNAs in the paraventricular nucleus are not affected by serotonin depletion. Brain Res 609:167–173
Hochol A, Tortorella C, Ricinski M, Ziolkowska A, Nussdorfer GG, Malendowicz LK (2007) Effects of neuropeptides B and W on the rat pituitary-adrenocortical axis: in vivo and in vitro studies. Int J Mol Med 19:207–211
Kawasaki M, Onaka T, Nakazato M, Saito J, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y (2006) Centrally administered neuropeptide W-30 activates magnocellular neurosecretory cells in the supraoptic and paraventricular nuclei with neurosecretion in rats. J Endocrinol 190:213–223
Kitamura Y, Tanaka H, Motoike T, Ishii M, Williams SC, Yanagisawa M, Sakurai T (2006) Distribution of neuropeptide W immunoreactivity and mRNA in adult rat brain. Brain Res 1093:123–134
Krahn DD, Gosnell BA, Majchrzak MJ (1990) The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry 27:1094–1102
Lam DD, Leinninger GM, Louis GW, Garfield AS, Marston OJ, Leshan RL, Scheller EL, Christensen L, Donato J Jr, Xia J, Evans ML, Elias C, Dalley JW, Burdakov DI, Myers MG Jr, Heisler LK (2011) Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab 13:584–591
Lee DK, Nguyen T, Porter CA, Cheng R, George SR, O’Dowd BF (1999) Two related G protein-coupled receptors: the distribution of GPR7 in rat brain and the absence of GPR8 in rodents. Brain Res Mol Brain Res 71:96–103
Levine AS, Winsky-Sommerer R, Huitron-Resendiz S, Grace MK, de Lecea L (2005) Injection of neuropeptide W into paraventricular nucleus of hypothalamus increases food intake. Am J Physiol Regul Integr Comp Physiol 288:R1727–R1732
Marti O, Marti J, Armario A (1994) Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav 55:747–753
Mazzocchi G, Rebuffat P, Ziolkowska A, Rossi GP, Malendowicz LK, Nussdorfer GG (2005) G protein receptors 7 and 8 are expressed in human adrenocortical cells, and their endogenous ligands neuropeptides B and w enhance cortisol secretion by activating adenylate cyclase- and phospholipase C-dependent signaling cascades. J Clin Endocrinol Metab 90:3466–3471
Mondal MS, Yamaguchi H, Date Y, Shimbara T, Toshinai K, Shimomura Y, Mori M, Nakazato M (2003) A role for neuropeptide W in the regulation of feeding behavior. Endocrinology 144:4729–4733
Mouri T, Itoi K, Takahashi K, Suda T, Murakami O, Yoshinaga K, Andoh N, Ohtani H, Masuda T, Sasano N (1993) Colocalization of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of the human hypothalamus. Neuroendocrinology 57:34–39
Niimi M, Murao K (2005) Neuropeptide W as a stress mediator in the hypothalamus. Endocrine 27:51–54
O’Dowd BF, Scheideler MA, Nguyen T, Cheng R, Rasmussen JS, Marchese A, Zastawny R, Heng HH, Tsui LC, Shi X et al (1995) The cloning and chromosomal mapping of two novel human opioid-somatostatin-like receptor genes, GPR7 and GPR8, expressed in discrete areas of the brain. Genomics 28:84–91
Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF (2004) Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology 145:3754–3762
Richard D, Lin Q, Timofeeva E (2002) The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol 440:189–197
Samson WK, Baker JR, Samson CK, Samson HW, Taylor MM (2004) Central neuropeptide B administration activates stress hormone secretion and stimulates feeding in male rats. J Neuroendocrinol 16:842–849
Schulz S, Laessle RG (2012) Stress-induced laboratory eating behavior in obese women with binge eating disorder. Appetite 58:457–461
Seki M, Kageyama H, Takenoya F, Hirayama M, Kintaka Y, Inoue S, Matsuno R, Itabashi K, Date Y, Nakazato M, Shioda S (2008) Neuropeptide W is expressed in the noradrenalin-containing cells in the rat adrenal medulla. Regul Pept 145:147–152
Shimomura Y, Harada M, Goto M, Sugo T, Matsumoto Y, Abe M, Watanabe T, Asami T, Kitada C, Mori M, Onda H, Fujino M (2002) Identification of neuropeptide W as the endogenous ligand for orphan G-protein-coupled receptors GPR7 and GPR8. J Biol Chem 277(39):35826–35832
Singh G, Maguire JJ, Kuc RE, Fidock M, Davenport AP (2004) Identification and cellular localisation of NPW1 (GPR7) receptors for the novel neuropeptide W-23 by [125I]-NPW radioligand binding and immunocytochemistry. Brain Res 1017:222–226
Skrzypski M, Pruszynska-Oszmalek E, Rucinski M, Szczepankiewicz D, Sassek M, Wojciechowicz T, Kaczmarek P, Kolodziejski PA, Strowski MZ, Malendowicz LK, Nowak KW (2012) Neuropeptide B and W regulate leptin and resistin secretion and stimulate lipolysis in isolated rat adipocytes. Regul Pept 176:51–56
Takenoya F, Hirayama M, Kageyama H, Funahashi H, Kita T, Matsumoto H, Ohtaki T, Katoh S, Takeuchi M, Shioda S (2005) Neuronal interactions between galanin-like-peptide- and orexin- or melanin-concentrating hormone-containing neurons. Regul Pept 126:79–83
Takenoya F, Kageyama H, Shiba K, Date Y, Nakazato M, Shioda S (2010a) Neuropeptide W: a key player in the homeostatic regulation of feeding and energy metabolism? Ann N Y Acad Sci 1200:162–169
Takenoya F, Yagi M, Kageyama H, Shiba K, Endo K, Nonaka N, Date Y, Nakazato M, Shioda S (2010b) Distribution of neuropeptide W in the rat brain. Neuropeptides 44(2):99–106
Tanaka H, Yoshida T, Miyamoto N, Motoike T, Kurosu H, Shibata K, Yamanaka A, Williams SC, Richardson JA, Tsujino N, Garry MG, Lerner MR, King DS, O’Dowd BF, Sakurai T, Yanagisawa M (2003) Characterization of a family of endogenous neuropeptide ligands for the G protein-coupled receptors GPR7 and GPR8. Proc Natl Acad Sci U S A 100:6251–6256
Taylor MM, Yuill EA, Baker JR, Ferri CC, Ferguson AV, Samson WK (2005) Actions of neuropeptide W in paraventricular hypothalamus: implications for the control of stress hormone secretion. Am J Physiol Regul Integr Comp Physiol 288:R270–R275
Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397
Yogo K, Oki Y, Iino K, Yamashita M, Shibata S, Hayashi C, Sasaki S, Suenaga T, Nakahara D, Nakamura H (2012) Neuropeptide W stimulates adrenocorticotrophic hormone release via corticotrophin-releasing factor but not via arginine vasopressin. Endocrinology 59:547–554
Acknowledgments
This study was supported in part by grants for the Grant-in-Aid for Scientific Research (C) and Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to FT 26350912, HK 24590241, and SH 90644261). This study was supported in part by Grant-in-Aid for challenging Exploratory Research for the Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to SS 22126004). The study was also supported in part by Grant-in-Aid for Challenging Exploratory Research of Japan (to SS 25670767).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takenoya, F., Wang, L., Kageyama, H. et al. Neuropeptide W-Induced Hypophagia is Mediated Through Corticotropin-Releasing Hormone-Containing Neurons. J Mol Neurosci 56, 789–798 (2015). https://doi.org/10.1007/s12031-015-0501-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0501-1