Abstract
Background
Cancers of the upper gastrointestinal tract represent a lethal disease entity comprising the esophagus, gastroesophageal junction, and stomach. The backbone of therapy in esophagogastric cancers has predominantly been chemotherapy-based. However, over the last decade, with the debut of immune checkpoint inhibitors, sophisticated molecular testing, and a more comprehensive understanding of the tumor microenvironment, immunotherapy has been incorporated into the treatment of localized and advanced esophagogastric cancers with promising results.
Purpose
This study aimed to review the unique tumor microenvironment and role of immunotherapy in esophagogastric cancers.
Methods
We conducted a systematic review of clinical and translational research for immunotherapy in esophagogastric cancers.
Results
This article will explore the unique tumor microenvironment in gastroesophageal cancers, the role of immunotherapy in localized and advanced disease, challenges in management, and new therapeutic approaches in clinical trials.
Conclusion
With further exploration into targeted therapy and immunotherapy, we anticipate the emergence of novel treatments that will improve survival and quality of life in patients with esophagogastric cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophagogastric cancers comprised esophageal (EC), gastroesophageal junction (GEJ), and gastric cancers (GC) and are challenging disease entities. As the 8th most common cancer, EC accounts for 604,000 new cases and 544,000 deaths each year [1]. GC is the 5th most common cancer worldwide and is responsible for over 1 million cases each year and 769,000 deaths worldwide [1]. Despite differences in epidemiology, localization, and molecular patterns, esophagus and stomach cancers are often grouped together in clinical trials and thus treated similarly in practice. Unfortunately, 5-year survival for patients with advanced disease is approximately 5%. While chemotherapy is the treatment backbone, the treatment landscape is evolving significantly with the advent of molecular testing and immunotherapy.
With the need for novel therapeutic strategies, increasing focus has shifted to studying the tumor microenvironment (TME), the complex molecular ecosystem that functions in the growth or inhibition of cancer cells [2]. In particular, the programmed death 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) immune checkpoints are negative regulators of T cell immune function, and its expression can function as “off-switches” for cancer to evade the immune system. Inhibition of these targets via immune checkpoint inhibitors (ICI) results in increased activation of the immune system and is the basis of immunotherapy treatment for many cancers, including EC and GC.
In clinical trials, assessment of programmed cell death ligand 1 (PD-L1) expression is often utilized through combined positive score (CPS) or tumor proportion score (TPS) to predict response to ICI. Interestingly, a few molecular signatures can also assist in predicting response. In particular, deficient mismatch repair (dMMR) with its unique genetic signature, high levels of microsatellite instability (MSI-H), and high tumor mutational burden (TMB-H) render tumors sensitive to PD-1-based ICI [3]. In addition, phase II data from integrated genomic analysis and genomic profiling of circulating tumor DNA (ctDNA) from patients with advanced gastric adenocarcinomas (AC) reveals the presence of Epstein-Barr virus (EBV)-positivity is associated with response to PD-1 ICI [4]. Besides the characterization of PD-L1 expression, dMMR/MSI-H, TMB-H, and EBV-positivity, the optimal way to identify patients who will respond to ICI is unclear.
This review will discuss the role of immunotherapy in patients with esophagogastric cancers based on histologic subtype, extent of disease, and molecular signatures. Novel strategies with non-traditional immunotherapy with bispecific antibodies targeting claudin, monoclonal antibodies against growth factors, and CAR-T will also be discussed.
Localized Esophageal Cancer
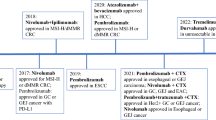
In patients with localized, resectable esophageal squamous cell carcinoma (SCC) and AC, the goal of treatment is curative (Table 1). The standard of care (SOC) treatment is neoadjuvant chemoradiotherapy followed by surgery based on the CROSS trial [5]. Unfortunately, the risk of recurrence remains high, as up to 75% do not achieve a pathological complete response (pCR) and have a worse prognosis compared to those with pCR [6]. Before the CheckMate-577 trial, SOC after neoadjuvant treatment and surgery was surveillance. The CheckMate-577 trial was a phase III randomized, double-blind, placebo-controlled trial for patients with stage II or III resectable esophageal or GEJ SCC or AC who completed neoadjuvant chemoradiotherapy followed by complete resection without evidence of a pCR. Participants were stratified according to PD-L1 expression and pathologic lymph-node status and randomized to adjuvant PD-1 inhibitor nivolumab or placebo for a maximum duration of 1 year. Median disease-free survival (DFS) was superior with nivolumab compared to placebo (22.4 vs. 11 months (mo)). Median overall survival (OS) results are not mature at this time. Based on these results, adjuvant nivolumab is now the SOC for patients without pCR after chemoradiotherapy and surgery. Immunotherapy is also being studied in other contexts for localized EC. The KEYNOTE-975 trial is currently in progress, evaluating the role of adjuvant pembrolizumab after definitive chemoradiotherapy alone (NCT04210115) [7].
Localized Gastric Cancer
For patients with localized, resectable GC, the SOC is perioperative chemotherapy with FLOT (fluorouracil, leucovorin, oxaliplatin, + docetaxel) based on the MAGIC trial [8]. To further assess predictive markers of response, a meta-analysis of four randomized trials (MAGIC, CLASSIC, ARTIST, ITACA-S) was performed to evaluate the benefit of perioperative chemotherapy in patients with resectable GC [9]. Interestingly, patients who had early-stage GC with dMMR, which is the biological footprint of MSI-H, did worse with chemotherapy compared to upfront surgery (five-year OS 75 vs. 83%). In a subset analysis, patients with MSI-low GC benefited more from perioperative chemotherapy than surgery alone, with a 5-year OS of 62 vs. 53%. However, an additional meta-analysis with a larger cohort of resected dMMR/MSI-H GC did not confirm the lack of benefit for adjuvant chemotherapy [10]. Patients with dMMR/MSI-H tumors who received adjuvant chemotherapy had a longer OS than surgery alone (OS at 3, 5, and 10 years 81, 84, and 64% vs. 64, 65, and 50%, respectively). Overall, the benefit of perioperative chemotherapy in patients with dMMR/MSI-H gastric and GEJ AC is debatable. The authors raise the question of whether perioperative ICI, rather than adjuvant chemotherapy, is the optimal therapy for dMMR/MSI-H gastric and GEJ cancers.
In the ongoing phase II NEONIPIGA trial, patients with dMMR/MSI-H resectable gastric and GEJ AC receive perioperative immunotherapy with neoadjuvant nivolumab + the CTLA-4 monoclonal antibody ipilimumab followed by surgery and adjuvant nivolumab for 9 months (NCT04006262) [11] (Table 2). Publication of early data shows that after a median follow-up of 14.9 months, 29 of 32 patients underwent surgery and had microscopically complete R0 resection with tumor-free biopsies. Three patients did not receive surgery and had a complete endoscopic response with tumor-free biopsies and a normal CT scan. As single high-dose anti-CTLA4 tremelimumab added to anti-PD-L1 durvalumab induces higher T cell expansion, the INFINITY single-arm multi-cohort phase II trial also evaluated the combination of CTLA-4 and PD-L1 inhibition with neoadjuvant tremelimumab + durvalumab for patients with MSI-H resectable gastric or GEJ AC (NCT04817826) [12]. In cohort 1, patients received tremelimumab (300 mg) + three cycles of durvalumab (T300/D). Eighteen patients were enrolled, one withdrew consent after one cycle, and two patients had a clinical complete response (cCR) and refused surgery. Among the 15 evaluable patients, pCR was 60%, and the major to complete pathological response rate was 80%. This study opens the discussion of nonoperative management and organ preservation in patients with clinical, pathologic, and molecular responses after tremelimumab + durvalumab. Cohort 2 includes patients treated with definitive T300/D without surgery if cCR is achieved. Updates for cohort 2 are not published. While this is promising, randomized trials comparing perioperative immunotherapy to SOC are needed.
Other studies combining PD-1 ICI with perioperative chemotherapy are currently in progress. These include the KEYNOTE-585, DANTE, and MATTERHORN trials, assessing the benefit of perioperative pembrolizumab, atezolizumab, and durvalumab, respectively [13,14,15]. In an interim analysis of KEYNOTE-585, neoadjuvant/adjuvant pembrolizumab + chemotherapy was associated with improved pCR compared to placebo + chemotherapy (12.9% vs. 2%) [16]. Despite improved pCR, pembrolizumab + chemotherapy did not improve survival over chemotherapy alone. Differences in event-free survival (EFS) and median OS were not statistically significant (EFS 44.4 mo with pembrolizumab vs. 25.4 mo with placebo; OS 60.7 mo with pembrolizumab vs. 58 mo with placebo). However, exploratory analysis suggests EFS benefit with pembrolizumab for CPS ≥ 10 (hazard ratio 0.70). Of note, most patients received doublet chemotherapy with fluorouracil + cisplatin (FP) or cisplatin + capecitabine (XP) rather than SOC triplet chemotherapy with FLOT, for which oxaliplatin may be more active with ICI. In the phase II DANTE trial, the addition of atezolizumab to perioperative FLOT was associated with improved tumor downstaging and pCR (24% vs 15%) compared to FLOT alone. Regression rates were further improved with higher PD-L1 expression (33% vs. 12% with CPS ≥ 10) and MSI-H tumors (63% vs. 27%). This prompted the transition to a phase III design with enrollment restricted to high immune responsiveness, i.e., MSI-H, PD-L1 CPS ≥ 1, TMB ≥ 10/MB, or EBV+ . Survival data is not mature [17]. Interim analysis of the MATTERHORN phase III trial assessing perioperative durvalumab + FLOT showed improved pCR (19% vs. 7%) and favorable downstaging (pT0 21% vs. 10%; pN0 47% vs. 33%) with the addition of durvalumab compared to FLOT alone [18]. Rates for surgery (87% vs. 86%) and R0 resection (84 vs. 86%) were similar. The role of immunotherapy in the perioperative setting is promising, with favorable pCR rates; however, the impact of improved pCR on survival is not clear [19].
Advanced Esophageal/Gastric Adenocarcinoma
The goal of treatment for advanced or unresectable esophagogastric cancers is to palliate symptoms and prolong survival. Immunotherapy treatment considerations require tumor histology and molecular testing for MSI/MMR, human epidermal growth receptor 2 (HER2), and PD-L1 expression. PD-L1 expression is often quantified by CPS, defined by the number of PD-L1-stained cells divided by the total number of viable tumor cells evaluated multiplied by 100 [20]. The less commonly used TPS is defined by the number of PD-L1-stained tumor cells divided by the total number of viable tumor cells multiplied by 100.
Initially, immunotherapy was reserved for third-line (3L) and beyond based on the KEYNOTE-059 trial, which showed durable responses with pembrolizumab in patients with previously treated gastric or GEJ cancers with two or more systemic therapies [21, 22]. In September 2017, pembrolizumab was granted accelerated Food and Drug Administration (FDA) approval; however, it was later withdrawn in April 2021 [23]. With the treatment landscape changing, it became clear that patients will likely have received immunotherapy before they need 3L treatment. Now, immunotherapy is primarily incorporated in the front-line setting.
In HER2-overexpressing esophagogastric cancers, adding trastuzumab improves overall survival [24]. Preclinical models show that trastuzumab increases T cell responses and upregulates PD-1 and PD-L1 expression of tumor infiltrating lymphocytes [25]. In mouse models, when combined with PD-1 ICI, trastuzumab results in increased immune-cell trafficking and tumor eradication. The synergy of HER2 and PD-1 prompted clinical trials combining trastuzumab and ICI. For HER2-overexpressing esophagogastric cancers, the Phase III KEYNOTE-811 trial is evaluating the addition of pembrolizumab to chemotherapy + trastuzumab [26]. In this trial, 692 patients with HER2-positive advanced gastric or GEJ AC were randomized to first-line (1L) platinum-containing chemotherapy + trastuzumab + pembrolizumab or chemotherapy + trastuzumab + placebo. Those receiving pembrolizumab had higher objective response rates (ORR, 74 vs. 52%) and more complete responders (11 vs. 3%) than placebo. Based on the first interim analysis, the FDA granted accelerated approval for pembrolizumab in combination with chemotherapy + trastuzumab for 1L treatment of advanced or metastatic HER2-positive gastric or GEJ AC regardless of PD-L1 expression. In the third interim analysis, progression-free survival (PFS) was longer with pembrolizumab compared to placebo (10 vs. 8.1 mo) among all patients and for tumors with PD-L1 CPS ≥ 1 (10.9 vs. 7.3 mo). There was no difference in PFS for tumors with CPS < 1 (9.5 vs. 9.5 mo). Overall survival favored pembrolizumab with OS 20 vs. 16.8 months among all patients and 20 vs. 15.7 months with CPS ≥ 1 [27]. Because PFS was limited to CPS ≥ 1, the FDA revised the indication of pembrolizumab and restricted its use to tumors with CPS ≥ 1 [28]. The European Medicines Agency (EMA) has also approved pembrolizumab for CPS ≥ 1 [29].
Treatment for patients with HER2-negative esophagogastric cancers depends on PD-L1 expression and the presence of dMMR/MSI-H. In general, chemotherapy is often combined with ICI for tumors with intermediate (CPS 5–9) or high (CPS ≥ 10) PD-L1 expression and dMMR/MSI-H. The benefit of nivolumab and pembrolizumab, in addition to cytotoxic therapy, has been shown in the CheckMate-649, KEYNOTE-590, and KEYNOTE-859 studies. In the phase III CheckMate-649 trial, 1581 patients with previously untreated HER2-negative, advanced, or metastatic esophagogastric AC were randomly assigned to nivolumab + chemotherapy (oxaliplatin plus either leucovorin + short-term infusional fluorouracil or capecitabine) and chemotherapy alone [30]. At a median follow-up of 13 months, nivolumab improved OS to 13.8 months compared to 11.6 months in the control group [31]. In subgroup analysis, while the median OS for CPS ≥ 5 was 14.4 vs. 11.1 months, there was no OS benefit for CPS < 1 (13.1 vs. 12.5 mo), CPS < 5 (12.4 vs. 12.3 mo), or CPS < 10 (12.4 vs. 12.5 mo). At a minimum 36-month follow-up, nivolumab continued to demonstrate OS and PFS benefit compared with chemotherapy in all randomized patients (OS 13.7 vs. 11.6 mo; PFS 7.7 vs. 6.9 mo) and patients with CPS ≥ 5 (OS 14.4 vs. 11.1 mo; PFS 8.3 vs. 6.1 mo). ORR for CPS ≥ 5 was 60% with nivolumab vs. 45% with chemotherapy alone. Responses were more durable in the nivolumab group with CPS ≥ 5 (median duration of response 9.6 vs. 7 mo) and all randomized patients (8.5 vs. 6.9 mo). Based on the initial report of OS benefit, nivolumab was FDA-approved in combination with fluoropyrimidine and platinum-containing chemotherapy for advanced or metastatic esophagogastric AC irrespective of PD-L1 expression. However, because the OS benefit was absent in tumors with low or absent PD-L1 expression, the EMA has restricted nivolumab approval to CPS ≥ 5 [32]. The NCCN recommendation for adding nivolumab to chemotherapy for CPS ≤ 5 is a category 2B recommendation [33].
KEYNOTE-590 was another phase III trial assessing the addition of ICI to chemotherapy with pembrolizumab for advanced esophagogastric cancer. Over 700 patients were randomized to pembrolizumab + chemotherapy (fluorouracil or cisplatin) and chemotherapy alone [34]. Both AC and SCC histological subtypes were included. In the initial analysis, pembrolizumab significantly improved OS compared to chemotherapy alone regardless of PD-L1 expression (12.4 vs. 9.8 mo); however, AC represented only 27% of the study population, and the survival benefit was likely driven more by SCC rather than AC (SCC OS 12.6 vs. 9.8 mo; AC OS 11.6 vs. 9.9 mo). When stratified by PD-L1 expression, the OS benefit was exclusively seen in tumors with CPS ≥ 10 (13.5 vs. 9.4 mo). For CPS < 10, there was no survival benefit with adding pembrolizumab (10.5 vs. 10.6 mo). Based on the initial survival data, the FDA approved pembrolizumab with platinum- and fluoropyrimidine-based chemotherapy for the treatment of advanced or metastatic esophageal and GEJ carcinoma regardless of PD-L1 expression [35]. On the other hand, the EMA has restricted approval to CPS ≥ 10 [36]. To better evaluate the role of pembrolizumab in esophagogastric AC, the KEYNOTE-859 trial investigated the addition of pembrolizumab in patients with untreated HER2-negative advanced gastric or GEJ AC [37]. In the study, 1579 patients were randomized to either pembrolizumab + chemotherapy (fluorouracil + cisplatin or capecitabine + oxaliplatin) or chemotherapy + placebo. At a median follow-up of 31 months, pembrolizumab improved OS (12.9 vs. 11.5 mo) in the entire study population regardless of CPS [38]. OS and PFS benefits were seen across subgroups of CPS ≥ 1, CPS ≥ 10, and MSI-H tumors. The NCCN recommendation for adding pembrolizumab to chemotherapy for CPS < 10 is a category 2B recommendation for both SCC and AC [33].
In a separate phase III study, KEYNOTE-062, 763 patients with previously untreated, advanced gastric or GEJ AC with CPS ≥ 1 were randomly assigned to pembrolizumab monotherapy, pembrolizumab + chemotherapy (cisplatin plus fluorouracil or capecitabine), or chemotherapy alone [39]. At a median follow-up of 29.4 months, pembrolizumab was non-inferior to chemotherapy (OS 10.6 vs. 11.1 mo). In an exploratory analysis, patients with CPS ≥ 10 experienced prolonged OS with pembrolizumab monotherapy compared to chemotherapy alone, although this was not statistically tested (17.4 vs. 10.8 mo). Pembrolizumab monotherapy is reasonable for patients unable to tolerate chemotherapy with positive PD-L1 expression.
HER2-negative advanced AC with intermediate PD-L1 expression (CPS 5–9) can be treated with combination immunotherapy and chemotherapy. Options include chemotherapy plus either nivolumab or pembrolizumab (NCCN category 2B for CPS < 10) based on the CheckMate-649 and KEYNOTE-859 trials [33]. As mentioned previously, in Checkmate-649, the survival benefit of adding nivolumab was seen with CPS ≥ 5 [30]. Additionally, the KEYNOTE-859 trial demonstrated an OS benefit with the addition of pembrolizumab to chemotherapy with CPS ≥ 1; however, it is unclear if the OS benefit is driven by those with CPS 5–9 [37]. Overall, more data is needed for this subgroup with intermediate expression. We recommend immunotherapy + chemotherapy for CPS 5–9 if there are no contraindications.
For patients with low or absent PD-L1 expression (CPS < 5), chemotherapy alone is recommended over chemoimmunotherapy as several randomized studies stratifying by PD-L1 expression show a lack of benefit with low or absent PD-L1 expression. Based on the combined analysis of data from the CheckMate-649, KEYNOTE-590, and KEYNOTE-062 trials, the addition of nivolumab or pembrolizumab for low or absent PD-L1 CPS expression (0–4) is not recommended [40]. Moreover, the addition of immunotherapy results in increased adverse events (AE). There are many differing expert opinions, and more data is needed, especially in those with intermediate PD-L1 expression.
Advanced Squamous Cell Carcinoma
Like advanced esophageal and gastric AC, advanced, unresectable, or metastatic SCC treatment is based on PD-L1 expression. For CPS ≥ 10, the addition of pembrolizumab to chemotherapy should be considered, given the survival benefit in the previously mentioned KEYNOTE-590 trial. For tumors with CPS ≥ 1, nivolumab with either chemotherapy or ipilimumab can also be considered based on CheckMate-648.
CheckMate-648 was an open-label, phase III trial that randomized 970 patients with previously untreated, unresectable, or metastatic esophageal SCC to either nivolumab + chemotherapy (fluorouracil + cisplatin), nivolumab + ipilimumab, or chemotherapy alone [41, 42]. Regardless of PD-L1 expression, adding nivolumab to chemotherapy improved OS (13.2 vs. 10.7 mo). The OS benefit was best seen with CPS ≥ 1 with OS 15.4 months compared to 9.1 months with chemotherapy alone. Nivolumab + ipilimumab also resulted in superior survival compared to chemotherapy alone in the entire population (12.7 vs. 10.7 mo). Like other cancers treated with immunotherapy, there was a delayed survival benefit of nivolumab + ipilimumab relative to nivolumab + chemotherapy and chemotherapy alone. In the Kaplan Meier analysis, while the survival curve for nivolumab + chemotherapy separates from the chemotherapy group early in the treatment, the combination nivolumab + ipilimumab overlaps chemotherapy until approximately 7 months, when the curve begins to separate. Further investigation is needed to characterize who may have early mortality compared to chemotherapy alone and may benefit from upfront chemotherapy or combination chemotherapy + immunotherapy [43]. Based on the CheckMate-648 trial, the FDA approved nivolumab in combination with either platinum + fluoropyrimidine-based chemotherapy or ipilimumab for the treatment of advanced or metastatic esophageal SCC, regardless of PD-L1 expression [44, 45]. Whether this should be carried into those with low PD-L1 expression is controversial; however, most patients with SCC in CheckMate-648 had tumors with CPS ≥ 1. The EMA has taken a more strict stance and restricts nivolumab to esophageal SCC with PD-L1 expression ≥ 1 [32].
Several meta-analyses have investigated the utility of ICI for low PD-L1 SCC. One meta-analysis with 1L trials of esophageal SCC evaluated by CPS noted a significant but modest benefit with combination chemotherapy + immunotherapy compared to chemotherapy alone for CPS < 10 [46]. The JUPITER-06 meta-analysis with five randomized clinical trials stratified by high or low PD-L1 expression showed a survival benefit with combination chemotherapy + immunotherapy compared to chemotherapy alone in patients with CPS < 10 [47]. In a separate meta-analysis that included 17 randomized phase III clinical trials for both first and second-line (2L) for SCC and AC, authors found that among patients with SCC, PD-L1 expression was the strongest predictor of benefit from immunotherapy [48]. The study also showed that PD-L1 expression was more common in SCC than AC and that those with SCC derived more benefit from immunotherapy than AC. Some argue that given the greater activity of immunotherapy in SCC, combination immunotherapy + chemotherapy can be considered for tumors with CPS < 10 as meta-analysis data suggests survival benefit in these patients, though less so than those with higher PD-L1 expression [48]. Many providers have a low threshold to hold immunotherapy if unfavorable features are present, such as lung disease predisposing to pneumonitis and CPS < 1. In addition, it is recommended to be selective in offering doublet immunotherapy in this patient population unless there are contraindications to chemotherapy [39, 41, 49, 50].
Deficient Mismatch Repair
Options for patients with dMMR esophagogastric cancers, most of which express PD-L1, include nivolumab or pembrolizumab plus cytotoxic therapy or immunotherapy alone. The addition of immunotherapy to chemotherapy or immunotherapy alone in those with dMMR/MSI-H esophagogastric cancer is supported by an exploratory analysis of pembrolizumab including 50 patients from KEYNOTE-062 (1L), 27 patients from KEYNOTE-061 (2L), and 7 patients from KEYNOTE-059 (≥ 3L) [51]. Patients with dMMR/MSI-H and CPS ≥ 1 who received chemotherapy + pembrolizumab had superior ORR (65 vs. 37%) and survival (OS not reached vs. 8.5 months) compared to chemotherapy alone. The authors also concluded that pembrolizumab monotherapy for dMMR/MSI-H tumors resulted in superior ORR (57% vs. 37%) and survival compared to chemotherapy alone, with median OS not reached vs. 8.5 months. Data from the previously mentioned CheckMate-649 trial and the KEYNOTE-158 trial with advanced dMMR/MSI-H non-colorectal cancers also support the role of immunotherapy in this patient population. The CheckMate-649 trial had a subset analysis with 44 patients with dMMR/MSI-H tumors [49]. Among these patients, those randomized to nivolumab + chemotherapy had a superior OS of 38.7 months compared to 12.3 months in the chemotherapy alone arm. The benefit was even more significant for patients with both dMMR and CPS ≥ 5, with a median OS of 44.8 vs. 8.8 months. The KEYNOTE-158 study with previously treated solid cancers with dMMR/MSI-H described susceptibility to pembrolizumab, resulting in its tumor agnostic approval [52]. GC represented 14.5% of the study population and was the second most common tumor type. Among those with GC, ORR was 31%, with PFS 3.2 months and median OS 11.0 months.
Challenges in Treatment
With the increasing use of immunotherapy, a substantial challenge is correctly identifying characteristics to predict response to immunotherapy. A common theme in treating upper gastrointestinal cancers is heavy reliance on PD-L1 expression to guide treatment. In esophagogastric cancer, CPS is a more sensitive prognostic marker and is thus used more widely than TPS [20]. The FDA-approved assays include PD-L1 IHC 22C3 pharmDx (used by Merck for pembrolizumab) and 28–8 pharmDx (used by Bristol Myers Squibb for nivolumab) [53]. Because PD-L1 expression is determined histomorphologically by a pathologist, there is a risk of interobserver variability. This was evident in an international study with 12 pathologists evaluating PD-L1 expression from 100 gastric and GEJ AC biopsies stained in a single laboratory using both 28–8 and 22C3. Despite the standard procedures and CPS training, there was high interobserver variability. Another challenge is the inconsistent concordance between primary tumors and metastases. In a retrospective analysis evaluating spatiotemporal heterogeneity in GC, 211 patients with 407 samples were evaluated [54]. Concordance for PD-L1 expression between primary and metastatic tumors was 61%. The concordance with primary tumors before and after chemotherapy was 57–63% and 73–75%, respectively. Intratumoral heterogeneity may also contribute to a low concordance rate in PD-L1 assessment [55]. The potential for interobserver variability, spatiotemporal heterogeneity, and the utilization of different assays by pharmaceutical companies could explain the differences in PD-L1 cutoffs for OS and PFS benefits in immunotherapy trials.
In GC, four tumor signatures predict response to immunotherapy, including the previously mentioned PD-L1 expression, dMMR/MSI-H, EBV-positivity, and TMB-H [56]. EBV+ and dMMR/MSI-H tumors have a higher sensitivity to immunotherapy, presumably due to their CD8+ T cell rich microenvironment and higher PD-L1 expression related to focal amplification of CD274 or IFN-gamma-mediated signaling [57, 58]. Highly mutated tumors (TMB-H) are more likely to harbor neoantigens that enhance immunogenicity and thus predict response to immunotherapy. Biomarkers beyond this are not fully developed and have prompted further investigation. In a prospective study, molecular characterization of tissue and ctDNA was performed on 61 patients with metastatic GC who were treated with pembrolizumab [57]. As expected, patients with MSI-H and EBV+ disease had dramatic responses to pembrolizumab with ORR 85.7% and 100%, respectively. Patients with PD-L1 positive disease had higher ORR than with PD-L1 negative tumors (50% vs. 0%). Decreases in ctDNA at 6 weeks post-treatment predicted favorable response and PFS. There was a high correlation between PD-L1 positivity and EBV-positivity and MSI-H. Because of this, routine testing for EBV-positivity may help identify patients with GC who will benefit from immunotherapy. The study also assessed distinct molecular subtypes and signatures defined through large genomic projects, the Cancer Genome Atlas (TCGA) project, and the Asian Cancer Research Group [59, 60]. The mesenchymal subtype at the gene profiling level was found to be a negative predictor of response to immunotherapy. Excluding MSI-H and EBV+ GC, the ORR for the mesenchymal subtype was 0% vs. 10% in the non-mesenchymal subtype. The lack of response was present despite corresponding tumors having high levels of the immune infiltrate signature. The mesenchymal subtype may contribute to immune escape and modulation of the TME, leading to decreased susceptibility to immune effector cells [61]. Further testing to confirm this correlation in a larger set of patients is needed. Currently, with the complex TME, no single biomarker is adequate to identify all patients with GC who will benefit from ICI [62].
Ongoing Studies/Future Directions
Despite the promise of immunotherapy in esophagogastric cancers, further novel treatments are needed to improve survival and quality of life. Tumors with high PD-L1 expression (CPS ≥ 5 62%), dMMR/MSI-H (4%), and/or TMB-H (13%) can be targeted with ICI, while HER2-positive cancers (22%) can be targeted with trastuzumab [56]. HER2-negative cancers without PD-L1 expression, dMMR/MSI-H, or TMB-H represent a unique group that does not fit within either category, and additional targeted therapies are needed.
Increasing attention has shifted to developing strategies to turn “cold” tumors with low PD-L1 expression into “hot” tumors. Dickkopf-1 (DKK1), a modulator of Wnt signaling, is overexpressed in many cancers and is associated with immunosuppressive effects [63]. Targeting DKK1 with the novel anti-DKK1 monoclonal antibody, DKN-01 results in a pro-inflammatory TME with the upregulation of PD-L1 levels. Its use has demonstrated antitumor activity in patients with advanced gastric and GEJ AC with low PD-L1 expression. DisTinGuish is a Phase II trial with 25 patients with HER2-negative gastric or GEJ AC who received 1L DKN-01, CAPOX (capecitabine and oxaliplatin), + tislelizumab, an Fc-optimized anti-PD-1 monoclonal antibody (NCT04363801) [64]. Most patients had low PD-L1 with CPS < 5 (70%). At 2 years of follow-up, DKN-01, CAPOX, + tislelizumab resulted in longer PFS and OS compared to the SOC regimen nivolumab + chemotherapy in both the overall population (PFS 11.3 vs. 7.7 mo; OS 19.5 vs. 13.8 mo) and in the PD-L1 low subgroup (PFS 10.7 vs. 7.5 mo; OS 18.7 vs 12.4 mo). Part C of the trial is evaluating mFOLFOX (modified folinic acid, fluorouracil, and oxaliplatin) or CAPOX + tislelizumab with or without DKN-01. The combination of DKN-01 + tislelizumab has shown promising therapeutic efficacy and may become the SOC for patients with PD-L1 low disease who otherwise have limited treatment options.
Another therapeutic target is claudin-18 isoform 2 (CLDN18.2), a tight junction protein normally expressed in gastric mucosa cells [65]. During malignant transformation, cell polarity is lost, and CLDN18.2 is exposed on the surface of gastric and GEJ AC, making it susceptible to antibodies. With up to 38% of patients with CLDN18.2-positive GC, there is growing interest in targeting the protein with Zolbetuximab. Zolbetuximab is a first-in-class chimeric monoclonal antibody that mediates cell death of CLDN18.2 positive cells through antibody-dependent and complement-dependent cellular cytotoxicity. Zolbetuximab has been efficacious in patients with CLDN18.2-positive HER2-negative advanced unresectable gastric and GEJ AC.
The SPOTLIGHT study was a phase III trial evaluating the combination of zolbetuzimab + mFOLFOX vs. mFOLFOX + placebo in patients with CLDN18.2 positive HER2-negative unresectable or metastatic gastric or GEJ AC [65]. The addition of zolbetuximab resulted in superior PFS compared to placebo (PFS 10.6 vs. 8.6 mo). Of note, the AE rate was high, and grade 3 or higher AEs occurred in 87% of patients vs. 78% in the placebo group. The most common grade 3 or higher AEs were nausea, vomiting, and decreased appetite. The GLOW trial was also a phase III study evaluating zolbetuximab with CAPOX vs. CAPOX + placebo for CLDN18.2 positive HER2-negative unresectable or metastatic gastric or GEJ AC [66]. PFS and OS favored the zolbetuximab arm, with PFS 8.21 vs. 6.8 months and OS 14.39 vs. 12.16 months in the placebo arm. Grade 3 or higher AEs were similar between arms. The ILUSTRO phase II trial is an ongoing multicohort study evaluating the safety and efficacy of zolbetuximab alone (cohort 1) or in combination with either mFOLFOX (cohort 2) or pembrolizumab (cohort 3) [67]. Cohort 1 with zolbetuximab monotherapy includes 27 patients. The ORR, disease control rate (DCR), PFS, and median OS are 0%, 44.4%, 1.54 months, and 5.62 months, respectively. Cohort 2 combining zolbetuximab + mFOLFOX has an ORR of 71.4%, DCR of 100%, and median duration of response of 15.9 months. Cohort 3 includes three patients receiving ≥ 3L zolbetuxumab + pembrolizumab. ORR, DCR, and median PFS are 0%, 66.7%, and 2.96 months, respectively. This study raises the question of whether immunotherapy or zolbetuximab should be utilized first in patients with both PD-L1-positive and CLDN18.2-positive tumors. Further investigation is needed to answer this. Additional monoclonal antibodies targeting CLDN18.2 include ASKB589 and osemitamab. ASKB859 is being studied with CAPOX and has shown promising results with ORR and DCR 75% and 100%, respectively (NCT04632108) [68]. The other CLND18.2 targeting antibody, Osemitamab, is thought to provide synergy when combined with ICI and is currently being studied with nivolumab, CAPOX (NCT04495296)[69], and mFOLFOX (NCT04396821)[70] in patients with gastric and GEJ AC.
Other trials targeting CLDN18.2 are underway, including bispecific antibodies and antibody–drug conjugates (ADC). In the gastric TME, CD137, or 4-1BB, is an inducible co-stimulatory molecule expressed on activated T-cells, natural killer cells, and regulatory T cells and is found near CLDN18.2. Signaling of 4-1BB results in activation of the MAPK signaling pathways and increased cytokine release. Therefore, a novel bispecific antibody TJ-CD4B, also known as ABL 111, was developed, targeting CLDN18.2 and 4-1BB to restrict activation within the TME [71]. In March 2022, the FDA granted an Orphan Drug Designation for TJ-CD4B to treat gastric and GEJ cancer [72]. The bispecific antibody is currently being evaluated in a phase I dose-escalation and dose-expansion study for patients with advanced solid tumors (NCT04900818) [73]. PT886 is an additional bispecific antibody targeting CLDN18.2 and CD47, an immunoglobulin overexpressed in multiple tumor types, and is associated with decreased phagocyte activity. PT886 is being studied in a phase I trial for advanced gastric, GEJ, and pancreatic AC (NCT05482893) [74]. The ADC, CMG901, composed of anti-CLDN18.2 monoclonal antibody and monomethyl auristatin E as the cytotoxic payload, was granted FDA fast-track designation in April 2022 as monotherapy for unresectable GC and GEJ cancer refractory to approved therapies. In the interim analysis of a phase Ia trial with 13 patients with advanced GC or GEJ and 14 patients with pancreatic cancer receiving CMG901, ORR and DCR were 75% and 100%, respectively [75]. The phase Ib dose-expansion phase is currently enrolling patients with solid cancers (NCT04805307).
Chimeric antigen receptor (CAR) T cells (CAR-T) have also entered the therapeutic landscape of esophagogastric cancers with a specific focus on targeting CLDN18.2. CT041 is composed of genetically engineered autologous T cells that express CLDN18.2 targeted CAR, which include a humanized single-chain variable fragment, a CD8α hinge region, a CD28 co-stimulatory domain, and a CD3ζ signaling domain [76]. In a phase II single-arm study, 37 patients with previously treated CLDN18.2-positive gastrointestinal cancers were treated with CT041 (NCT03874897) [76]. The ORR and DCR reached 48.6% and 73%, respectively. At 6 months, the survival rate was 81%. Results show promising efficacy with an acceptable toxicity profile in those with heavily pre-treated gastrointestinal cancers, particularly those with GC. In a separate trial, 11 patients with previously treated CLDN18.2-positive metastatic GC (five patients) or pancreatic cancer (six patients) received CT041 (NCT04404595) [77]. In an interim analysis published in June 2022, three patients with GC were evaluated for response. One patient achieved a complete response, and two patients had a partial response with an ORR of 100%. There were no dose-limiting toxicities, treatment-related deaths, severe cytokine release syndrome (CRS), or immune effector cell-associated neurogenic syndrome (ICANS) observed. The preliminary results of this study revealed encouraging safety and therapeutic efficacy.
Another immunotherapy strategy is PD-1 and anti-T cell immunoglobulin and ITM (TIGIT) blockade [78]. The dual inhibition yields increased tumor antigen-specific CD8+ T cell expansion and potent antitumor activity. The EDGE-Gastric trial (NCT05329766) is a study exploring the safety and efficacy of combination anti-TIGIT monoclonal antibody, domvanalimab, anti-PD-1 monoclonal antibody, zimberelimab, and chemotherapy for 1L treatment of unresectable esophageal, gastric, and GEJ AC. In an interim analysis (June 2023), 41 patients were evaluated. ORR was promising, particularly in patients with PD-L1 high expression (ORR intent-to-treat 59%; PD-L1 high ORR 80%, PD-L1 low ORR 46%). In the PD-L1 high group, median PFS was not reached, and 93% were progression-free at 6 months. With this encouraging data, the STAR-221 trial (NCT05568095) will compare domvanalimab + zimberelimab + chemotherapy to nivolumab + chemotherapy for 1L advanced esophageal, gastric, and GEJ AC [79].
Another target, fibroblast growth factor receptor 2b (FGFR2b), is overexpressed in about 30% of HER2-negative GC [80]. Bemarituzumab is a first-in-class monoclonal antibody blocking FGFR2b that has demonstrated efficacy in FGFR2b-overexpressing advanced gastric and GEJ AC when combined with FOLFOX (oxaliplatin + leucovorin + fluorouracil) [80]. Preclinical studies show that bemarituzumab modulates the TME, inducing natural killer cell-dependent increases in PD-L1, providing the rationale for combining this regimen with nivolumab [81]. FORTITUDE-102 is a phase Ib/III trial in progress evaluating the combination of bemarituzumab with FOLFOX + nivolumab vs. FOLFOX + nivolumab in the 1L setting of advanced or metastatic gastric and GEJ AC (NCT05111626) [82]. If this trial is positive, combining chemotherapy, nivolumab, and bemarituzumab could be a new SOC in patients with FGFR2b-overexpressing AC.
Like FGFR2b, the immune modulatory capabilities and anti-tumor properties make anti-angiogenic tyrosine kinase inhibitors (TKI) an additional ideal class for synergizing with immunotherapy. TKIs induce immunogenic modulation, resulting in tumor cells sensitization to killing by T-cells and immune subset conditioning, increasing the function of effector immune elements and decreasing the number and function of immune suppressor cells [83]. The LEAP-015 trial is a randomized, open-label phase III study evaluating the efficacy of lenvatinib + pembrolizumab + chemotherapy for the 1L treatment of HER2-negative advanced esophagogastric AC (NCT04662710) [84]. In the safety run-in portion of the trial, 15 patients received lenvatinib with pembrolizumab and chemotherapy. Treatment was associated with a manageable safety profile. Part 2, evaluating the efficacy and safety of this combination, is not yet published.
Conclusions
The treatment landscape for esophagogastric cancers is rapidly evolving. There is a shift away from traditional non-targeted chemotherapy and more focus on the addition of targeted therapy and immunotherapy. Immunotherapy has been integrated into the management of localized esophageal and unresectable or metastatic esophageal, gastric, and GEJ cancers. There are differences in ICI approvals from the FDA and EMA as subset analysis of randomized clinical trials shows benefit is often restricted to specific levels of PD-L1 expression. Molecular testing has provided valuable advances in personalized medicine to better predict response to immunotherapy. PD-L1 expression, dMMR/MSI-H, EBV-positivity, and TMB-H are associated with response to PD-1-based ICI. EBV-positivity is not routinely tested, but given the positive predictive value, its testing in clinical practice should be considered. At this time, there is no single biomarker adequate to identify all patients with esophagogastric cancer who will benefit from ICI, likely due to the complex TME. Additional challenges in biomarkers include the spatiotemporal heterogeneity in PD-L1 expression with CPS and TPS. Esophagogastric cancers without PD-L1 expression, dMMR/MSI-H, TMB-H, or HER2-positivity represent a unique category with few options for targeted therapy. Studies to further target this subset of patients are ongoing. Targeting CLDN18.2 has become increasingly popular as CLDN18.2-based monoclonal antibody therapy, and CAR-T have shown promising results in GC. As more investigation is dedicated to predictive biomarkers and targeted therapy, we anticipate the emergence of novel treatments to improve survival in those with upper gastrointestinal cancers.
Data Availability
No datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Wang DK, Zuo Q, He QY, Li B. Targeted immunotherapies in gastrointestinal cancer: from molecular mechanisms to implications. Front Immunol. 2021;12:705999.
Shimozaki K, Nakayama I, Hirota T, Yamaguchi K. Current strategy to treat immunogenic gastrointestinal cancers: perspectives for a new era. Cells. 2023;12(7).
Sidaway P. Immunotherapy-responsive gastric cancers identified. Nat Rev Clin Oncol. 2018;15(10):590-.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84.
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384(13):1191–203.
Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, et al. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol (London, England). 2021;17(10):1143–53.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Pietrantonio F, Miceli R, Raimondi A, Kim YW, Kang WK, Langley RE, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37(35):3392–400.
Nie RC, Chen GM, Yuan SQ, Kim JW, Zhou J, Nie M, et al. Adjuvant chemotherapy for gastric cancer patients with mismatch repair deficiency or microsatellite instability: Systematic Review and Meta-Analysis. Ann Surg Oncol. 2022;29(4):2324–31.
André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2023;41(2):255–65.
Pietrantonio F, Raimondi A, Lonardi S, Murgioni S, Cardellino GG, Tamberi S, et al. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023;41(4_suppl):358-.
Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future oncology (London, England). 2019;15(9):943–52.
Al-Batran S-E, Lorenzen S, Thuss-Patience PC, Homann N, Schenk M, Lindig U, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol. 2022;40(16_suppl):4003-.
Janjigian YY, Van Cutsem E, Muro K, Wainberg Z, Al-Batran SE, Hyung WJ, et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future oncology (London, England). 2022;18(20):2465–73.
Shitara K. LBA74 Pembrolizumab plus chemotherapy vs chemotherapy as neoadjuvant and adjuvant therapy in locally-advanced gastric and gastroesophageal junction cancer: The phase III KEYNOTE-585 study. Ann Oncol. 2023;34.
Al-Batran S-E, Lorenzen S, Thuss-Patience PC, Homann N, Schenk M, Lindig U, et al. A randomized, open-label, phase II/III efficacy and safety study of atezolizumab in combination with FLOT versus FLOT alone in patients with gastric cancer and adenocarcinoma of the oesophagogastric junction and high immune responsiveness: The IKF-S633/DANTE trial, a trial of AIO in collaboration with SAKK. J Clin Oncol. 2023;41(16_suppl):TPS4177-TPS.
Janjigian. LBA73 - Pathological complete response (pCR) to durvalumabplus 5-fl uorouracil, leucovorin, oxaliplatin and docetaxel(FLOT) in resectable gastric and gastroesophageal junctioncancer (GC/GEJC): Interim results of the global, phase IIIMATTERHORN study. Ann Oncol. 2023.
Data are still insufficient to recommend immune checkpoint inhibitors in perioperative therapy for gastric/gastro-oesophageal junction cancers [cited 2023]. Available from: https://dailyreporter.esmo.org/esmo-congress-2023/gastrointestinal-cancers/data-are-still-insufficient-to-recommend-immune-checkpoint-inhibitors-in-perioperative-therapy-for-gastric-gastro-oesophageal-junction-cancers.
Yamashita K, Iwatsuki M, Harada K, Eto K, Hiyoshi Y, Ishimoto T, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23(1):95–104.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4(5):e180013.
FDA grants accelerated approval to pembrolizumab for advanced gastric cancer. 2017. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-advanced-gastric-cancer.
Tucker N. Pembrolizumab withdrawn from US market as option for third-line gastric or GEJ adenocarcinoma. 2021 [November 19, 2023]. Available from: https://www.targetedonc.com/view/pembrolizumab-withdrawn-from-us-market-as-option-for-third-line-gastric-or-gej-adenocarcinoma.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci USA. 2011;108(17):7142–7.
Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30.
Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023.
FDA amends pembrolizumab’s gastric cancer indication. 2023. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-amends-pembrolizumabs-gastric-cancer-indication.
EMA adopts a positive opinion for a new indication for pembrolizumab. 2023. Available from: https://www.esmo.org/oncology-news/ema-adopts-a-positive-opinion-for-a-new-indication-for-pembrolizumab.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
Janjigian YY, Shitara K, Moehler MH, Garrido M, Gallardo C, Shen L, et al. Nivolumab (NIVO) plus chemotherapy (chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): 3-year follow-up from CheckMate 649. J Clin Oncol. 2023;41(4_suppl):291-.
Opdivo: European Medicines Agency. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo.
National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology (NCCN Guidelines): esophageal and esophagogastric junction cancers. Available at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed on 19 Dec 2023.
Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–71.
FDA approves pembrolizumab for esophageal or GEJ carcinoma. 2021. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-esophageal-or-gej-carcinoma.
European Medicines Agency. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda.
Tabernero J, Bang YJ, Van Cutsem E, Fuchs CS, Janjigian YY, Bhagia P, et al. KEYNOTE-859: a Phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol (London, England). 2021;17(22):2847–55.
Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–95.
Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80.
Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40(4):392–402.
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–62.
Kato K, Ajani JA, Doki Y, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab (NIVO) plus chemotherapy (chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): 29-month (mo) follow-up from CheckMate 648. J Clin Oncol. 2023;41(4_suppl):290-.
Chao I. Abstract: O-3 Nivolumab (NIVO) plus chemotherapy (chemo) or ipilimumab (IPI) vs chemo as first-line treatment for advanced esophageal squamous cell carcinoma (ESCC): Expanded efficacy and safety analyses from CheckMate 648. Ann Oncol. 2022;33.
Opdivo-nivolumab injection. 2023. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4de2-abfa-4d0a4ae4e394.
Yervoy: ipilimumab injection. 2023. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2265ef30-253e-11df-8a39-0800200c9a66.
Yap DWT, Leone AG, Wong NZH, Zhao JJ, Tey JCS, Sundar R, et al. Effectiveness of immune checkpoint inhibitors in patients with advanced esophageal squamous cell carcinoma: a meta-analysis including low PD-L1 subgroups. JAMA Oncol. 2023;9(2):215–24.
Wu HX, Pan YQ, He Y, Wang ZX, Guan WL, Chen YX, et al. Clinical benefit of first-line programmed death-1 antibody plus chemotherapy in low programmed cell death ligand 1-expressing esophageal squamous cell carcinoma: a post hoc analysis of JUPITER-06 and meta-analysis. J Clin Oncol. 2023;41(9):1735–46.
Yoon HH, Jin Z, Kour O, Kankeu Fonkoua LA, Shitara K, Gibson MK, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol. 2022;8(10):1456–65.
Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942–8.
Yoon HH, Dong H, Shi Q. Impact of PD-1 blockade in nonresponders: pitfalls and promise. Clin Cancer Res. 2022;28(15):3173–5.
Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7(6):895–902.
Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33(9):929–38.
Robert ME, Rüschoff J, Jasani B, Graham RP, Badve SS, Rodriguez-Justo M, et al. High interobserver variability among pathologists using combined positive score to evaluate PD-L1 expression in gastric, gastroesophageal junction, and esophageal adenocarcinoma. Mod Pathol. 2023;36(5): 100154.
Zhou KI, Peterson B, Serritella A, Thomas J, Reizine N, Moya S, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res. 2020;26(24):6453–63.
Ye M, Huang D, Zhang Q, Weng W, Tan C, Qin G, et al. Heterogeneous programmed death-ligand 1 expression in gastric cancer: comparison of tissue microarrays and whole sections. Cancer Cell Int. 2020;20:186.
Pihlak R, Fong C, Starling N. Targeted therapies and developing precision medicine in gastric cancer. Cancers (Basel). 2023;15(12).
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Nakano H, Saito M, Nakajima S, Saito K, Nakayama Y, Kase K, et al. PD-L1 overexpression in EBV-positive gastric cancer is caused by unique genomic or epigenomic mechanisms. Sci Rep. 2021;11(1):1982.
Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449–56.
Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11(7):824–46.
Chen Y, Jia K, Sun Y, Zhang C, Li Y, Zhang L, et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat Commun. 2022;13(1):4851.
Haas MS, Kagey MH, Heath H, Schuerpf F, Rottman JB, Newman W. mDKN-01, a novel anti-DKK1 mAb, enhances innate immune responses in the tumor microenvironment. Mol Cancer Res. 2021;19(4):717–25.
Klempner SJ, Sonbol BB, Wainberg ZA, Uronis HE, Chiu VK, Scott AJ, et al. A phase 2 study (DisTinGuish) of DKN-01 in combination with tislelizumab + chemotherapy as first-line (1L) therapy in patients with advanced gastric or GEJ adenocarcinoma (GEA). J Clin Oncol. 2023;41(16_suppl):4027-.
Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401(10389):1655–68.
Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29(8):2133–41.
Klempner SJ, Lee KW, Shitara K, Metges JP, Lonardi S, Ilson DH, et al. ILUSTRO: phase II multicohort trial of zolbetuximab in patients with advanced or metastatic Claudin 18.2-positive gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2023;29(19):3882–91.
Zhang M, Gong J, Wang J, Shi J, Zhu H, Wang Y, et al. A phase I/II study of ASKB589 (anti-claudin 18.2 [CLDN18.2] monoclonal antibody) in patients with solid tumors. J Clin Oncol. 2023;41:397-.
A trial to evaluate safety, tolerability, pharmacokinetics and preliminary efficacy of TST001 in advanced or metastatic solid tumors. ClinicalTrials.gov identifier: NCT04495296. Updated March 3, 2023. Accessed on 19 Dec 2023. https://www.clinicaltrials.gov/study/NCT04495296?term=NCT04495296&rank=1.
A trial to evaluate safety and tolerability of TST001 in advanced or metastatic solid tumors. ClinicalTrials.gov identifier: NCT04396821. Updated December 13, 2023. Accessed on 19 Dec 2023. https://www.clinicaltrials.gov/study/NCT04396821?term=NCT04396821&rank=1.
Gao J, Wang Z, Jiang W, Zhang Y, Meng Z, Niu Y, et al. CLDN18.2 and 4–1BB bispecific antibody givastomig exerts antitumor activity through CLDN18.2-expressing tumor-directed T-cell activation. J Immunother Cancer. 2023;11(6).
I-Mab announces first patient dosed in phase 1 clinical trial of Claudin 18.2 and 4–1BB bispecific antibody TJ-CD4B in solid tumors in China: I-Mab Biopharma. 2022. Available from: https://www.i-mabbiopharma.com/i-mab-announces-first-patient-dosed-in-phase-1-clinical-trial-of-claudin-18-2-and-4-1bb-bispecific-antibody-tj-cd4b-in-solid-tumors-in-china/.
Study of TJ033721 in subjects with advanced or metastatic solid tumors. ClinicalTrials.gov identifier NCT04900818. Updated June 22, 2023. Accesed on 19 Dec 2023. https://clinicaltrials.gov/study/NCT04900818?cond=TJ033721&rank=1#study-plan.
PT886 for treatment of patients with advanced gastric, gastroesophageal junction and pancreatic adenocarcinomas. ClinicalTrials.gov identifier: NCT05482893. Updated June 15, 2023. Accessed on 19 Dec 2023. https://clinicaltrials.gov/study/NCT05482893?term=NCT05482893&rank=1.
Xu R. A phase 1a dose-escalation, multicenter trial of anti-claudin 18.2 antibody drug conjugate CMG901 in patients with resistant/refractory solid tumors. J Clin Oncol. 2023;41(4_suppl):352-.
Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28(6):1189–98.
Botta GP, Becerra CR, Jin Z, Kim DW, Zhao D, Lenz H-J, et al. Multicenter phase Ib trial in the U.S. of salvage CT041 CLDN18.2-specific chimeric antigen receptor T-cell therapy for patients with advanced gastric and pancreatic adenocarcinoma. J Clin Oncol. 2022;40(16_suppl):2538-.
Janjigian YY, Oh D-Y, Pelster M, Wainberg ZA, Sison EAR, Scott JR, et al. EDGE-Gastric Arm A1: phase 2 study of domvanalimab, zimberelimab, and FOLFOX in first-line (1L) advanced gastroesophageal cancer. J Clin Oncol. 2023;41(36_suppl):433248-.
A clinical trial of a new combination treatment, domvanalimab and zimberelimab, plus chemotherapy, for people with an upper gastrointestinal tract cancer that cannot be removed with surgery that has spread to other parts of the body (STAR-221).
Wainberg ZA, Enzinger PC, Kang Y-K, Yamaguchi K, Qin S, Lee K-W, et al. Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT). J Clin Oncol. 2021;39(3_suppl):160-.
Xiang H, Chan AG, Ahene A, Bellovin DI, Deng R, Hsu AW, et al. Preclinical characterization of bemarituzumab, an anti-FGFR2b antibody for the treatment of cancer. MAbs. 2021;13(1):1981202.
Wainberg ZA, Cutsem EV, Moehler MH, Kang Y-K, Yen P, Finger E, et al. Trial in progress: phase 1b/3 study of bemarituzumab + mFOLFOX6 + nivolumab versus mFOLFOX6 + nivolumab in previously untreated advanced gastric and gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-102). J Clin Oncol. 2022;40(16_suppl):TPS4165-TPS.
Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron. 2015;2(1).
Yanez PE, Ben-Aharon I, Rojas C, Eyzaguirre DA, Hubert A, Araya H, et al. First-line lenvatinib plus pembrolizumab plus chemotherapy versus chemotherapy in advanced/metastatic gastroesophageal adenocarcinoma (LEAP-015): safety run-in results. J Clin Oncol. 2023;41(4_suppl):411-.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript. Material preparation, data collection and analysis were performed by N.B.B. and S.S.K. The first draft of the manuscript was written by N.B.B. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
S.S.K has served on advisory boards for Merck, Eisai, Bristol Myers Squibb,, Daiichi Sankyo and received research funding from Merck.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balmaceda, N.B., Kim, S.S. Immunotherapy in Esophagogastric Cancer: Treatment Landscape, Challenges, and New Directions. J Gastrointest Canc 55, 153–167 (2024). https://doi.org/10.1007/s12029-023-01000-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-023-01000-8