Abstract
Purpose
The prognostic nutritional index (PNI), like other systemic inflammatory markers, has been shown to be a prognostic factor in various cancer patients. In this study, we aimed to show whether PNI calculated before adjuvant chemotherapy is a prognostic factor for overall survival (OS) and disease-free survival (DFS) in patients with lymph node-positive stage II-III gastric cancer.
Methods
The PNI was calculated using the albumin and lymphocyte count. The PNI cut-off value was found to be 39.5. They were divided into two groups as being ≤ 39.5 (PNI low group) and > 39.5 (PNI high group).
Results
Our study included 168 patients with lymph node-positive stage II-III gastric cancer who received adjuvant chemotherapy. Of the patients, 116 (69.0%) were 65 years or younger, and 52 (31.0%) were over 65 years old. Of the patients, 117 (69.6%) were pT3, 51 (30.4%) were pT4. Seventy-three (43.4%) patients had pN1-2 disease and 95 (56.6%) patients had pN3 disease. The number of stage II patients was 73 (43.5%) and the number of stage III patients was 95 (56.5%). There were 73 patients with PNI ≤ 39.5 and 95 patients with PNI > 39.5. The mOS of the patients with low PNI group was 39.5 months, while the OS of the patients with high PNI group was 96.8 months (p = 0.002). In the group of patients with PNI low group, mDFS 24.4 months was significantly higher than those with PNI high group was 50.7 months (p = 0.021). The PNI score was statistically significant in univariate and multivariate analyzes for both DFS and OS.
Conclusion
PNI can be used as an independent prognostic factor for both OS and DFS in patients lymph node-positive, stage II-III gastric cancer who will receive adjuvant chemotherapy.
Key Points
-
1.
Prognostic nutritional index, an inflammatory marker, has been shown to be a prognostic factor in cancer patients.
-
2.
The prognostic nutritional index is an independent predictor of both disease-free survival and overall survival in patients with lymph node-positive gastric cancer receiving adjuvant chemotherapy.
-
3.
The prognosis is worse in those with a low prognostic nutritional index. Both overall survival and disease-free survival times are significantly longer in those with a high prognostic nutritional index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the 5th most common cancer and the 3rd most common cause of cancer-related deaths [1]. However, the long-term outcomes of patients with GC remain poor, particularly for those with advanced disease. The 5-year survival rate in patients with early GC is 85–100%, while it is only 5–20% for advanced GC patients [2]. Adjuvant treatments are used after resection to improve survival in patients with locally advanced GC [3]. Although there is variability in the results of meta-analyses of phase III studies showing the efficacy of adjuvant therapy in the literature, there are few meta-analyses that support a significant survival advantage for adjuvant chemotherapy, and it has been shown that the risk of death is reduced by 15% in patients receiving adjuvant chemotherapy [4,5,6]. Currently, the Tumor-Node-Metastasis (TNM) classification is the most generally accepted predictor of long-term outcome and selection of adjuvant therapies for GC in clinical practice [7]. Other biomarkers should be identified to more precisely predict individual outcomes and develop individualized treatment strategies for patients with GC. The relationship between cancer development and inflammation has increased interest in the prognostic significance of inflammatory markers [8]. In cancer patients, serum albumin level is an indicator of nutritional status and is also considered a biomarker of immune inflammatory reaction [9]. It has been reported that there is a significant relationship between serum albumin level and C-reactive protein (CRP) level, which is considered one of the markers of systemic inflammation [10]. In addition, lymphocyte is widely accepted as an important index on both immune inflammatory status and body nutrition [11]. Based on these features, a prognostic nutritional index (PNI) was created by combining the serum albumin level with the total lymphocyte count [12]. In this study, we aimed to show whether the PNI calculated before adjuvant chemotherapy in gastric cancer is a prognostic factor for OS and DFS of patients.
Matrials and Methods
Study Population
For our study, the files of 1425 GC patients registered in the archive of the medical oncology outpatient clinic of our hospital were scanned. Patients who underwent primary tumor surgery + D2 lymph node dissection between January 2015 and December 2018, had pathological T3/T4 tumor and pathological lymph node positive, pathological stage II or stage III tumor, received at least 3 cycles of adjuvant chemotherapy and were followed for at least 3 years, a total of 168 patients were included. Those with stage I or stage IV tumors, pathological stage II-III and lymph node negative and not receiving adjuvant chemotherapy, incomplete file data, secondary malignancy or active autoimmune disease were excluded from the study. Our study was approved by the local ethics committee (Approval number:2022/3974). This study was conducted in agreement with the Declaration of Helsinki-Ethical principle.
Data Collection and Measurements
Gender, age, tumor localization, histological type, Her 2 status, tumor size, pT stage, pN stage, pathological stage, lymphovascular invasion (LVI) and perineural invasion, stage, type of adjuvant chemotherapy, adjuvant radiotherapy (RT), death or recurrence were recorded from the files. In addition, the laboratory parameters measured before adjuvant chemotherapy, lymphocyte and albumin values were recorded. The prognostic nutritional index score was calculated using the formula (10 × serum Albumin, g/dL) + (0.005 × blood lymphocyte count, unit/L) from lymphocyte and albumin values. OS was defined as the time from diagnosis to death from any cause. DFS was defined as the time from the start of adjuvant chemotherapy in patients to relapse.
Statistical Analysis
SPSS (SPSS 22.0; IBM Inc., Chicago, IL, USA) program was used for statistical analysis. Descriptive statistics were presented as median (min-max) and percentage. Chi-square test was applied for categorical variables. Kaplan-Meier analysis was performed for OS and DFS analyses, and log rank test was used. Univariate and multivariate analyzes were used to evaluate the prognostic value of each variable for OS and DFS. Receiver operating characteristic (ROC) curve analyzes were performed to determine the optimal cutoff value of the PNI. The optimal cut-off values for PNI were 39.5 with 50% sensitivity and 61% specificity [AUC = 0.558 (95% confidence interval [CI]: 0.471–0.646)]. The PNI cut-off value was found to be 39.5. They were divided into two groups as being ≤ 39.5 and > 39.5. Clinicopathological features and survival outcomes were compared between the groups. A p value < 0.05 was considered to be statistically significant.
Results
In our study, there were 168 patients who were operated on and received adjuvant chemotherapy. The general characteristics of the patients included in our study are given in Table 1. Of the patients, 113 (67.2%) were male and 55 (32.8%) were female. One hundred sixteen (69.0%) patients were 65 years or younger, 52 (31.0%) patients were older than 65 years. The Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 53 patients was 0, ECOG PS 1 of 90 patients and ECOG PS 2 of 25 patients. There was no statistically significant relationship between ECOG PS and survival and metastasis. There were 3 groups according to primary tumor location, 34 (20.2%) patients had gastroesofagial junction-cardia tumor, 60 (35.8%) patients had corpus tumor, and 74 (44.0%) patients had distal gastric cancer. Signet ring cell component was detected in 29 (17.1%) of 168 patients. Nine patients were Her-2 3+. The Her-2 status of 40 patients was not evaluated. They were divided into two groups according to the median tumor size as ≤ 55 mm and > 55 mm. There were 87 (51.7%) patients with a tumor size of ≤ 55 mm and 81 (48.3%) patients with a tumor size of > 55 mm. One hundred seventeen (69.6%) of the patients were pT3, 51 (30.4%) of them were pT4. Seventy three (43.4%) patients had pN1-2 disease and 95 (56.6%) patients had pN3 disease. There were 97 (57.8%) patients with grade 1–2 and 71 (42.2%) patients with grade 3. Perineural invasion was positive in 68.5% of the patients and LVI was positive in 70% of the patients. There were 73 (43.5%) patients with stage II and 95 (56.5%) patients with stage III. The 51.2% of patients received FOLFOX/CAPEOX, 24.4% of FUFA/Capecitabine and 24.4% of patients received CF ± taxane adjuvant chemotherapy. 133 patients received adjuvant RT. During follow-up, 88 (52.4%) of the patients developed recurrence/metastasis. Ninety-two (54.8%) patients died during follow-up.
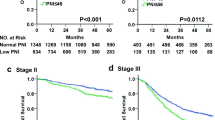
Our study group was divided into two groups as PNI ≤ 39.5 and PNI > 39.5. There were 73 patients with PNI ≤ 39.5 and 95 patients with PNI > 39.5. Metastasis was observed in 46 (52.4%) patients in the PNI low group and in 42 (25%) patients in the PNI high group (p = 0.990). There was a statistically significant difference between the groups in terms of age, histology, and final status (p < 0.05 for all) (Table 1). The mOS of patients with PNI ≤ 39.5 was 39.5 (21.6–57.4) months, and those with > 39.5 had a mOS of 96.8 (74.8-114.8) months. This difference was statistically significant (p = 0.002) (Fig. 1). In the group of patients with PNI ≤ 39.5, mDFS (24.4 [5.6–43.1] months) was significantly higher than those with > 39.5 (50.7 [40.5–78.3] months) (p = 0.021) (Fig. 2). Median OS and DFS, PNI were found to be statistically significantly lower in the low group. In univariate analysis, PNI level (≤ 39.5, > 39.5), sex (male, female), age (≤ 65, > 65), histology, tumor size (≤ 55 mm, > 55 mm), LVI, perineural invasion, grade, pT stage, pN stage, pathological stage, adjuvant RT (yes, no), adjuvant chemotherapy type were evaluated. PNI, tumor size, LVI, perineural invasion, pN stage, pT stage, pathological stage were found to be significant for OS (p < 0.05 for all) (Table-2). In the multivariate analysis for OS, PNI, tumor size, pN stage, pT stage were found to be significant as prognostic markers (p = 0.019, p = 0.045, p = 0.028, p = 0.008, respectively) (Table-2). A low PNI (hazard ratio [HR],0.598; 95% CI, 0.388–0.919, P = 0.019) was found to be an independent prognostic factor that adversely affected OS, as a result of cox multivariate analysis. In the univariate analysis for DFS, PNI, LVI, perineural invasion, grade, pT stage, pN stage, and pathological stage were found to be statistically significant (p < 0.05 for all) (Table-3). In the final multivariate analysis for DFS, PNI (≤ 39.5, > 39.5), perineural invasion status, and pT stage prognostic marker were determined (p = 0.032, p = 0.040, p = 0.020, respectively) (Table-3). A low PNI (hazard ratio [HR],0.639; 95% CI, 0.424–0.963, P = 0.032) was found to be an independent prognostic factor that adversely affected DFS, as a result of cox multivariate analysis.
Discussion
Prognostic nutritional index has been shown to be a prognostic factor in many types of cancer. Lower PNI was found to be associated with worse prognosis. In our study, we found that patients with low PNI had significantly shorter OS and DFS. We also show that PNI are independent predictors for OS and DFS. These results are compatible with the literature.
In recent years, studies have increasingly focused on assessing the prognostic significance of PNI as an indicator of the patient’s immunological and nutritional status. Numerous studies have shown that PNI can be used as a prognostic factor for survival in cancer patients [13]. The prognostic effect of PNI has been extensively studied in cancer patients as well as in other diseases. Because the cancer microenvironment is a dynamically changing environment, the cut-off value for PNI was different in each study, and clear values for practical use could not be determined [14]. For our study, the PNI cut-off value calculated by statistical analysis was 39.5. Nutritional factors play an important role in GC prognosis and nutritional status is thought to be a reflection of changes in the tumor microenvironment. It is quite natural to discuss the effects of nutritional factors in this disease process and treatment in malignant tumors of the stomach, which is the most important organ related to digestion and nutrition. It is thought that patients with high PNI are in good nutritional status and have better tolerance to treatment and, as a result, better adherence to treatment, which is associated with better long-term prognosis [14].
Regarding the prognostic significance of PNI in GC, a limited number of studies are available and a definite consensus has not been established. In one of the first studies evaluating PNI in GC, the relationship between the preoperative PNI value and the clinicopathological features of the patients was investigated. In this study, it was reported that low preoperative PNI value was significantly correlated with greater tumor depth, lymph node metastasis, presence of LVI, and PNI value was an independent prognostic indicator in GC [15]. In another studies, Migita K. et al., in his study, the differences in the causes of survival and death between the PNI-high and PNI-low groups at each stage of GC were investigated. In patients with stage I-III disease, the PNI-low group had a significantly lower rate of OS and relapse-free survival than the PNI-high group [16]. In a meta-analysis of 10 studies involving 3396 patients with GC, it was reported that a low PNI was an important predictor of poor OS and postoperative complications. It has been reported that low PNI is significantly associated with worse OS in patients with stage I, II and III GC, but not in stage IV. In addition, low PNI has been reported to be significantly associated with more advanced tumor characteristics such as older age, deeper tumor depth, positive lymph node metastasis, more advanced TNM stages, and positive LVI [17]. In a recent study investigating whether PNI is a prognostic factor for survival in elderly GC patients after gastrectomy, the cut-off value for PNI was 46.5, and the 5-year overall survival rate of patients with a PNI score < 46.5 and a PNI score ≥ 46.5 was found to be statistically significantly, 38.2% and 49.3% respectively. According to multivariate analysis, PNI score was found to be an independent prognostic factor. It has been reported that PNI can be used to predict OS in elderly GC patients [18]. At the same time, malnutrition patients have a lower tolerance for adverse drug reactions during chemotherapy, which requires interruption or discontinuation of chemotherapy, resulting in a worse response to chemotherapy. As a simple and usable nutritional test, PNI can be used as an indicator of individual nutritional status and is widely used to predict the prognosis of various malignancies as well as to assess whether perioperative and postoperative complications will develop [14, 19, 20]. There is no study in the literature showing that PNI predicts the efficacy of adjuvant therapy in patients with GC. Our study is the first to investigate this situation. In our study, mOS 39.5 months and mDFS 24.4 months in patients with low PNI were found to be significantly lower than the group with high PNI (mOS 96.8 months and mDFS 50.7 months).
In a recent study, it was reported that the pre-treatment systemic immun index (SII)-PNI score is an important indicator for predicting the chemosensitivity of patients who have locally advanced after capecitabine plus oxaliplatin chemotherapy combined with sintilimab immunotherapy, and may help identify high-risk groups and predict prognosis [21]. Based on this latest study, it was kind of confirmed that pre-treatment PNI could also predict chemosensitivity. For this reason, we have shown that we can predict the survival times of patients who receive adjuvant chemotherapy in GC, and whether they will relapse or not, with the PNI value checked before the treatment.
There are some limitations in our study. Since our design was retrospective, we could not reach some parameters such as comorbidities, weight change, medications used. Previous studies have always found different cutoff points for PNI. Comparison of studies could be more accurate if the exact cut-off value had been defined and validated. Therefore, these data should be validated in a prospective study.
Conclusion
We showed that the PNI value measured before treatment can be used as an independent prognostic factor for both OS and DFS in patients who have undergone standard primary tumor surgery, lymph node-positive, stage II-III gastric cancer who will receive adjuvant chemotherapy. PNI, which is calculated by lymphocyte and albumin values before treatment, can be useful in identifying patients who will benefit from adjuvant chemotherapy as an easy, simple and applicable marker.
Data Availability
The data presented in this study can be obtained from the corresponding author.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–49.
Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–30.
Ronellenfitsch U, Schwarzbach M, Hofheinz R, GE Adenocarcinoma Meta-analysis Group. ;. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. 2013;(5):CD008107.
Diaz-Nieto R, Orti-Rodríguez R, Winslet M. Post-surgical chemotherapy versus surgery alone for resectable gastric cancer. Cochrane Database Syst Rev. 2013;9. CD008415.
Oba K, Paoletti X, Alberts S, GASTRIC group, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: a meta-analysis. J Natl Cancer Inst. 2013;105(21):1600–7.
Gokce A. Prognostic significance of Extracapsular Invasion in Gastric Cancer. Selcuk Med J. 2021;37(4):307–12.
Kowalewska M, Nowak R, Chechlinska M. Implications of cancer-associated systemic inflammation for biomarker studies. Biochim Biophys Acta. 2010;1806(2):163–71.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54.
McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–3.
Wolfson M, Strong CJ, Minturn D, Gray DK, Kopple JD. Nutritional status and lymphocyte function in maintenance hemodialysis patients. Am J Clin Nutr. 1984;39(4):547–55.
Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–7.
Zhang Q, Bao J, Zhu ZY, Jin MX. Prognostic nutritional index as a prognostic factor in lung cancer patients receiving chemotherapy: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(18):5636–52.
Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10(1):17373.
Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440–3.
Migita K, Takayama T, Saeki K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20(8):2647–54.
Yang Y, Gao P, Song Y, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. 2016;42(8):1176–82.
Takahashi T, Kaneoka Y, Maeda A, Takayama Y, Fukami Y, Uji M. The preoperative prognostic nutrition index is a prognostic indicator for survival in elderly gastric cancer patients after gastrectomy: a propensity score-matched analysis. Updates Surg. 2020;72(2):483–91.
Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15(2):945–50.
Alwarawrah Y, Kiernan K, MacIver NJ. Changes in Nutritional Status Impact Immune Cell metabolism and function. Front Immunol. 2018;9:1055.
Ding P, Guo H, Sun C, et al. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22(1):121.
Funding
None.
Author information
Authors and Affiliations
Contributions
Author Contributions: MK, design and the conduct of the study. MK, MME, AD; data colection. MK; wrote manuscript text. MZK, MK; Performed statistical analysis. MA, MA, MKE; Performed final review and correction. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was conducted retrospectively from data obtained for clinical purposes and was in line with the 1964 Declaration of Helsinki. This study was approved by Necmettin Erbakan University Research Ethics Committee (Approval number:2022/3974).
Consent to Participate
Written informed consent was not obtained as the study was a retrospective study.
Competing interests
The authors report there are no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Korkmaz, M., Eryılmaz, M.K., Er, M.M. et al. Is the Prognostic Nutritional Index a Prognostic Marker for the Survival of Patients with Lymph-Node Positive Stage II-III Gastric Cancer Who Receive Adjuvant Chemotherapy?. J Gastrointest Canc 54, 962–969 (2023). https://doi.org/10.1007/s12029-023-00972-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-023-00972-x