Abstract
Purpose
With the aging of society, the mean age of patients with gastric cancer (GC) in Japan has increased. However, there are few documented outcomes for young patients with stage IV GC. We investigated the clinical characteristics and prognosis of such patients aged < 40 years using a dataset from an integrated population-based cohort study.
Methods
We conducted this multicenter population-based cohort study to determine whether earlier onset of GC was a poor prognostic factor. We enrolled patients with metastatic GC aged < 40 years (young group) and those aged between 60 and 75 years (middle-aged group). Patients were histologically diagnosed as having gastric adenocarcinoma. We evaluated the overall survival (OS) of both groups and the hazard ratio (HR) for OS based on age. The adjusted HR with 95% confidence interval (CI) was evaluated using the Cox proportional hazards model after adjusting for confounding factors, including sex, histology, number of metastatic lesions, surgical resection, and chemotherapy.
Results
This study enrolled 555 patients. The patients were classified into the young (n = 20) and the middle-aged group (n = 535). The median OS durations were 5.7 and 8.8 months in the young and middle-aged groups, respectively (p = 0.029). The adjusted HR (95% CI) of the young group was 1.88 (1.17–3.04, p = 0.009).
Conclusion
Age was an independent prognostic factor in patients with stage IV GC. Further studies investigating the genomic characteristics of GC and exploring more effective chemotherapeutic agents are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mean age of patients at onset of gastric cancer (GC) ranges from 60 to 70 years [1,2,3]. However, this age has gradually increased in Japan [4], possibly because of the drastic decline in the prevalence of Helicobacter pylori infection among the younger generation. H. pylori infection significantly affects GC development [5, 6]. Nevertheless, approximately 2–10% of patients with GC are aged ≤ 40 years [7].
Previous reports have demonstrated that the prognosis of young patients with resectable stage I–III GC is similar to or better than that of middle-aged or older patients [3, 8,9,10]. Younger patients have fewer comorbidities and a higher tolerance for surgery and adjuvant chemotherapy than older patients [11, 12]. Additionally, they can undergo intensive chemotherapy followed by gastrectomy with adequate lymph node dissection, which improves long-term prognosis [13, 14]. However, some young patients present with rapidly progressive disease and distant metastasis. These patients are diagnosed with stage IV GC at the initial presentation. Previous studies on the prognosis of stage IV GC have reported 1-year and 5-year survival rates of 15.6–20.0% and 0–7.9%, respectively [12, 15,16,17]. Pathological differences have been observed between younger patients with GC and older patients with atrophic gastritis caused by H. pylori infection [18]. Moreover, previous reports have shown that poorly differentiated adenocarcinoma, diffuse invasive type, and lymphatic or distant metastasis are more common in younger than in older patients [2, 3, 19]. A treatment strategy for stage IV disease in younger patients should be established. However, most previous studies have focused on the surgical outcomes of young patients with resectable GC. These studies are limited because data were obtained from a single institute, and there are few documented outcomes of young patients with stage IV GC [11,12,13,14,15,16,17].
We investigated the clinical characteristics and prognosis of young patients with stage IV GC using a dataset from an integrated population-based cohort study. We hypothesized that the survival time of young patients was worse than that of middle-aged patients with stage IV GC.
Patients and Methods
Study Design and Cohort Development
This was a population-based study. All the nine hospitals designated for cancer treatment in the Fukushima Prefecture participated in this study. First, patients with stage IV GC were enlisted using hospital-based cancer registries. Subsequently, individual patient data, including age, sex, body mass index, performance status, the Charlson comorbidity index [20], discovery of symptoms, site, morphological type, histological type, metastatic sites, number of metastatic lesions, operation type, and chemotherapy, were obtained. We merged the datasets from each participating institute after anonymizing the information.
We enrolled patients in this study if they were diagnosed with GC (International Classification of Diseases, Tenth Revision, C16.0–16.9) and had histologically proven adenocarcinoma (differentiated type, undifferentiated type, and mixed type) from a primary lesion between 2008 and 2015. Patients who were lost to follow-up, had multiple primary cancers, or did not undergo biopsy were excluded.
The protocol, registered at the University Hospital Medical Information Network (UMIN000033718), was approved by the institutional review board (IRB) of all the participating hospitals. This board waived the informed consent requirement in accordance with the Japanese government’s Ethical Guidelines for Medical and Health Research Involving Human Subjects, which allow an opt-out approach.
Definition of Gastric Cancer Patient Groups
Figure 1 shows the age distribution histogram of 1366 patients diagnosed with stage IV GC in this period. In this cohort, the median age of the patients was 71 years. In addition, the median ages of the patients in the first, second, third, and fourth quartiles were 16, 62, 78, and 98 years, respectively. The patients aged < 40 years and those aged 60–75 years were classified as “young” and “middle-aged” patients, respectively. The middle-aged group in this study was set based on the histogram and the previous study [3]. The age when patients were diagnosed with GC was considered in the analysis.
Outcomes and Statistical Analyses
The primary outcome was the young group’s hazard ratio (HR) for overall survival (OS). After adjusting for sex, histological type, number of metastatic lesions, primary lesion resection, and chemotherapy as confounding factors, we calculated the HR and 95% confidence interval (CI) of both groups using the Cox proportional hazards model. Kaplan–Meier curves were used to illustrate the cumulative incidence of deaths in the young and middle-aged groups, and a log-rank test was performed to compare the OS of these patient groups. Descriptive statistics were also evaluated. Continuous variables were compared using Student’s t-test, and categorical variables were compared using Fisher’s exact test. All the statistical tests were two-sided, and p-values ≤ 0.05 were considered statistically significant. All the statistical analyses were performed using R software version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Enrolled Patients
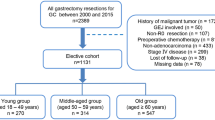
Figure 2 presents the patient enrollment flowchart. A total of 555 patients were enrolled in this study. Twenty patients were included in the young group, and 535 patients were included in the middle-aged group. Table 1 shows patient characteristics, and Table 2 shows treatment details in the two groups. Histological types were more differentiated in the middle-aged group and poorly differentiated in the young group (p = 0.003). The young group had a lower transition rate to third-line chemotherapy than the middle-aged group (p = 0.11). The chemotherapeutic agents administered to 17 of the young patients included oral fluoropyrimidine plus cisplatin (n = 11), oral fluoropyrimidine plus oxaliplatin (n = 1), oral fluoropyrimidine plus paclitaxel (n = 1), oral fluoropyrimidine alone (n = 2), and paclitaxel alone (n = 1). In total, 171 (30.8%) patients were screened for Her2. Of these, 19 (3.4%) patients were positive, and trastuzumab was administered to 10 patients. The young patients were all negative for Her2. There was no significant difference in the median time of transition from first line to second line chemotherapy between the two groups (139 and 150.5 days in the young and middle-aged groups, respectively; p = 0.193).
Adjusted Hazard Ratios and Overall Survival Curves
Table 3 shows the adjusted HRs for all the patients. With the HR of the middle-aged group as a reference, the HR (95% CI) of the young group was 1.88 (1.17–3.04, p = 0.009). Figure 3 shows the OS and at-risk population for both groups. The median OS values were 5.7 months and 8.8 months in the young and middle-aged groups, respectively (p = 0.029).
Discussion
This study yielded four important results. First, age < 40 years was identified as an independent risk factor for survival. Second, the predominant histological type in the young group was poorly differentiated, and the typical metastatic pattern was peritoneal dissemination. Third, the proportion of patients who received third-line chemotherapy was lower in the young than in the middle-aged group. Finally, the findings suggest that the survival of young patients is worse than that of middle-aged patients.
Previous studies have reported that the prognosis of patients aged < 40 years with stage I–III GC is comparable to or better than that of patients aged ≥ 40 years [4, 8,9,10, 21]. In this study, we focused on patients with stage IV GC and found that the young group had a worse prognosis than the middle-aged group. Young patients were more likely to have undifferentiated type GC, resulting in a higher incidence of peritoneal dissemination than hematogeneous metastasis. Peritoneal dissemination can progress more rapidly than liver or lymph node metastasis, and the switch to chemotherapy is often unsuccessful. In this study, the rate of third-line treatment in young patients was lower than that in middle-aged patients, reflecting the difficulty of treating peritoneal dissemination.
The prevalence of H. pylori infection among young Japanese people is low. Therefore, GC development in patients aged < 40 years may involve carcinogenesis pathways and biological properties that are different from those of common GC secondary to atrophic gastritis [18]. The molecular mechanisms of gastric carcinogenesis have recently been elucidated, and potential therapeutic targets have been identified based on the classification of molecular subtypes [22]. The genomically stable GC subtype is more common in younger patients, has the highest resistance to fluorouracil, and is associated with poor prognosis [23]. In addition, the chromosomal instability GC subtype, which is associated with extensive gastric mucosal atrophy owing to H. pylori infection, is more sensitive to chemotherapy and presents a lower recurrence rate after adjuvant therapy than other subtypes [23]. These molecular differences may be related to differences in chemotherapy efficacy and GC progression. Young patients with stage IV GC who already have distant metastasis at the time of diagnosis require shorter intervals between examinations and an earlier evaluation of treatment effects.
Previous studies have shown that younger age was not a poor prognostic factor for stage I–III GC [15, 21]. However, for stage IV GC, younger age was a poor prognostic factor in our study. Early-onset disease includes rare cases of rapid progression. Patient survival is short when the disease is detected at stage IV.
This study had a few limitations. First, information on H. pylori infection and genetic information (CDH1 mutation, RhoA, microsatellite instability, and loss of heterozygosity) was not collected. Second, the number of patients in the young group was quite small, which made the analyses statistically unstable. However, cases of young patients with GC are rare, and stage IV GC cases are uncommon [9, 19]. Indeed, young patients with stage IV GC accounted for approximately 1.5% of our cohort. The strength of our study was to focus on the outcome of the quite rare population of GC.
In conclusion, younger age (< 40 years) was an independent prognostic factor for patients with stage IV GC. Although this is a rather rare population among patients with stage IV GC, further studies investigating the genomic characteristics of GC and exploring more effective chemotherapeutic agents are required.
Author Contribution
Category 1.
Conception and design of the study: R. Y., M. H., H. K., and H. K.; acquisition of data: M. H., H. K., H. K., and S. H.; and analysis and/or interpretation of data: R. Y. and M. H.
Category 2.
Drafting of the manuscript: R. Y. and M. H. and revising the manuscript critically for important intellectual content: R. Y. and M. H.
Category 3.
Approval of the version of the manuscript to be published: R. Y., M. H., H. K., H. K., K. T., A. M., S. Y., Y. T., S. S., K. K., S. H., T. K., T. I., and N. Y.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
The data that support the findings of this study are available from the corresponding author, M. H., upon reasonable request.
References
Theuer CP, de Virgilio C, Keese G, et al. Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg. 1996;172:473–7. https://doi.org/10.1016/S0002-9610(96)00223-1.
Song P, Wu L, Jiang B, Liu Z, Cao K, Guan W. Age-specific effects on the prognosis after surgery for gastric cancer: a SEER population-based analysis. Oncotarget. 2016;7:48614–48624. https://doi.org/10.18632/oncotarget.9548
Takatsu Y, Hiki N, Nunobe S, Ohashi M, Honda M, Yamaguchi T, Nakajima T, Sano T. Clinicopathological features of gastric cancer in young patients. Gastric Cancer. 2016;19:472–8. https://doi.org/10.1007/s10120-015-0484-1.
Honda M, Wong SL, Healy MA, Nakajima T, Watanabe M, Fukuma S, Fukuhara S, Ayanian JZ. Long-term trends in primary sites of gastric adenocarcinoma in Japan and the United States. J Cancer. 2017;8:1935–42. https://doi.org/10.7150/jca.19174.
Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol. 2013;7:35–40. https://doi.org/10.1586/egh.12.67.
Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017;19:36. https://doi.org/10.1007/s11894-017-0575-8.
Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Białek A, Kulig J, Kaczmarczyk M, Starzyńska T. Analysis of clinicopathologic characteristics of gastric cancer in patients ≤40 and ≥40 years of age. Scand J Gastroenterol. 2020;55:62–6. https://doi.org/10.1080/00365521.2019.1699597.
Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg. 2007;94:737–42. https://doi.org/10.1002/bjs.5600.
Wang Z, Xu J, Shi Z, Shen X, Luo T, Bi J, Nie M. Clinicopathologic characteristics and prognostic of gastric cancer in young patients. Scand J Gastroenterol. 2016;51:1043–9. https://doi.org/10.1080/00365521.2016.1180707.
Zhang J, Gan L, Xu MD, Huang M, Zhang X, Gong Y, Wang X, Yu G, Guo W. The prognostic value of age in non-metastatic gastric cancer after gastrectomy: a retrospective study in the US and China. J Cancer. 2018;9:1188–99. https://doi.org/10.7150/jca.22085.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJH. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. https://doi.org/10.1016/S1470-2045(10)70070-X.
Hsieh FJ, Wang YC, Hsu JT, Liu KH, Yeh CN. Clinicopathological features and prognostic factors of gastric cancer patients aged 40 years or younger. J Surg Oncol. 2012;105:304–9. https://doi.org/10.1002/jso.22084.
Ramos MFKP, Pereira MA, Sagae VMT, Mester M, Morrell ALG, Dias AR, Zilberstein B, Ribeiro Junior U, Cecconello I. Gastric cancer in young adults: a worse prognosis group? Rev Col Bras Cir. 2019;46: e20192256. https://doi.org/10.1590/0100-6991e-20192256.
Lee JG, Kim SA, Eun CS, Han DS, Kim YS, Choi BY, Song KS, Kim HJ, Park CH. Impact of age on stage-specific mortality in patients with gastric cancer: a long-term prospective cohort study. PLoS ONE. 2019;14: e0220660. https://doi.org/10.1371/journal.pone.0220660.
Isobe T, Hashimoto K, Kizaki J, Miyagi M, Aoyagi K, Koufuji K, Shirouzu K. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep. 2013;30:43–9. https://doi.org/10.3892/or.2013.2467.
Tavares A, Gandra A, Viveiros F, Cidade C, Maciel J. Analysis of clinicopathologic characteristics and prognosis of gastric cancer in young and older patients. Pathol Oncol Res. 2013;19:111–7. https://doi.org/10.1007/s12253-012-9530-z.
Nakamura R, Saikawa Y, Takahashi T, Takeuchi H, Asanuma H, Yamada Y, Kitagawa Y. Retrospective analysis of prognostic outcome of gastric cancer in young patients. Int J Clin Oncol. 2011;16:328–34. https://doi.org/10.1007/s10147-011-0185-7.
Li J. Gastric cancer in young adults: a different clinical entity from carcinogenesis to prognosis. Gastroenterol Res Pract. 2020;2020:9512707. https://doi.org/10.1155/2020/9512707.
Rona KA, Schwameis K, Zehetner J, et al. Gastric cancer in the young: an advanced disease with poor prognostic features. J Surg Oncol. 2017;115:371–5. https://doi.org/10.1002/jso.24533.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. https://doi.org/10.1016/0895-4356(94)90129-5.
Zhao B, Mei D, Lv W, Lu H, Bao S, Lin J, Huang B. Clinicopathologic features, survival outcome, and prognostic factors in gastric cancer patients 18–40 years of age. J Adolesc Young Adult Oncol. 2020;9:514–21. https://doi.org/10.1089/jayao.2019.0162.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. https://doi.org/10.1038/nature13480.
Sohn BH, Hwang JE, Jang HJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the Cancer Genome Atlas project. Clin Cancer Res. 2017;23:4441–9. https://doi.org/10.1158/1078-0432.CCR-16-2211.
Acknowledgements
We would like to express our gratitude to Seria Sato, Koji Uehara, Nobuko Kanno, Mika Yusa, Kazuhira Saito, Tomoko Oya, Yosinobu Yamazaki, Yoko Endo, Chieko Tairako, and Yumi Inaba for their contribution to data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All the study procedures were performed in accordance with the ethical standards of the respective committees on human experimentation (institutional and national), as well as with the Declaration of Helsinki of 1964 and its later versions. The study was approved by the institutional review boards of all the participating institutes.
Consent to Participate
The anonymous nature of the data allowed the requirement for informed consent to be waived.
Consent for Publication
All the authors provided consent for the publication of this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamamoto, R., Honda, M., Kawamura, H. et al. Clinical Features and Survival of Young Adults with Stage IV Gastric Cancer: a Japanese Population-Based Study. J Gastrointest Canc 54, 56–61 (2023). https://doi.org/10.1007/s12029-021-00797-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-021-00797-6