Abstract
Background
Pretherapy serum neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR) have been shown to predict prognosis in patients with pancreatic ductal adenocarcinoma (PDAC). However, the published literature is conflicting; hence, we aimed to evaluate their role in predicting survival outcomes in operated patients of PDAC.
Methods
A retrospective analysis was performed in all operated cases of PDAC who underwent curative resection between 2011 and 2018. The pretherapy NLR, PLR, and LMR were calculated and analyzed with respect to pathological and survival outcomes
Results
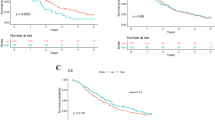
One hundred thirty-four operated patients were included. The median overall survival for NLR of less than 2, 2.7, and 5 was 30.8, 27.2, and 27.5 months and for NLR of more than 2, 2.7, and 5 was 22.9, 21.6, and 21.5 months, respectively, and was statistically insignificant (p-value—0.32, 0.91, 0.34, respectively). Similarly, the PLR was not significant for a cutoff of 150 (p-value—0.27), and LMR was not significant for a cutoff of 2.8 (p-value—0.13) and 4.8 (p-value—0.11). On univariate analysis age, CA 19–9 levels, perineural invasion, margin positivity, lymph node positivity, and TNM stage were found to have a significant correlation with overall survival. However, on multivariate analysis, only TNM stage was found to be significant.
Conclusion
The NLR, PLR, and LMR do not correlate with overall survival in operated patients with PDAC in this study. A combination of inflammatory markers or their dynamic testing might probably achieve prognostic significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With a continuous progress in development of newer and effective cancer-related therapies, much improvement in survival has been achieved in many cancers. However, there has been hardly any headway when it comes to pancreatic ductal adenocarcinoma (PDAC), and it is still one of the most fatal cancers, with a 5-year survival rate of 5–10% [1]. The main hindrance being difficulty is in its early detection, as most patients are asymptomatic in early stages of cancer and the inherent aggressive tumor biology of PDAC. At the time of diagnosis, 10–20% of tumors are operable; 30–40% are borderline resectable (BRPC), while remaining are either locally advanced or metastatic [2]. For advanced disease, palliative chemotherapy and/or radiotherapy remain the only treatment option.
Operable pancreatic cancers are generally divided into resectable and borderline resectable depending upon radiological features and relation to surrounding vessels, predicting the risk of margin positivity. This distinction is important, in order to offer neoadjuvant treatment in BRPCs, which has shown to increase the percentage of R0 resections and reduce lymph node positivity, thus reducing the risk of local recurrence [3]. A prognostic marker which can predict margin positivity in a seemingly upfront resectable tumor will help in deciding the criteria of resectability and need of neoadjuvant chemotherapy in this patient population. However, this requires that patients are risk stratified before starting treatment. Radiological evaluation is not entirely accurate in distinguishing resectable from borderline tumors, as radiological findings do not always correlate with intraoperative findings and histopathological parameters. Contrast-enhanced computed tomography (CECT) has poor sensitivity (77%) to predict vascular invasion, and endoscopy ultrasound (EUS) has high false positives or negatives (33%) to predict resection [4, 6].
A number of systemic inflammation-based prognostic markers and scores have been studied to predict cancer-specific survival in several digestive cancers [5]. Out of these, CA19-9 has been most commonly used in pancreatic cancers. However, use of CA 19–9 may not be accurate as few patients are non-secretors (10–15%); also, some patients with obstructive jaundice will have spuriously elevated CA 19–9 levels [6]. Previous studies concluded that the level of serum neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR) might be associated with prognosis in several tumor types, including pancreatic cancers [7,8,9,10,11,12]. Although they are easy to measure, the number of neutrophils, monocytes, and lymphocytes is influenced by the immune status of body, which in turn is activated by host of other factors like active infection, chronic inflammatory diseases, and lifestyle-related habits [13]. In the present study, we aimed to assess the value of inflammation-based markers, namely, NLR, PLR, and LMR in predicting prognosis of operated PDAC patients.
Materials and Methodology
Data was collected from a prospectively maintained surgical database of patients who underwent curative pancreatectomy for PDAC, between January 2010 and June 2019. Inclusion criteria were patients with histologically proven PDAC with imaging findings consistent with resectable or borderline resectable pancreatic cancers as per the AHPBA criteria [14], documented patient Eastern Cooperative Oncology Group (ECOG) performance status score of < 2, who underwent curative intent surgery, either upfront or after neoadjuvant therapy. Exclusion criteria included patient with metastatic disease on presentation, patients lost to follow-up, patient who died in the immediate post-operative period, and those in whom data was not available on pre-treatment NLR, LMR, and PLR. Each patient was reviewed by multidisciplinary joint team (MDT) comprising of HPB surgeon, medical oncologist, radiation oncologist, radiologist, and pathologist, for planning treatment. Patients with borderline resectable cancers were offered neoadjuvant chemotherapy/chemoradiation or upfront surgery as per discretion of MDT.
The NLR was calculated from the differential leucocyte count by dividing the absolute neutrophil count by the absolute lymphocyte count. Similarly, the PLR (platelet count by the absolute lymphocyte count) and the LMR (absolute lymphocyte count by the absolute monocyte count) were also calculated. The ratios were then divided into two groups using cutoff values; for NLR, we used cutoff values of 2.7 (mean NLR), 2, and 5. For PLR, 150, and for LMR, 2.8 and 4.8 were used as cutoffs. These cutoffs were based on literature review of previous large studies which have shown difference in survival using these values [7,8,9,10,11,12]. Patients who were upfront resectable had their blood samples collected within 1 month before surgery, and those planned for NACT had their values obtained from blood sample taken just before starting chemotherapy. All patients who were planned for neoadjuvant chemotherapy or chemoradiation or surgery showed no signs of systemic inflammation or infection as noted at the time of laboratory testing. For patients having obstructive jaundice, all blood test results were re-assessed after procedures such as endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous trans-hepatic biliary drainage (PTBD), once jaundice had resolved, before planning surgery.
Statistical Test
Continuous variables were compared using Student’s t-test and categorical variables were compared using the chi-squared test. Survival curves were calculated using the Kaplan–Meier method and compared using log-rank test to evaluate difference in survival. Survival curves were compared using the cutoff values already mentioned. P values less than 0.05 were considered statistically significant. The factors associated with OS in univariate analysis were used for the performance of the multivariate Cox-regression analysis. The statistical analyses were performed with the Statistical Package of the Social Sciences (SPSS), version 17.0.
Results
Overall, 1144 patients underwent surgery between 2010 and 2018 for pancreatic and periampullary tumors. Seven hundred eight patients had tumors of periampullary origin, and 260 were of non-adenocarcinoma histology. Follow-up data was not available for 20 patients, and pretherapy blood parameter records were not available of 5 patients. There were 17 post-operative deaths. After excluding these cases, 134 patients were available for final analysis who underwent curative resections for pancreatic adenocarcinoma. All patients underwent either pancreatico duodenectomy (PD) or radical antegrade modular pancreatico-splenectomy (RAMPS).
There were slightly more females than males, and most of the cancers were pancreatic head adenocarcinomas (85.8%). Seventy-six patients (56.7%) were upfront resectable, and 58 (43.3%) were borderline resectable according to radiological evaluation. Forty (29.9%) patients received neoadjuvant therapy (37—chemotherapy, 3—chemoradiation). Adjuvant chemotherapy was given to 110 patients (82.1%). Thirty-two (24.3%) out of 132 patients had margin positivity. Most patients were T3 (43.3%), and there were an almost equal number of node-positive (54.5%) and node-negative (45.5%) patients (Tables 1 and 2).
The mean absolute lymphocyte, monocyte, neutrophil, and platelet counts were 1.9 × 109/l, 0.4 × 109/l, 5 × 109/l, and 288 × 109/l, respectively, and the mean NLR, LMR, and PLR values were 2.7, 195, and 4.8, respectively. Kaplan–Meier method was used to calculate survival curves, and survival was compared using log-rank test. Age more than 65 years (p-0.008), CA 19–9 more than 200 U/mL (p-0.002), presence of perineural invasion (p-0.036), margin positivity (p-0.044), lymph node positivity (p-0.039), and higher TNM stage (p-0.002) were found to have worse OS (Fig. 1). On univariate analysis stage of disease (p-0.001, HR—3.54, 95% CI 1.69–7.44), age with cutoff of 65 years (p-0.010, HR—2.03, 95% CI 1.18–3.49), CA 19–9 for a cutoff 200 U/mL (p-0.016, HR—1.83, 95% CI 1.31–2.96) perineural invasion (p-0.040, HR—0.59, 95% CI 0.36–0.97), and lymph node positivity (p-0.04, HR—0.60, 95% CI 0.37–0.98) were found to have significant correlation with overall survival. However, on multivariate analysis, only TNM stage (p-0.02, HR—2.97, 95% CI 1.13–0.7.77) was found to be significant. None of the inflammation-based markers, namely, NLR, PLR, and LMR were found to correlate with overall survival (Table 3).
Discussion
There is a significant difference in the biological behavior and prognosis of pancreatic cancers, with patients within same stage also having varied final outcomes. To identify some markers which can predict survival may help us further prognosticate these patients and ultimately assist in decision-making. An ideal prognostic marker should give us information on the long-term prognosis of patients, help us in making a comprehensive treatment plan including sequencing of surgery, chemotherapy, and or radiotherapy and also in predicting margin and lymph nodal status before surgery [2]. The prognostic marker should best correlate with the pathological and clinical stage of disease. The most accurate information on outcomes following surgery are obtained from post-operative histological parameters like tumor size, nodal status, margin status, and pathological stage of the disease [15]. However, to make an effective treatment plan, patients need to be risk stratified before surgery [7]. In the present study age, CA19-9, perineural invasion, margin positivity, lymph node positivity, and TNM stage of disease were found to have a significant association with overall survival. None of the inflammation-based marker ratios, namely, NLR, PLR, or LMR was found to have correlation with overall survival.
Inflammation is one of the hallmarks of carcinogenesis and plays an important role in tumor progression [16]. Neutrophils are among the first cells to migrate to site of inflammation. They, in turn, release chemokines and protease which further recruit other effector cells [17]. On the other hand, lymphocyte count correlates with the systemic immune response that the host can mount, and tumor-infiltrating lymphocytes (specifically T cells) are responsible for mounting the antitumor response within the microenvironment [18]. As far as platelets and cancer cells are concerned, they are known to interact with each other during tumorigenesis. Cancer cells bring about platelet adhesion, platelet activation, and degranulation with resultant prosurvival and proangiogenic signals which may promote cancer spread [19, 20]. Literature on use of these blood cell–based markers is inconsistent with few studies showing either no or some implication [15, 21,22,23,24,25,26] while others concluding their usefulness in prognostication of disease [6,7,8, 27, 28]. Although most studies including meta-analysis have shown favorable outcomes with the use of proinflammatory markers for prognosis, there is considerable variation in the type of marker used, timing of blood sample collection, and the cutoff value of marker.
In a recently published retrospective study assessing the role of NLR and PLR for resectable pancreatic cancers, no significant association was found between these markers and survival for a range of cutoff values [21]. Similarly, prospective study by Jamieson et al. studying the prognostic value of tumor and patient-related factors in patients undergoing potentially curative surgery for PDACs did not find either NLR or PLR to be associated significantly with survival for cutoff values of 5 and 150, respectively [15]. Other studies assessing multiple parameters have shown survival to be unrelated to at least one or more of these markers (NLR, PLR, or LMR). In one of the studies with 74 resected pancreatic cancers, NLR was found to be significantly associated with disease-free survival at a cutoff of 5, but no difference was seen with C reactive protein (CRP) or PLR [22]. One of the larger retrospective studies found significant association between NLR (cutoff of 5) and overall survival for both resected and inoperable pancreatic cancers. However, no significance was seen for PLR (cutoff of 150) [27]. Likewise, retrospective cohort study by Sierzega et al. (442 patients) found NLR and LMR to be associated with survival, but no relation was seen with PLR [29] (Table 4).
There are at least 3 meta-analyses conducted to look into the prognostic significance of these markers [10,11,12]. One of the initial meta-analyses by Yang et al. (11 studies and 1804 patients) had a diverse patient population with few studies consisting of locally advanced cancers treated with chemotherapy, others having resected pancreatic cancer, and others still having a combination of both resected and locally advanced patients [11]. Another meta-analysis of 1519 patients with 8 studies [12] which looked into only resected pancreatic cancers found a significant association between NLR (cutoff values ranging from 2 to 5) and survival. Nevertheless, most of these were studies conducted in Asian population, and this may be a confounding factor in validating the outcomes of this analysis into other population groups. A recently published larger meta-analysis of 8252 patients [10] consisting of heterogeneous study groups also found a significant association between NLR and survival. Even the subgroup analysis of surgery alone trials, as also analysis based on ethnic origin of the patients, showed positive results. Again, the surgery alone trials were predominantly conducted in Asian countries, and the subgroup of Caucasian trials predominantly involved non-surgical treatment, thus making comparison between study groups debatable. Surgery alters the natural course of disease process; thus, it might not be correct to club resected and inoperable patients together as a common cohort while analyzing the results of the study. Moreover, the cutoff value of NLR and sample size of included cohorts varied from 2 to 5 and 28 to 474, respectively.
With considerable improvement in survival over past decades and corresponding decrease in morbidity [30], identification of prognostic markers should help us in further improving patient outcomes. However, our study did not find any significant association between these markers and overall survival and, although the survival was better with low NLR and high LMR for all cutoffs, it did not reach statistical significance. One of the reasons for this may be that, although, individually, this test may not be predictive of survival, a combination of two or more of these variables may be a better marker to assess prognosis. The systemic immune-inflammation index (SII), calculated with lymphocyte, neutrophil, and platelet counts, has been shown to be an independent negative predictor of disease-free survival in resectable PDAC [31] as well as OS when evaluated as a continuous variable rather than preoperative value in patients given neoadjuvant chemotherapy [32]. It has also been shown to be better than neutrophil-to-lymphocyte ratio (NLR) or the platelet-to-lymphocyte ratio (PLR) for prediction of OS in patients with resectable PDAC [33]. Also, as inflammatory markers are dynamic, they may change during the treatment course. Thus, instead of assessing these variables at a point in time, looking at change of values over the course of treatment might be a better way of utilizing their potential. This may be of significance as neoadjuvant approach is being increasingly used to treat borderline resectable pancreatic cancers. Glazer et al. in their study of BRPCs did not find NLR value alone (pre- and post-chemo) as predictive of survival, but an increase in values over course of chemotherapy to be associated with poor survival post-resection [28].
The main limitation of our study is its single-center, retrospective design with a relatively small sample size. Larger, multicenter studies will give a better answer to the applicability of these markers. To summarize, although literature is replete with studies that have shown correlation of survival with proinflammatory markers, their applicability is still contentious. Many of the studies had heterogeneous population groups with regards to management (surgical or non-surgical), cutoff value used, the timing of sample collection, presence of associated inflammatory condition, and tumor stage. Thus, there is still an ambiguity involved in using these markers for the purpose of prognostication. Further larger trials using combination of markers measured preoperatively for upfront resectable patients or assessing response of these markers following neoadjuvant treatment for BRPC are needed. Till robust data on their use is available, it may be counterproductive if these markers are used for definitive decision making.
Conclusion
The NLR, PLR, and LMR were not found to correlate with overall survival in operated patients with pancreatic ductal adenocarcinomas in our study. A combination of inflammatory markers or their dynamic testing might probably achieve prognostic significance.
References
Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43–9.
Kim WJ, Lim TW, Park PJ, Choi SB, Kim WB. Prognostic impact of the combination of the neutrophil-to-lymphocyte ratio and serum carbohydrate antigen 19–9 in patients with pancreas head cancer. ANZ J Surg. 2019;89(7–8):E302–7.
Ueda M, Endo I, Nakashima M, et al. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009;33:104–10.
Tamburrino D, Riviere D, Yaghoobi M, Davidson BR, Gurusamy KS. Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2016;9:CD011515
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–63.
Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate antigen 19–9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a National Cancer Database Study. J Am Coll Surg. 2016;223:52–65.
Recio-Boiles A, Nallagangula A, Veeravelli S, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios inversely correlate to clinical and pathologic stage in patients with resectable pancreatic ductal adenocarcinoma. Ann Pancreat Cancer. 2019;2:8.
Giakoustidis A, Neofytou K, Costa Neves M, et al. Identifying the role of neutrophil-to-lymphocyte ratio and platelets-to-lymphocyte ratio as prognostic markers in patients undergoing resection of pancreatic ductal adenocarcinoma. Ann Hepatobiliary Pancreat Surg. 2018;22(3):197–207.
Onoe S, Maeda A, Takayama Y, et al. The prognostic impact of the lymphocyte-to-monocyte ratio in resected pancreatic head adenocarcinoma. Med Princ Pract. 2019;28(6):517–25.
Zhou Y, Cheng S, Fathy AH, et al. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther. 2018;11:1899–908.
Yang JJ, Hu ZG, Shi WX, et al. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21:2807–15.
Mowbray NG, Griffith D, Hammoda M, Shingler G, Kambal A, Al-Sarireh B. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB (Oxford). 2018;20(5):379–84.
Buyukkaya E, Karakas MF, Karakas E, et al. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost. 2014;20(2):159–63.
Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–33.
Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, et al. A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2318–28.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Weitzman SA, Weitberg AB, Clark EP, Stossel TP. Phagocytes as carcinogens: malignant transformation produced by human neutrophils Science. 1985;227:1231–3.
Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4:84–95.
Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35:254–61.
Egan K, Crowley D, Smyth P, O'Toole S, Spillane C, Martin C, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One 2011;6:e26125.
Chawla A, Huang TL, Ibrahim AM, Hardacre JM, Siegel C, Ammori JB. Pretherapy neutrophil to lymphocyte ratio and platelet to lymphocyte ratio do not predict survival in resectable pancreatic cancer. HPB (Oxford). 2018;20(5):398–404.
Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868–72.
Hamed MO, Roberts KJ, Smith AM, Morris SG. Elevated pre-operative neutrophil to lymphocyte ratio predicts disease free survival following pancreatic resection for periampullary carcinomas. Pancreatology. 2013;13(5):534–8.
La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–23.
Clark EJ, Connor S, Taylor MA, Madhavan KK, Garden OJ, Parks RW. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford). 2007;9:456–60.
Sanjay P, de Figueiredo RS, Leaver H, Ogston S, Kulli C, Polignano FM, et al. Preoperative serum C-reactive protein levels and post-operative lymph node ratio are important predictors of survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. JOP. 2012;13:199–204.
Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–21.
Glazer ES, Rashid OM, Pimiento JM, Hodul PJ, Malafa MP. Increased neutrophil-to-lymphocyte ratio after neoadjuvant therapy is associated with worse survival after resection of borderline resectable pancreatic ductal adenocarcinoma. Surgery. 2016;160(5):1288–93.
Sierzega M, Lenart M, Rutkowska M, et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Ann Surg Oncol. 2017;24(3):808–15.
Shrikhande SV, Shinde RS, Chaudhari VA, Kurunkar SR, Desouza AL, Agarwal V, Bhandare MS. Twelve Hundred Consecutive Pancreato-Duodenectomies from Single Centre: Impact of Centre of Excellence on Pancreatic Cancer Surgery Across India. World J Surg. 2020 Aug;44(8):2784-2793
Aziz MH, Sideras K, Aziz NA, et al. The systemic-immuneinflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg. 2018;270:139–46.
Murthy P, Zenati MS, Al Abbas AI, et al. Prognostic value of the Systemic Immune-Inflammation Index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer. Ann Surg Oncol. 2020;27(3):898–906.
Jomrich G, Gruber ES, Winkler D, et al. Systemic Immune-Inflammation Index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. 2020;24(3):610–8.
Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203.
Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72.
Author information
Authors and Affiliations
Contributions
VG, MB VC, and SS were involved in the conception and design of the work. VG and MB are involved in acquisition, analysis, interpretation of data, and manuscript preparation. MB, VC, and SS are involved in manuscript editing, manuscript review. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The data of the present study were collected in the course of common clinical practice, and accordingly, the signed informed consent was obtained from each patient for any surgical and clinical procedure. The study protocol was in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments. Because this was a retrospective study, formal consent for this study is not required, and no approval of the institutional research committee was needed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, V., Chaudhari, V., Shrikhande, S.V. et al. Does Preoperative Serum Neutrophil to Lymphocyte Ratio (NLR), Platelet to Lymphocyte Ratio (PLR), and Lymphocyte to Monocyte Ratio (LMR) Predict Prognosis Following Radical Surgery for Pancreatic Adenocarcinomas? Results of a Retrospective Study. J Gastrointest Canc 53, 641–648 (2022). https://doi.org/10.1007/s12029-021-00683-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-021-00683-1