Abstract
Aims
To investigate pretreatment platelet and neutrophil counts as well as a combined platelet-neutrophil (PN) index for prognostic information in patients with rectal adenocarcinoma that received neoadjuvant treatment.
Patients and Methods
Charts from 164 patients with localized rectal adenocarcinoma were retrospectively reviewed, and 112 patients with complete data were included in the study. Patients were stratified in groups according to their neutrophil counts, platelet counts, and a combined platelet/neutrophil (PN) index. Baseline parameters of the groups were compared using the x2 test. Pathologic responses on the surgical specimen of patients with lower platelet counts (≤ 350 × 109/L), lower neutrophil counts (≤ 7.5 × 109/L), and a lower PN index were compared with those of patients with higher platelet counts (> 350 × 109/L), higher neutrophil counts (> 7.5 × 109/L), and a higher PN index using the x2 test. Kaplan-Meier curves of overall and progression free survival were constructed and compared with the log-rank test.
Results
A total of 33 (29.5%) patients belonged to the high-PN index group, and 79 (70.9%) patients belonged to the low-PN index group. A significant difference was present between the two groups with regard to pathologic response. Patients with both high platelet and high neutrophil counts were less likely to have a complete pathologic response than those in the low-PN index group (P = 0.039). Additionally, tumor location and tumor stage were significantly associated with complete pathologic response to neoadjuvant treatment. Patients with a complete response were more likely to present with a low tumor (≤ 5 cm from the anal verge). Likewise, patients diagnosed with stage II disease were more likely to experience complete response than those diagnosed with stage III (x2 test P = 0.016). There was no significant difference in overall and progression free survival between the two platelet groups (log-rank P = 0.73 and 0.40, respectively) and the two PN index groups (log-rank P = 0.92 and 0.43, respectively).
Conclusion
In this retrospective analysis, the combination of higher platelet and neutrophil counts at the time of diagnosis had predictive value with respect to complete pathologic response to neoadjuvant treatment in locally advanced rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tumor microenvironment is recognized as a key contributor to tumor progression and metastasis. Tumor cell interactions with endothelial cells, pericytes, fibroblasts, platelets, and immune cells shape the tumor microenvironment. In addition, in the circulation, platelets interactions have been found to protect cancer cells from shear stress and immune surveillance, to facilitate cancer cell adherence to endothelial cells, and to promote vascular integrity [1]. As a result, thrombocytosis is often associated with cancer, as the cytokines that stimulate thrombopoiesis are elevated in the circulation of some cancer patients.
Besides thrombocytosis, which has been found to be an adverse prognostic factor in many common cancers, neutrophil counts have also been studied for pro-tumorigenic effects [2]. Many studies have documented the platelet to lymphocyte and/or neutrophil to lymphocyte ratios (PLR and NLR, respectively) for use as prognostic tools owning to the fact that platelets and neutrophils have been found to be pro-tumorigenic, while lymphocytes may be protective against tumor growth [3]. However other studies have not confirmed circulating blood cells as predictive markers [4]. Given that these markers are readily available and inexpensive, their value as prognostic factors deserves further investigation.
Despite recent reductions in disease incidence and mortality rates related to screening and treatment progress, colorectal cancer (CRC) remains the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related deaths in the world [5]. The global burden of CRC is expected to increase by 60% to more than 2.2 million new cases and 1.1 million deaths by 2030 [6]. Among CRC, the treatment of localized rectal cancer is particular in that neo-adjuvant combination chemoradiotherapy is the standard of care. The therapeutic benefits of preoperative (neoadjuvant) chemoradiotherapy in rectal cancer include disease down-staging and improved local control [7]. The use of prognostic factors including pathologic stage, positive surgical margins, and pretreatment elevated carcinoembryonic antigen (CEA) aids in predicting which individual patients will respond better to therapy. However, the ability of these markers to predict outcomes is imperfect. For this reason, it is necessary to discover additional accurate and reliable biomarkers for predicting response to treatment of rectal adenocarcinomas. The purpose of this study was therefore, to evaluate pretreatment platelet and neutrophil counts individually as well as a combined platelet-neutrophil index (PN index) and their relationship to pathologic response to neoadjuvant therapy in patients with localized rectal adenocarcinoma. The study also investigated overall survival (OS) and progression free survival (PFS) with respect to pathologic response to treatment and hematologic markers.
Patients and Methods
Charts from 164 patients treated for localized rectal cancer between 2011 and 2017 in two cancer programs were retrospectively reviewed. Inclusion criteria were a localized rectal cancer diagnosis, treatment with neoadjuvant therapy, and complete follow-up. Follow-up was considered complete if the patient was followed until death or seen within the last 6 months of data collection. Of these 164 patients, 112 patients had localized disease at diagnosis and received neoadjuvant therapy with complete follow-up and were therefore, included in the analyses.
Baseline characteristics including age, sex, tumor location (distance from the anal verge; high defined as > 5 cm and low defined as ≤ 5 cm), clinical presentation (bowel obstruction, perforation, or a change in bowel habits considered as a high-risk presentation and diagnosis with screening or bleeding/anemia classified as low-risk presentation), Eastern Cooperative Oncology Group (ECOG) performance status (PS), clinical stage, blood hematologic, and biochemical markers [carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), albumin, platelets, neutrophils, and lymphocytes] at diagnosis and at surgery were extracted. Other data recorded consisted of the time interval from the end of neoadjuvant treatment to surgery in days (interval to surgery, IS), pathologic response, date of disease progression, date of death, or date of last follow-up.
Patients were classified into high and low groups according to their platelets and neutrophil counts at diagnosis. Thresholds used for platelets were ≤ 350 × 109/L versus > 350 × 109/L, while the thresholds for neutrophils were ≤ 7.5 × 109/L versus > 7.5 × 109/L. The platelet-neutrophil (PN) index was then established by creating high and low strata of pretreatment platelet and neutrophil counts. The high-PN index group was defined as having at least one of the pretreatment platelet or neutrophil counts above their respective thresholds. Conversely, the low group was defined as having both pretreatment platelet and neutrophil counts below their thresholds. Baseline characteristics for the two groups were compared and tested for significance using the x2 test.
Pathologic response to neoadjuvant therapy was categorized as either complete or incomplete (the latter including both patients with partial and no pathologic response). The baseline characteristics of the two pathologic response groups were compared and tested for significance using the x2 test.The significant variables were then included in the multivariate analysis using logistic regression to determine which variables retained independent significance. Two multivariate models were performed including the significant variables from the univariate analysis: the first including the PN index and the second using the individual platelets and neutrophil counts (with the same thresholds) separately.
Progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression. Overall survival (OS) was evaluated from the date of diagnosis to death. Both variables were censored at the date of last contact without progression or death for PFS and OS, respectively. Kaplan-Meier curves of PFS and OS were constructed and the log-rank test was employed to compare the outcomes between the two groups of each of the following: high and low levels of platelets at diagnosis, neutrophils at diagnosis, PN index, and complete pathologic response versus no complete response.
For all statistical tests, the p value was considered significant at a level of < 0.05. Data analysis was completed using Microsoft Excel and statistical calculations were completed using non-commercial online tools available from http://www.statpages.org/.
Results
The median age of the patients in the series was 61 years old. Of the 112 patients, 72 patients (64.3%) were 65 years old or younger, and 40 patients (35.7%) were older than 65 years old (Table 1). Seventy patients (62.5%) in the series were male, and 42 patients (37.5%) were female. Twenty-seven patients (24.1%) were in the high-platelet group at diagnosis (> 350 × 109/L), and 85 patients (75.9%) were in the low-platelet group (≤ 350 × 109/L) (Table 1). Thirty-three patients (29.5%) had a high-PN index (either or both platelets and neutrophils > 350 × 109/L and 7.5 × 109/L respectively), and 79 patients (70.5%) were in the low-PN index group (both platelets and neutrophils ≤ 350 × 109/L and ≤ 7.5 × 109/L respectively) (Table 2). There were no significant differences between groups in the platelet strata regarding age (above or below 65 years old), gender, high-risk clinical presentation, tumor location (high or low in the rectum), CEA, and stage (Table 1). Similarly, there were no significant differences in these parameters between the high- and low-PN index groups (Table 2).
Twenty-seven patients (24.1%) were found to have a complete pathologic response at surgery, while 85 (75.9%) had an incomplete pathologic response (Table 3). In the univariate analysis for possible associations with complete or lack of complete pathologic response, platelets and neutrophils and their composite represented by the PN index showed a significant correlation. Among patients with high pretreatment platelet counts (> 350 × 109/L), two patients (7.4%) obtained a complete pathologic response at surgery, whereas 25 patients (92.6%) did not (Table 3). From the patients with low platelets (≤ 350 × 109/L) at diagnosis, 25 patients (29.4%) and 60 (70.6%) experienced complete and incomplete responses at surgery, respectively (x2 test P = 0.02). Additionally, of the 18 patients in the high pretreatment neutrophil group, one patient (5.6%) responded completely to treatment, while 17 patients (94.4%) did not. Twenty-six patients (27.7%) from the low-neutrophil group had a complete pathologic response, whereas 68 patients (72.3%) did not (P = 0.045). Regarding the PN index, 33 patients (29.5%) belonged to the high-PN index group, while 79 patients (70.5%) were in the low-PN index group. Among the high-PN index patients, three patients (9.1%) experienced complete pathologic response at surgery, while 30 patients (90.9%) obtained less than a complete response. Among those in the low-PN Index, 24 patients (30.4%) experienced a complete pathologic response, and 55 patients (69.6%) had an incomplete response (P = 0.016).
In addition to the platelet and neutrophil counts and the combined PN index, tumor location and tumor stage were significantly associated with complete pathologic response to neoadjuvant treatment. Eleven of 66 patients (16.7%) presenting with a high-rectal adenocarcinoma (> 5 cm from the anal verge) experienced a complete response to treatment while 16 of 46 patients (34.8%) with a low tumor (≤ 5 cm from the anal verge) responded completely to treatment (x2 test P = 0.027). Likewise, 18 of 52 patients (34.6%) diagnosed with stage II disease and 9 of 60 (15.0%) patients with stage III disease experienced complete response (x2 test P = 0.016). In contrast, age above 65 years old, gender, and high-risk clinical presentation were not significantly associated with complete pathologic response (Table 3).
The multivariate model that included all the aforementioned significant variables from the univariate analysis confirmed statistical significance of stage and PN index (0.035 and 0.039, respectively). However, tumor location was borderline non-significant (P = 0.052), and both platelet and neutrophil counts at diagnosis did not retain significance in the multivariate analysis (Tables 4 and 5).
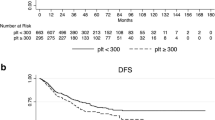
In the survival analysis of the 112-patient cohort, 27 patients had died with a median overall survival (OS) of 30.6 months, while 85 patients were alive at last follow-up with a mean follow-up time of 46.3 months. Regarding the PFS of the cohort, 32 patients suffered progression of disease at a mean of 22.3 months, while 80 patients who did not progress had a median follow-up of 41.9 months. The Kaplan-Meier survival analysis disclosed no significant differences for PFS or OS regarding the high and low platelet groups (log-rank P = 0.40 and 0.73, respectively). The same was observed for the high- and low-PN index groups (log-rank P = 0.43 and 0.92, respectively). The PFS of patients with a complete response was significantly better than that of patients with incomplete response (log-rank P = 0.022) (Fig. 1). This did not hold true for OS which was not statistically different (P = 0.1).
Discussion
Accurate and effective serum biomarkers to predict patients’ response to neoadjuvant treatment of rectal cancer could potentially improve both quality of care and survival by way of tailoring intensity of treatment to specific subsets of patients according to disease risk. This risk stratification may well be paramount in the initial treatment decisions. For patients unlikely to respond to neoadjuvant therapy, serum biomarkers could provide the opportunity for consideration of alternative treatment strategies. Similarly, but on the other end of the spectrum, for patients likely to respond to neoadjuvant therapy, an accurate prediction may help select patients for non-operative programs that spare patients from life-altering abdominoperineal resection.
A recent meta-analysis of predictive factors for pathologic response to neoadjuvant chemoradiation in rectal cancer concluded that at present there is a lack of robust clinical or radiologic biomarkers for such a response [8]. Molecular profiling may become a promising tool in the future. However, it is currently unavailable in most practices. The need for identification of novel practical and accurate predictive biomarkers for neoadjuvant chemoradiation in rectal cancer thus remains.
Various circulating blood cell counts including, neutrophils, lymphocytes, platelets as well as the combinations of them have been investigated as prognostic factors in cancer. These circulating blood cells may be involved in the immune response in rectal and other cancer patients. In fact they are known as incoming contributors of the internal tumor micro-environment [9].
Neutrophils have a dual role in malignancy [10, 11]. Tumor-suppressing effects may be derived from production of cytokines and chemokines with antitumoral effects as well as various protein-degrading enzymes. However, several mechanisms relate neutrophils to cancer promotion. Among them is the production of a non-specific inflammatory environment in tumors which favors pro-tumorigenic signaling pathways [12]. Neutrophils are also the source of neutrophil extracellular traps, networks of neutrophil-derived DNA expelled in the circulation. While these have a physiologic role in combatting microbial infections, they have also been observed in some cancers and may be associated with worse prognosis [13]. Concurring with these pro-tumorigenic effects of neutrophils, several studies associate neutrophilia with more aggressive and more advanced cancers [14].
Platelets also have an active role in tumor promotion. Platelets entering the tumor microenvironment may exchange information with tumor cells and become so called tumor-educated platelets (TEPs). TEPs have been observed in the serum of patients with various cancers and DNA derived from them could be used to discriminate between patients with cancers and healthy controls with high accuracy [15]. Platelets have been shown to induce changes involving loss of adhesion proteins such as E-cadherin that ultimately aid in neoplastic cells acquiring a trans-differentiated phenotype called epithelial-mesenchymal transition (EMT) [16]. Furthermore, platelets contain alpha and dense granules that secrete growth factors such as transforming growth factor-beta (TGF-ß), epithelial growth factor (EGF), platelet-derived growth factor (PDGF), and hepatocyte growth factor (HGF), among others. These growth factors have been shown to contribute to the onset of EMT and activate signaling pathways enabling cancer capabilities and therefore, promote malignancy.
Platelets’ role in vascular integrity inadvertently supports a vascular network to tumor growth and potential metastasis. Factors contained within platelet granules including vascular endothelial growth factor (VEGF), platelet factor-4 (PF-4), and interleukin-6 (IL-6) may be delivered to tumor cells to enhance angiogenesis. IL-6 plays an additional role in inducing liver thrombopoietin [17]. More importantly, it is this effect that leads to the thrombocytosis often associated with solid cancers. In gastrointestinal cancers (including colorectal carcinoma), IL-6 is typically measured at higher levels when compared to controls and may be involved in chemotherapy response [18].
Both neutrophil and platelet levels have been the subject of research on prediction of survival outcomes in rectal cancer. For example, in a study done by investigators at the University of Tokyo, hematologic data for patients with advanced rectal cancer were examined at various time points before, during, and after neoadjuvant treatment. These included markers such as hemoglobin, white blood cells with their subpopulations, and platelets [19]. Similar to our study, the aim of that study was to establish serum-predictive biomarkers for treatment response. It found that lymphocytes and neutrophils showed opposing associations with tumor response while platelets showed no predictive association. Specifically, in cases with complete response, neutrophil counts were low while lymphocyte counts tended to be higher.
We recently reported that a ratio of neutrophils times platelets divided by the lymphocytes count was predictive of survival in patients with metastatic colorectal cancer [20]. Others have reported that the main value of the neutrophil to lymphocyte ratio in cancer prognostication derives from neutrophils, while lymphocytes had little contribution [21]. In a study of these peripheral blood elements, Watt et al. reported that Neutrophil Platelet Score (NPS), calculated similarly to our index but with a different cutoff for platelets at 400 × 109/L, was prognostic for survival outcomes in operable colorectal cancer patients [22].
The findings among recent studies do seem to suggest adverse prognostic association between thrombocytosis, neutrophilia, and cancer. These findings are supplemented by preclinical data in mice supporting a synergism between neutrophils and activated platelets in extravasation into inflamed tissues [23]. Thus, further analysis of baseline platelet and neutrophil counts in cancer treatment response has a strong rationale and could improve on the predictive value of currently available markers.
In our current study, we aimed to further understand the predictive value of platelets and neutrophils in locally advanced rectal cancer treated with neoadjuvant therapy. We also investigated associations between isolated and combined (PN index) pretreatment thrombocytosis and neutrophilia and the effectiveness of neoadjuvant therapy in a series of 112 patients. We considered the relationship between pathologic response and both overall and progression free survival. A significant difference was found regarding the PN index wherein, patients with either or both high platelet and high neutrophil counts were less likely to have a complete pathologic response than those in the low-PN index group (P = 0.039). In keeping with the literature that reports pathologic stage as a main prognostic factor and treatment response determinant, we also found tumor stage to be significant for treatment response. Patients diagnosed with earlier stage disease were more likely to respond completely to treatment than those diagnosed with stage III disease (P = 0.035). Although pretreatment neutrophils, platelets, and their composite represented by the PN index were not associated with survival outcomes, PFS was significantly longer in patients with a complete pathologic response to neoadjuvant treatment than their counterparts with incomplete response (log-rank test P = 0.0221).
Limitations of the current study include its retrospective nature and relatively small patient population. Patients were registered from only two centers, and it remains unknown whether results are more generalizable in other populations. In addition, platelet and neutrophil counts may be influenced by several other factors beyond the tumor microenvironment and its cytokine effects, such as bone marrow cell production and survival of blood cells in the circulation. Another limitation of this study and other studies of retrospective nature is that they do not inform on subtypes of circulating cells that could have discordant influences on cancer progression.
Nonetheless, our study shows that the combination of higher platelet and neutrophil counts at the time of diagnosis has predictive value with respect to treatment response in localized rectal cancer. Provided that further analyses with additional and larger patient populations are obtained, it is possible that the combination of platelet and neutrophil counts as a PN index may become a useful, inexpensive, and pragmatic approach to treatment response prediction.
References
Voutsadakis IA. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J Gastrointest Oncol. 2014;6:34–40.
Steele M, Voutsadakis IA. Pre-treatment platelet counts as a prognostic and predictive factor in stage II and III rectal adenocarcinoma. World J Gastrointest Oncol. 2017;9:42–9.
Kim JH, Lee JY, Kim HK, Lee JW, Jung SG, Jung K, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol. 2017;23:505–15.
Clark TL, White DA, Osborne ME, et al. Predicting response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer with serum biomarkers. Ann R Coll Surg Engl. 2017;99:373–7.
Haggar F, Boushey R. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–7.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91.
Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, et al. Review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci. 2016;12:1022–31.
Ryan JE, Warrier SK, Lynch AC, Ramsay RG, Phillips WA, Heriot AG. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Color Dis. 2015;18:234–46.
Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–6.
Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res. 2015;2015:983698.
Vogt Sionov R, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8:125–58.
Nowarski R, Gagliani N, Huber S, Flavell RA. Innate immune cells in inflammation and cancer. Cancer Immunol Res. 2013;1:77–84.
Berger-Achituv S, Brinkmann V, Abed UA, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48.
Su Z, Mao YP, OuYang PY, Tang J, Xie FY. Initial hyperleukocytosis and neutrophilia in nasopharyngeal carcinoma: incidence and prognostic impact. PLoS One. 2015;10:e0136752.
Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, et al. RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–76.
Steinestel K, Eder S, Schrader A, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Transl Med. 2014;3:17.
Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–8.
Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–5.
Yasuda K, Sunami E, Kawai K, Nagawa H, Kitayama J. Laboratory blood data have a significant impact on tumor response and outcome in preoperative chemoradiotherapy for advanced rectal cancer. J Gastrointest Cancer. 2012;43:236–43.
Mercier J, Voutsadakis IA. The platelets-neutrophils to lymphocytes ratio: a new prognostic marker in metastatic colorectal cancer. J Gastrointest Oncol. 2018;9:478–86.
Watt DG, Martin JC, Park JH, Horgan PG, McMillan DC. Neutrophil count is the most important prognostic component of the differential white cell count in patients undergoing elective surgery for colorectal cancer. Am J Surg. 2015;210:24–30.
Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC. The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS One. 2015;10:e0142159.
Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–8.
Funding
This study was partially funded by a Northern Ontario School of Medicine Dean’s summer student research award (to A. Policicchio).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Policicchio, A., Mercier, J., Digklia, A. et al. Platelet and Neutrophil Counts as Predictive Markers of Neoadjuvant Therapy Efficacy in Rectal Cancer. J Gastrointest Canc 50, 894–900 (2019). https://doi.org/10.1007/s12029-018-0173-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-018-0173-5