Abstract
Hepatocellular carcinoma has still been one of the cancer with increasing incidence and highest mortality rate in the world. Although many new promising developments have been defined in hepatocarcinogenesis, with a short survival the treatment of patients with advanced hepatocellular carcinoma is an emerging issue. On the recent decade, only one anti-angiogenic agent sorafenib improved overall survival with costing a hardly manageable toxicity. Novel immunotherapeutic agents, especially immune checkpoint inhibitors are on the edge of more effective but less toxic treatments for these patients. In this article the activity of immune checkpoint inhibitors, anti-CTLA-4 and anti-PD1 antibodies for the treatment of patients with advanced hepatocellular cancer will be reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancer among men and also third the most common cause of cancer-related deaths (9.5 death/100.000) following lung and breast cancer among both sexes in the world [1]. HCC has different underlying etiologies; including hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol, aflatoxin B, and nonalcoholic steatohepatitis [2]. These risk factors can cause diverse molecular changes that induces to hepatocarcinogenesis. The prognosis and treatment of HCC depends on anatomic stage, biological grade, and functional status of underlying liver diseases. The treatment of the patients with advanced HCC is an unmet need. Recently, immunotherapy especially with immune checkpoint inhibitors is the new hope on the horizon.
Pre-immunotherapy era
Clinical staging for HCC is complex, and specific treatments are assigned based on Barcelona Clinic Liver Cancer staging (BCLC) [3]. Patients with very early (0) and early (A) staged disease are generally treated with curative intend; tumor resection, liver transplantation and radiofrequency ablation (RFA)/percutaneous ethanol injection (PAI), and the expected 5-year overall survival (OS) varies from 40 to 70%. On the other hand, patients with intermediate (C) and advanced staged (D) disease are managed with locoregional approaches like transarterial chemoembolization (TACE)/transarterial radioembolization (TARE) and systemic therapy (TKI inhibitor; sorafenib), respectively. With these palliative treatments, 3-year survival ratio ranges from 10 to 30% in the randomized clinical trials (RCT) [4].
For patients with BCLC stage C disease, the only approved treatment is sorafenib, based on results from the randomized phase III SHARP trial comparing sorafenib vs placebo in advanced, unresectable HCC (uHCC) [5, 6]. This is the first time that an agent demonstrated improved median OS (10.7 vs 7.9 months) in advanced incurable HCC in a randomized phase III trial. When compared with sorafenib in phase III REFLECT trial, lenvatinib is not inferior in OS and achieves statistically significant and clinically meaningful improvements in PFS, TTP, and ORR, as first-line therapy for uHCC. It may be a potential treatment option without a safer toxicity profile [7].
In the setting of advanced HCC following sorafenib failure, two agents targeting angiogenesis have yielded improved results in recent phase III clinical trials. Ramicurumab, a VEGFR2 antibody, prolonged the median OS among patients with baseline AFP > 400 ng/mL (7.8 vs 4.2 months) when compared to placebo [8]. The other promising agent, regorafenib, a small molecule inhibitor of VEGFR1-3, TIE2, PDGFRβ, FGFR, KIT, RET, and RAF reduced the risk of death (38%) and progression (54%) and yielded median OS (10.6 vs 7.8 months) when compared to placebo [9].
Immunotherapy
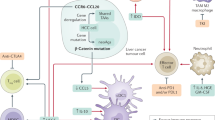
The immune system is responsible for eliminating tumor cells. Normally, with release of cancer cell antigens, antigen presenting cells (APCs) process and present these antigens via MHC I and MHC II to TCR on T-cells. With co-signaling interaction of T-cell and APCs, CD4 and CD8-positive T-cell are primed and activated in lymph nodes. Activated T-cells reach to tumor tissue and begin to infiltrate tumor. T-cells recognize tumor cells and kill them via perforin and granzyme [10]. Unfortunately, many tumor cells can escape from the immune system by multiple mechanisms [11]. Two major escaping mechanisms related to immune checkpoints are co-inhibitory CTLA-4 and PD-1 pathways [12, 13]. CTLA-4 antigen inhibits the interaction between APC and T-cell activation in lymph nodes and PD-1 antigen on active T-cells interacts with PD-L1 on tumor cells, and inhibits tumor cell kill.
HCC is an immunogenic tumor and expresses many tumor-associated antigens. The tumor-associated antigens recognized by CTLs included cyclophilin B, squamous cell carcinoma antigen recognized by T cells (SART) 2, SART3, p53, multidrug resistance-associated protein (MRP) 3, alpha-fetoprotein (AFP), and human telomerase reverse transcription (hTERT) [14, 15]. Co-inhibitory antigen PD L1 are clearly detected on both immune cells and HCC cells. PD-L1 expression (observed in ~ 74% of HCC cases) predicts recurrence/survival in HCC patients after resection [16].
HBV and HCV infections cause immunosuppression. Antiviral T cells in HBV and HCV infection are anergic and can impair immune responses [17]. PD-1 pathway-mediated immunosuppressive mechanisms are also evidenced in patients with virally infected chronic liver diseases [18].
Immunotherapy has emerged as an exciting treatment modality for HCC. Wide varieties of other immune-based treatment approaches are used to manipulate the immune system and, thereby, improve outcomes. These include vaccine-based approaches, oncolytic viruses, various cytokines, as well as cell-based approaches (e.g., elimination of suppressor cells and in vitro modulation of immune cells).
However, the most mature immunotherapy data in advanced HCC to date are related to immune checkpoint inhibitors targeting CTLA-4 and PD-1 pathways. Antibodies against CTLA-4, PD-1, and PD-L1 antigens have shown promising activity in early phase trials.
Immune Checkpoint Inhibitors
CTLA4 Blockade
Sangro B, et al. reported that CTLA-4 blockade with tremelimumab in patients with HCC and HCV achieved promising results; ORR 17.6%, disease control rate (DCR) 76.4%, median TTP 6.48 months [19]. Sangro et al. also demonstrated a decrease of > 200-fold in serum HCV viral load at day 210 in 12 patients treated with tremelimumab [19]. First results of phase II study, tremelimumab plus TACE/RFA/stereotactic body radiotherapy (SBRT), showed 6 and 12-month probabilities of tumor progression-free survival for the refractory HCC population were 57.1 and 33.1%, respectively, with median time to tumor progression of 7.4 months (95% CI 4.7 to 19.4 months). Median overall survival was 12.3 months (95% CI 9.3 to 15.4 months) [20].
PD-1 and PD-L1 Blockade
Recently, a plenty of PD 1 and PD L1 inhibitors demonstrated significant clinical activity in the treatment of many incurable metastatic tumors including melanoma, lung cancer, renal cell carcinoma, head-neck cancer, breast cancer, colorectal cancer, and urothelial cancer.
With a shorter than 6-month survival, treatment of the patients with advanced HCC is an emerging issue.
Nivolumab is a fully human immunoglobulin (IgG4) monoclonal antibody inhibitor of PD-1 receptor. The safety and efficacy results were analyzed across dose-escalation and dose-expansion cohorts in Study CheckMate 040 [21, 22]. The dose escalation cohort consisted of 48 uninfected and infected (HBV and HCV) advanced HCC patients and dose-expansion cohort consisted of 112 uninfected (54 sorafenib naïve/intolerant, 58 sorafenib progressor) and 102 infected (51 HBV and 51 HCV) advanced HCC patients. Majority of the patients (76%) had extrahepatic diseases and received systemic treatment (75%). AFP level was ≥ 200 ng/dL in 40% of the patients. Of 262 patients, 68% had any grade and 19% had grade ¾ toxicity, predominantly skin adverse effects and laboratory abnormalities, transaminitis, and increase in lipase levels. Disease was controlled in 68% of the patients. Objective responses (16%) were occurred irrespective of infection status (uninfected or infected HCV or HBV), prior sorafenib treatment, and PD L1 expression on tumor cells. Median duration of response was 17 months in escalation cohort and not reached in expansion cohort [23]. Nivolumab demonstrated limited antiviral activity in infected patients with advanced HCC. Nivolumab is currently under investigation in a phase III RCT (CheckMate 459) vs sorafenib in the first-line setting (NCT02576509).
With these promising results, a phase III study of another PD1 inhibiting agent, pemrolizumab is ongoing (NCT02702401). There is a rationale to block other immune checkpoints (such as Lag-3 and TIM-3) and to evaluate antibodies that agonistically bind costimulatory receptors on immune cells (OX40, GITR, CD137). While early phase studies evaluating such agents are ongoing, combinations of immunotheroupatic agents both with themselves (CTLA4 and PD1 /PD L1 inhibitors, costimulatory receptors) and other treatments (sorafenib/TACE/TARE) will be challenging for the shadowed side of the HCC treatment.
References
International Agency for Research on Cancer. GLOBOCAN. Estimated Global Cancer Incidence, Mortality and Prevalence Worldwide in. 2012:2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed August 04, 2017
Whittaker S, et al. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005.
Llovet JM, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711.
Subramanian S, et al. A review of hepatocellular carcinoma (HCC) staging systems. Chin Clin Oncol. 2013;2:33.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Kane RC, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14:95–100.
Cheng A-L et al. Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 35, 2017 (suppl; abstr 4001).
Zhu AX, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–70.
Bruix J, et al. Efficacy and safety of regorafenib versus placebo in patients with hepatocellular carcinoma (HCC) progressing on sorafenib: results of the international, randomized phase 3 RESORCE trial. Annals of Oncol, 27, 2016 suppl LBA-3, ii140–ii141.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol. 2011;54(4):830–4.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer—preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–9.
Mizukoshi E, Nakamoto Y, Arai K, et al. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–16.
Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–26.
Umemoto Y, et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol. 2015;50(1):65–75.
Grakoui A, et al. Turning on the off switch: regulation of anti-viral T cell responses in the liver by the PD-1/PD-L1 pathway. J Hepatol. 2006;45(4):468–72.
Schmidt J, et al. T-cell responses in hepatitis B and C virus infection: similarities and differences. Emerg Microbes Infect. 2013;2(3):e15.
Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–8.
Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–51.
El-Khoueiry AB, et al. Phase 1/2 safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma: interim analysis of the CheckMate 040 dose-escalation study. Poster presentation at ASCO. 2016; poster 4012
Sangro B, Melero I, Yau T et al. Safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma: interim analysis of dose-expansion cohorts from the phase 1/2 checkmate 040 study. In:Presented at the 52nd Annual Meeting of the American Society of Clinical Oncology (ASCO); June 3–7, 2016; Chicago, IL, USA, poster 4078.
Crocenzi T S. Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J Clin Oncol 35, 2017 (suppl; abstr 4013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahin, B. Enlighting the Shadow for Advanced Hepatocellular Carcinoma: Immunotherapy with Immune Checkpoint Inhibitors. J Gastrointest Canc 48, 288–290 (2017). https://doi.org/10.1007/s12029-017-9996-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-017-9996-8