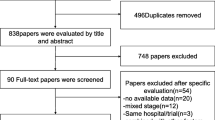
Abstract
Purpose
Microsatellite instability in colorectal cancer (CRC) and its long-term outcomes remains poorly studied in Asians. We investigate the prognostic significance of microsatellite instability in an Asian population and assess its clinical impact in patients who undergo adjuvant chemotherapy.
Methods
Six hundred fifty-four consecutive CRC patients who underwent surgical resection between January 2010 and December 2012 were recruited. Survival was estimated using the Kaplan-Meier approach. Univariate Cox proportional hazard models were used to estimate the hazard ratios for variables associated with survival. A subgroup analyses was performed for stage III patients who underwent chemotherapy to evaluate the prognostic significance of microsatellite instability in this group.
Results
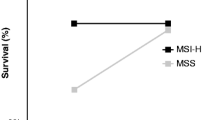
Five hundred ninety-one (90.4%) patients were microsatellite stable (MSS) while 63 (9.6%) were microsatellite instable (MSI). Three years recurrence-free survival (RFS) and disease-specific survival (DSS) were 83.7 versus 73.7% (p = 0.295) and 87.1 versus 91.2% (p = 0.307) in MSS and MSI tumors, respectively. Among stage III patients who received adjuvant therapy, MSI status was found to be an adverse prognostic factor for RFS (HR 2.74 (95% CI 1.43–5.26), p = 0.002). This remained significant on multivariate analysis (HR 2.38 (95% CI 1.15–4.93), p = 0.018). Adjuvant chemotherapy was associated with survival benefit for patients with MSS tumors (HR 0.35, 95% CI 0.17–0.69, p = 0.002) but not MSI tumors (HR 0.67, 95% CI 0.08–8.15, p = 0.750).
Conclusions
MSI status is not a prognostic indicator in the general CRC population but appears to be an adverse prognostic indicator for RFS in stage III CRC patients who received adjuvant chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most frequent cause of cancer death worldwide [1]. Among genetic abnormalities involved in carcinogenesis, microsatellite instability is a major pathway of cancer development, accounting for almost all colorectal cancers occurring in Lynch syndrome, which makes up 2–5% of all colorectal cancers, and approximately 15% of sporadic colorectal cancers [2, 3].
The incidence of mismatch repair (MMR) deficiency in CRC and its long-term outcomes remains poorly studied in Asians. A previous meta-analysis has found that microsatellite instability status was a significant prognostic factor in CRC [4]. It also alluded to a lack of benefit of adjuvant fluorouracil-based chemotherapy among microsatellite instable (MSI) patients with stage II disease [5, 6]. Prognostic factors among the Western population may not be applicable to Asians as survival of CRC is known to vary among major ethnic groups [7]. Asians have been shown to display improved survival as compared to other races [7, 8]. These differences have persisted despite adjustments for confounding variables such as age, grade, histology, and socio-economic status, suggesting that biologic factors may account for the disparity in survival [8]. It remains to be seen if the prognostic significance of MSI tumors demonstrated in Western populations remain applicable to an Asian population since few studies have been done [9, 10].
We aim to investigate the prognostic significance of microsatellite instability in an Asian population and assess its clinical impact in CRC patients who undergo adjuvant chemotherapy.
Materials and Methods
Subjects
Consecutive CRC patients who underwent surgical resection in Singapore General Hospital between January 2010 and December 2012 for histological confirmed CRC were recruited. Immunohistochemistry (IHC) staining for MMR proteins was performed routinely for all patients starting from January 2010. Patients who presented with recurrent cancer, inflammatory bowel disease, familial adenomatous polyposis, or other polyposis syndromes were excluded. The study protocol was approved by the Institutional Review Board of Singapore General Hospital.
Pre-operative staging included computerized tomography (CT) scanning of the thorax, abdomen, and pelvis. Rectal tumors were additionally staged with MRI or endorectal ultrasound where feasible. Pre-operatively, all patients underwent complete colonoscopy, where possible, to exclude synchronous cancers. Obstructive lesions that precluded a complete endoscopic evaluation of the colon were evaluated for synchronous lesions using CT colonography or barium enema pre-operatively.
Staging of disease was according to AJCC Cancer Staging Manual, 6th edition after surgical resection, and comprises information from histological review of the resected specimen and radiological investigations of distant metastases [11].
Immunohistochemical Staining
Immunohistochemistry (IHC) staining was routinely performed for all patients undergoing elective resection for CRC. IHC was performed using the standard streptavidin-biotin-peroxidase procedure. Specifically, 5-μm-thick sections of 10% formalin-fixed, paraffin-embedded tumor or tissue were first de-paraffinized in xylene, rehydrated in graded alcohols, and washed in double-distilled water. Endogenous peroxidase activity was blocked by incubation with 3% H2O2. The slides were then placed in 10 mM citrate buffer at pH 6 and boiled in a microwave for 15 min for antigen retrieval. After treatment with 10% normal goat serum for 10 min to block nonspecific protein binding, primary monoclonal antibodies against MLH1 (clone G168-728, diluted 1:250, PharMingen, San Diego, CA), MSH2 (clone FE11, diluted 1:50, Oncogene Research Products, Cambridge, MA), MSH6 (clone GRBP.P1/2.D4, diluted 1:200; Serotec Inc., Raleigh, NC) were applied. Antigen-antibody reaction was visualized using the avidin-biotinylated horseradish peroxidase complex (LSAB kit, Dako) and diaminobenzidine as the chromogen. Slides were counterstained with hematoxylin. Normal colonic crypt epithelium adjacent to the tumor, lymphoid, and stromal cells served as internal positive controls for staining. Appropriate external positive (normal colon mucosa) and negative (MSI tumors known to lack MLH1 or MSH2 protein expression) controls were used.
For PMS2 (clone A164, 1:30 dilution)—heat retrieval was done at 100 °C for 20 min using Epitope retrieval 2 buffer solution, with 20 min antibody incubation at room temperature on Leica Bond-III autostainer using Bond Polymer Detection kit.
All specimens with IHC staining were reviewed by a dedicated gastrointestinal pathologist. Patients are classified as MSI when one of the four DNA mismatch repair proteins is stained negative. Patients are classified microsatellite stable (MSS) when all four DNA mismatch repair proteins are stained positive.
Adjuvant Therapy Regime
Adjuvant therapy was offered for all stage III patients who were deemed fit enough to undergo adjuvant chemotherapy. Adjuvant chemotherapy was also offered to stage II patients with high-risk factors such as perineural invasion, lymphovascular invasion, and obstructed or perforated tumors. Adjuvant therapy comprised 6 months of fluoropyrimidine (5-fluorouracil or capecitabine) with or without oxaliplatin.
Follow-up Regime
Post-operatively, the patients were followed up at 3-monthly intervals for the first 2 years, 6-monthly for the next 2 years and then yearly thereafter as per the NCCN Guidelines [12]. At each consultation, CEA levels were measured and full history and physical examination (including digital rectal examination) were performed. Regular periodic computed tomography imaging was performed annually for all high-risk stage II and stage III tumors. Patients with suspicious symptoms and signs of rising CEA trend on follow-up will be evaluated earlier with colonoscopy and/or radiological imaging.
The cohort of patient was followed up till December 2015 for the purpose of this study.
Statistical Analyses
All analyses were performed using R 3.1.1 (2014 Vienna, Austria). Recurrence-free survival (RFS) was calculated from date of surgery to recurrence or last follow-up. Disease-specific survival (DSS) was calculated from date of surgery to death from colorectal cancer or till the point of last follow-up. RFS and DSS were estimated using the Kaplan-Meier approach and 3-year survival probabilities with 95% confidence limits were summarized. Univariate Cox proportional hazard models were used to estimate the hazard ratios for each of the demographic and clinical characteristics associated with RFS and DSS, for the overall population. A subgroup analyses was performed for stage III patients who underwent chemotherapy to evaluate the prognostic significance of MMR deficiency status in this group. All tests were two-sided and the significance level was set at 5% throughout.
Results
A total of 654 patients were included in the study, out of which 591(90.4%) were MSS and 63 (9.6%) were MSI. Demographic characteristics of the study cohort are illustrated in Table 1. MSI tumors comprised of a larger proportion of right-sided tumors compared to MSS tumors (50.8 versus 14.9%, p < 0.001). In a median follow-up of 32 months (range 16 to 50 months), 3 years RFS and DSS were 83.7 versus 73.7% (p = 0.295) and 87.1 versus 91.2% (p = 0.307) in patients with MSS and MSI tumors, respectively (Fig. 1). Of the 95 patients (14.5%) who had either local or systemic recurrence, 83 patients (87.4%) had MSS tumors and 12 patients (12.6%) had MSI tumors. The overall mortality was 10.2% (67 out of 654). Mortality was 10.7% (63 out of 591) in the MSS group and 6.3% (4 out of 63) in the MSI group (p = 0.393).
Univariate and multivariate analyses for RFS and DSS are illustrated in Table 2 and Table 3, respectively. In terms of RFS, factors significant on univariate analysis include tumor grade, TNM stage, perineural infiltration, vascular emboli, and apical node involvement. However, in multivariate analysis, only TNM stage, perineural infiltration, and vascular emboli remained significant predictors of RFS. (Table 2) For DSS, factors significant in univariate analysis include mucinous tumors, tumor grade, TNM staging, perineural invasion, presence of vascular emboli, apical node involvement, and BRAF status. Age, TNM stage IV status, the presence of vascular emboli, apical node involvement, and BRAF status remained significant predictors of DSS after all the variables significant in the univariable analysis were entered into a single model. (Table 3).
Subgroup Analyses for Stage III Patients Who Have Undergone Adjuvant Chemotherapy
Two hundred twenty-four (34.3%) patients underwent adjuvant therapy and comprised of 21 (55.2%) patients in stage II and 203 (67.9%) patients in stage III. The 203 stage III patients that received adjuvant chemotherapy were out of a cohort of 299 stage III patients and those who did not receive chemotherapy either refused adjuvant treatment or were deemed not fit enough for treatment. Stage II patients were excluded from this analysis due to the small numbers of patients with stage II disease who underwent adjuvant chemotherapy. Among the 203 stage III patients who received adjuvant chemotherapy, 17 were MSI while 186 were MSS. Chemotherapy regimes did not differ significantly between the groups with MSS and MSI tumors (5FU/capecitabine monotherapy 37.6 versus 29.4%; 5FU/capecitabine in combination with oxaliplatin 60.2 versus 64.7% and 5FU/capecitabine in combination with irinotecan 2.2 versus 5.9%, p = 0.322).
In stage III patients, adjuvant chemotherapy was associated with improved DSS (HR 0.38 (95% CI 0.2–0.73), p = 0.004) but not RFS (HR 0.61 (95% CI 0.32–1.12), p = 0.108).
The prognostic significance of MMR deficiency status on stage III patients who received adjuvant therapy was assessed. MSI was found to be an adverse prognostic factor for RFS (HR 2.74 (95% CI 1.43–5.26), p = 0.002) (Table 4) but not DSS (HR 1.56 (95% CI 0.55–4.41), p = 0.401). MSI status remained an adverse prognostic factor for RFS in multivariate analysis (HR 2.38 (95% CI 1.15–4.93), p = 0.018). These results for univariate and multivariate analyses for RFS among the stage III patients are illustrated in Table 4.
When stage III patients were stratified based on adjuvant therapy status, MSI remained an adverse prognostic factor and had poorer recurrence-free survival in those who received adjuvant chemotherapy (HR 2.60 (95% CI 1.27–5.35), p = 0.009) (Table 5). Adjuvant chemotherapy was associated with survival benefit for patients with MSS tumors (HR 0.35, 95% CI 0.17–0.69, p = 0.002) but not those with MSI tumors (HR 0.67, 95% CI 0.08–8.15, p = 0.750).
Discussion
In our institution, IHC was initially performed for young CRC patients of age ≤50 years old, but has been routinely implemented in all age groups since the year 2010. We have published our initial report of a purely ≤50 years old cohort and noted the presence of 21% MMR deficient CRC on IHC screening [13]. This study highlighted the importance of MMR screening and the high incidence of MMR detected despite an absence of strong family history of CRC [13]. Our current study reports a consecutive series of 654 sporadic, unrelated CRC patients across all ages in which IHC staining of MMR proteins was routinely performed. This study’s main aim is to investigate the prognostic significance of MSI in an Asian population and assess the clinical impact of MSI in colorectal cancer patients who undergo adjuvant chemotherapy.
Our series reports a large IHC-screened Asian cohort and significantly adds on to the literature on the prognostic significance of MSI in Asians. Previously published meta-analyses were from cohorts of Western populations, and the applicability of these findings to an Asian population is thus unknown as few studies have been performed [9, 10]. In our study cohort, MSI status interestingly had no prognostic significance for both RFS and DSS. It was only in subset analysis of stage III patients who had received adjuvant therapy that MSI tumors were found to have poorer RFS (HR 2.74, 95% CI 1.43–5.26, p = 0.002), a finding which persisted on multivariate analysis (HR 2.38, 95% CI 1.15–4.93, p = 0.018). Studies among other Asian populations have revealed mixed findings. Jung et al. had illustrated in a cohort of 1232 patients that MSI status was associated with improved 5-year cancer-specific survival rates (88.2 versus 61.2%, p < 0.001) [9]. In contrast, Shin et al. failed to demonstrate any prognostic significance of MSI status [10]. Interestingly, Shin et al. also concluded that MSI status may confer an adverse prognostic impact, albeit in patients with stage II disease [10]. In this study, adjuvant chemotherapy, predominantly of the 5-FU regime, was administered to 87.8% of the population with stage II disease. This prompted the authors to hypothesize that the poorer outcome for stage II MSI CRCs might be attributable to the non-beneficial effect of 5-FU adjuvant chemotherapy in this subset of patients [10].
The selection of patients for adjuvant chemotherapy remains challenging and traditional clinical and histopathological factors dominate decision-making. While it is routine for all stage III patients to be offered adjuvant chemotherapy, and has quite clearly proven an overall survival advantage in many studies including our study cohort, it is difficult to predict the patients in which adjuvant chemotherapy is unlikely to benefit. In our study, survival benefit of adjuvant chemotherapy was observed mainly for patients with MSS tumors (HR 0.35, 95% CI 0.17–0.69, p = 0.002) but not those with MSI tumors (HR 0.67, 95% CI 0.08–8.15, p = 0.750). Our study findings are not unique and these results have been mirrored in several other studies although these included patients with stage II disease as well [14,15,16,17,18]. While these findings may suggest the possible attenuation of benefit of adjuvant chemotherapy in stage III tumors with MSI, it will require validation by further large cohort studies in a randomized setting. We are unfortunately unable to analyze the impact of MSI on stage II adjuvant treatment due to a small sample size. Based on our study findings, there is insufficient evidence to suggest that MSI status should be used to guide decision for adjuvant treatment. This is consistent with the findings of a recently published systematic review of 9212 patients with CRC [19].
There are molecular hypothesis for chemoresistance and poorer prognosis in MSI tumors. While MSI-H CRCs are molecularly heterogeneous tumors, in vitro experiments have noted that preservation of MMR function in cancer cells can lead to apoptotic effect of 5-FU and thus explain the molecular basis of resistance of 5-FU based chemotherapy in CRCs with MSI [20,21,22]. Another plausible explanation is the presence of CpG Island Methylator Phenotype (CIMP) within the MSI tumor population. This is a distinct subset of CRC characterized by repression of tumor suppressor genes as a result of promoter methylation. These CIMP-H tumors are frequently associated with older age, frequent BRAFV600E mutations, poor differentiation, and signet ring cell components in MSI CRCs. CIMP-H tumors, therefore, may contribute to the poorer prognosis in CRCs with MSI and are known to respond poorly to 5-FU chemotherapy [21]. Data remains conflicting and non-conclusive.
The main limitation of our study is that the sample size of patients with MSI tumors was small. This may have led to a type 2 error when benefit of adjuvant chemotherapy was assessed after stratification by MMR deficiency status. However, as only 10–15% of colorectal cancers are MSI, a small sample size is a common problem among most clinical studies done on MMR deficiency. Treatment effects of adjuvant chemotherapy are also not based on a randomized setting and interpretation of results may thus have inherent bias. We also did not have information regarding polymerase chain reaction testing of microsatellite instability in our cohort as IHC was the main stay of MMR deficient screening in our institution during the study duration. However, this is unlikely to have resulted in significant miscategorization of MSI tumors in our study as IHC and MSI testing via polymerase chain reaction are considered to have equivalent accuracy in literature [23]. Nonetheless, our series remains one of the few in Asia which studies the prognostic value of microsatellite instability and its impact on adjuvant therapy and would be an invaluable addition to the scant literature on this topic in our region.
Conclusion
MSI is not a prognostic indicator in the general CRC population. Subset analysis suggests MSI may be an adverse prognostic indicator for recurrence-free survival in stage III CRC patients who received adjuvant chemotherapy. Further studies are required to determine if MSI status should be considered during decision-making for adjuvant chemotherapy.
References
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365(9454):153–65. doi:10.1016/S0140-6736(05)17706-X.
Buecher B, Cacheux W, Rouleau E, Dieumegard B, Mitry E, Lievre A. Role of microsatellite instability in the management of colorectal cancers. Dig Liver Dis. 2013;45(6):441–9. doi:10.1016/j.dld.2012.10.006.
Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002;62(9):2447–54.
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–18. doi:10.1200/JCO.2005.01.086.
Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355(9217):1745–50. doi:10.1016/S0140-6736(00)02261-3.
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57. doi:10.1056/NEJMoa022289.
Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomark Prev. 2008;17(8):1950–62. doi:10.1158/1055-9965.EPI-07-2774.
Le H, Ziogas A, Taylor TH, Lipkin SM, Zell JA. Survival of distinct Asian groups among colorectal cancer cases in California. Cancer. 2009;115(2):259–70. doi:10.1002/cncr.24034.
Jung SH, Kim SH, Kim JH. Prognostic impact of microsatellite instability in colorectal cancer presenting with mucinous, signet-ring, and poorly differentiated cells. Ann Coloproctol. 2016;32(2):58–65. doi:10.3393/ac.2016.32.2.58.
Shin US, Cho SS, Moon SM, Park SH, Jee SH, Jung EJ, et al. Is microsatellite instability really a good prognostic factor of colorectal cancer? Ann Coloproctol. 2014;30(1):28–34. doi:10.3393/ac.2014.30.1.28.
BD ESB, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed; 2009.
Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–35. doi:10.1016/S1473-3099(12)70323-7.
Chew MH, Koh PK, Tan M, Lim KH, Carol L, Tang CL. Mismatch repair deficiency screening via immunohistochemical staining in young Asians with colorectal cancers. World J Surg. 2013;37(10):2468–75. doi:10.1007/s00268-013-2134-2.
Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45(10):1890–6. doi:10.1016/j.ejca.2009.04.018.
Ooki A, Akagi K, Yatsuoka T, Asayama M, Hara H, Takahashi A, et al. Combined microsatellite instability and BRAF gene status as biomarkers for adjuvant chemotherapy in stage III colorectal cancer. J Surg Oncol. 2014;110(8):982–8. doi:10.1002/jso.23755.
Saridaki Z, Souglakos J, Georgoulias V. Prognostic and predictive significance of MSI in stages II/III colon cancer. World J Gastroenterol. 2014;20(22):6809–14. doi:10.3748/wjg.v20.i22.6809.
Tougeron D, Mouillet G, Trouilloud I, Lecomte T, Coriat R, Aparicio T, et al. Efficacy of adjuvant chemotherapy in colon cancer with microsatellite instability: a large multicenter AGEO study. J Natl Cancer Inst. 2016;108(7) doi:10.1093/jnci/djv438.
Yang L, Sun Y, Huang XE, Yu DS, Zhou JN, Zhou X, et al. Carcinoma microsatellite instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for stage II rectal cancer. Asian Pac J Cancer Prev. 2015;16(4):1545–51.
Webber EM, Kauffman TL, O’Connor E, Goddard KA. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer. 2015;15:156. doi:10.1186/s12885-015-1093-4.
Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003;106(1):66–73. doi:10.1002/ijc.11176.
Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol. 2014;20(15):4230–43. doi:10.3748/wjg.v20.i15.4230.
Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001;61(13):5193–201.
Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10(4):293–300. doi:10.2353/jmoldx.2008.080031.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tan, W.J., Hamzah, J.L., Acharyya, S. et al. Evaluation of Long-Term Outcomes of Microsatellite Instability Status in an Asian Cohort of Sporadic Colorectal Cancers. J Gastrointest Canc 49, 311–318 (2018). https://doi.org/10.1007/s12029-017-9953-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-017-9953-6