Abstract
Background
Delayed cerebral ischemia associated with cerebral vasospasm (CVS) in aneurysmal subarachnoid hemorrhage significantly affects patient prognosis. Levosimendan has emerged as a potential treatment, but clinical data are lacking. The aim of this study is to decipher levosimendan’s effect on cerebral hemodynamics by automated quantitative measurements of brain computed tomography perfusion (CTP).
Methods
We conducted a retrospective analysis of a database of a neurosurgical intensive care unit. All patients admitted from January 2018 to July 2022 for aneurysmal subarachnoid hemorrhage and treated with levosimendan for CVS who did not respond to other therapies were included. Quantitative measurements of time to maximum (Tmax), relative cerebral blood volume (rCBV), and relative cerebral blood flow (rCBF) were automatically compared with coregistered CTP before and after levosimendan administration in oligemic regions.
Results
Of 21 patients included, CTP analysis could be performed in 16. Levosimendan improved Tmax from 14.4 s (interquartile range [IQR] 9.1–21) before treatment to 7.1 s (IQR 5.5–8.1) after treatment (p < 0.001). rCBV (94% [IQR 79–103] before treatment and 89% [IQR 72–103] after treatment, p = 0.63) and rCBF (85% [IQR 77–90] before treatment and 87% [IQR 73–98] after treatment, p = 0.98) remained stable. The subgroup of six patients who did not develop cerebral infarction attributed to delayed cerebral ischemia showed an approximately 10% increase (rCBV 85% [IQR 79–99] before treatment vs. 95% [IQR 88–112] after treatment, p = 0.21; rCBF 81% [IQR 76–87] before treatment vs. 89% [IQR 84–99] after treatment, p = 0.4).
Conclusions
In refractory CVS, levosimendan use was associated with a significant reduction in Tmax in oligemic regions. However, this value remained at an abnormal level, indicating the presence of a persistent CVS. Further analysis raised the hypothesis that levosimendan causes cerebral vasodilation, but other studies are needed because our design does not allow us to quantify the effect of levosimendan from that of the natural evolution of CVS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Delayed cerebral ischemia (DCI) in the setting of aneurysmal subarachnoid hemorrhage (aSAH) is a complex phenomenon with a largely unknown physiopathology involving neuroinflammation, microthrombosis, vascular dysfunction, and even spreading depolarizations [1, 2]. Cerebral vasospasm (CVS) is a transient phenomenon that results in extensive constriction of cerebral arteries and is also closely associated with the occurrence of DCI. Although the exact relationship with DCI remains unclear, CVS can cause an imbalance between local oxygen delivery and consumption and remains the most important therapeutic target to prevent DCI [3, 4].
Levosimendan is a calcium-sensitizing inodilator that appears attractive as a novel therapeutic approach for CVS [5]. Levosimendan is most commonly used for decompensated chronic heart failure cases [6, 7], showing favorable results in terms of in-hospital mortality rates and hospitalization duration. Other uses have been discovered, such as treatment for cardiogenic shock, postoperative cardiac surgery, and right ventricular failure, owing to its positive inotropic effects without raising myocyte oxygen consumption [7, 8]. In continuous infusion, levosimendan has a reduced half-life of about 1.5 h [6]. Although some of its metabolites are quickly eliminated in the feces and urine, this molecule has a unique specificity of having an active metabolite, OR-1896, whose maximum concentration is achieved between 48 and 72 h after the treatment begins, and its elimination takes much longer, with a half-life of about 70–80 h [6]. Thus, the efficacy duration on the heart and vessels after a 24-h infusion of levosimendan is estimated to be 1 week [6], with maximum efficacy at approximately the 24th h. This pharmacological feature of levosimendan, because of its extended duration of action, presents an especially interesting aspect in cases of CVS, as this complication typically persists for approximately 10 days [9].
By opening the ATP-sensitive K+ channel in smooth muscle fibers surrounding the vascular bed, it induces sustained arterial vasodilation, even in combination with norepinephrine infusion. Its effect has already been described in vitro in animal models of CVS of the basilar artery in rabbits [10] and cerebral arteries in rats [11, 12]. To date, there have been few case reports indicating neurological improvement in patients after the use of levosimendan [13, 14]. Also, a recent case series of 18 patients with aSAH treated with levosimendan for Tako-Tsubo cardiomyopathy showed an increase in mean vessel diameter between days 1 and 5–7 [15].
In our neurointensive care unit, levosimendan has been used off-label since 2018 in rare cases of clinical deterioration attributable to a catastrophic CVS, with a refractory course in which no further conventional treatment is deemed appropriate, and as the last compassionate therapeutic option.
This analysis aimed to decipher the effects on cerebral hemodynamics of levosimendan by automated comparison of computed tomography perfusion (CTP) in our local patient cohort by performing quantitative measurements in oligemic regions before and after this treatment. This was a retrospective observational study with perfusion imaging analysis before and after treatment. In addition, the evolution of cerebral tissue oxygen pressure (PtiO2) was studied, and clinical outcomes were recorded.
Methods
Criteria for the Inclusion and Management of CVS
We retrospectively reviewed the medical prospective database of all consecutive patients admitted to our center for aSAH from January 2018 to July 2022. This study complies with national and European guidelines on the protection of personal health data (RGPD), as our clinical data warehouse is quality certified in EHDEN. Approval for this study was obtained from the local ethics committee. As required by national regulations, systematic inquiries were made to ensure that patients did not object to the use of their data. For this type of study, formal consent is not required.
All patients with aSAH hospitalized in our department are secured within 48 h of bleeding, either through embolization or surgical clipping depending on the aneurysm’s conformation. This is followed by neurological monitoring and prevention of DCI with nimodipine orally. Many patients with aSAH have external ventricular drains, and PtiO2 sensors are installed in patients with the most severe aSAH who remain comatose after securing the aneurysm. CTP is used as a diagnostic test for CVS when clinical suspicion is present, which is based on clinical findings, transcranial Dopplers, and neuromonitoring. CTP is not performed systematically but only in cases in which symptomatic CVS is suspected. CTP is also used for assessing the effectiveness of our therapies: we rely on concordant clinical and paraclinical parameters as well as improvement in arterial caliber and resolution of perfusion abnormalities.
We included in the present study all patients treated with levosimendan for clinical deterioration attributable to severe CVS who were refractory to other therapies. The therapeutic algorithm used in our unit is explained later in the text.
The only exclusion criterion was the absence of CTP performed both before and after treatment with levosimendan or unusable perfusion sequences (e.g., due to patient movement). Clinical deterioration was defined as a new onset of focal neurological impairment, a drop of at least 2 points on the Glasgow Coma Scale (GCS), or a sustained drop in PtiO2 below 20 mm Hg without confounding factors. In such cases, CVS could also be suspected using transcranial Doppler technology by observing an acceleration of the mean velocities in the middle cerebral arteries above 120 cm/s, an increase of 50 cm/s from their baseline value, or a maximum velocity in the same arteries above 200 cm/s. CVS was finally diagnosed by an experienced neuroradiologist either by computed tomography [CT] angiography and CTP or directly by cerebral arteriography.
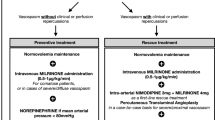
CVS management follows a precise algorithm: the first step is to induce hypertension and optimize blood volume. If there is no clinical improvement and there are no contraindications, treatment with intravenous milrinone is then initiated. If these two steps fail to produce any effect, endovascular angioplasty is performed as a final measure. The procedure combines chemical angioplasty with nimodipine and/or milrinone and mechanical angioplasty at the operator’s discretion. If this endovascular procedure proves ineffective, additional similar procedures may be performed. Discontinuation of these repeated endovascular therapies is decided on a collegial basis between the critical care team and the interventional neuroradiology team only when the expected benefit no longer appears to be more than futile. In these situations, levosimendan infusion is proposed as a last resort. Our local protocol mandates at least one attempt of endovascular procedure before abandoning it, unless marked otherwise. We strive to provide two attempts, but this may not be technically feasible (e.g., due to brain navigation difficulties).
Levosimendan was administered at a rate of 0.4 µg/kg/min for a 24-h infusion concomitantly with the complete cessation of milrinone in such extremely rare situations in which the disease progression was catastrophic and unresponsive to previous treatments. Chemical or mechanical angioplasty was not used after this infusion, which was deemed the final therapeutic alternative. The dosage was selected by our center after consulting with a knowledgeable cardiac surgery critical care team who frequently administers this drug to patients with heart failure. The dosage is primarily based on the percentage of patients who respond, which is maximum at 0.4 µg/kg/min. It also marks the point at which hypotensive episodes begin to be worrisome [8].
This decision to attempt compassionate treatment was made collaboratively by the intensive care and neuroradiology teams because of the catastrophic nature of the disease following the reappearance of clinical symptoms despite ongoing treatment.
Radiologic Method of CTP Comparison
For the radiological data, repetitive CTP scans were obtained on a 64-slice CT scanner (Optima 660; GE Healthcare). CTP acquisitions were obtained with the following parameters: 80 kV, 150 mA, 250 mm FOV, 512 × 512 matrix, 16 slices covering 80 mm, 3-s time resolution (time rotation, 0.5 s). Images were acquired after injection of a 1-mL/kg bolus of iodinated contrast agent (XENETIX 300 mg iodine/mL) injected at a rate of 5 mL/s.
For each patient, we obtained a pair of CTP scans corresponding to the last CTP scan before and the first CTP scan after levosimendan administration (pre-CTP and post-CTP). Parametric maps (time to maximum [Tmax], cerebral blood volume [CBV], cerebral blood flow [CBF]) were obtained from Olea Sphere 3.0 (Oléa Medical) using the Bayesian deconvolution method. The extraction of quantitative data from these maps was then fully automated to eliminate any operator-dependent bias. The parametric maps generated by the Olea software were postprocessed offline. Brain extraction was performed using Functional Magnetic Resonance Imaging of the Brain Software Library (FSL) 6.0 [16]. Affine linear registration was performed between pre-CTP and post-CTP using advanced normalization tools [17]. Registration accuracy was visually verified for each patient.
Regions of interest (ROIs) with significant hypoperfusion defined as Tmax > 6 s were automatically calculated from the pre-CTP and propagated to the post-CTP using the transform matrix for coregistration. Example of this method is shown in Fig. 1. Mean Tmax, CBV, and CBF were quantified within these coregistered regions. Tmax values were kept as absolute values, whereas CBV and CBF were normalized to obtain relative parameters (relative CBV [rCBV]; relative CBF [rCBF]) using the following ratio: \(\frac{\mathrm{mean\,CBV}/\mathrm{CBF\,in\,ROI }}{\mathrm{mean\,CBV}/\mathrm{CBF\,in\,the\,whole\,brain}}\).
Cerebral hemodynamic indicators during levosimendan treatment. The Wilcoxon signed-rank test was used to compare before/after parameters. Stars were used to show statistically significant comparisons. a Individual cerebral oxygen pressure (PtiO2) as a function of the start of levosimendan infusion for the eight patients with Licox probe. The general trend was plotted using generalized additive models with smoothing in R. b Time to computed tomography perfusion (CTP). c Comparison of time to maximum (Tmax) before and after levosimendan infusion in the two CTP scans. Orange dots correspond to patients who did not develop delayed cerebral ischemia. d Comparison of relative cerebral blood volume (rCBV) before and after levosimendan infusion in the two CTP scans. Orange dots correspond to patients who did not develop delayed cerebral ischemia. e Comparison of relative cerebral blood flow (rCBF) before and after levosimendan infusion in the two CTP scans. Orange dots correspond to patients who did not develop delayed cerebral ischemia. f Example of automatic segmentation of the oligemic region of interest, propagated from the first to the second CTP scan
We also analyzed cerebral arterial diameter measurements by locating the most constricted area of the most affected cerebral artery on the angioscan performed at the time of the initial CTP scan prior to levosimendan administration. This artery’s diameter was then measured by a senior neuroradiologist using three angioscans: one conducted on admission during aSAH diagnosis, one before levosimendan infusion (i.e., the first CTP scan), and one just after (i.e., the second CTP scan).
Radiological results are presented as number (percentage) or median (interquartile range [IQR]) as adjusted. The Wilcoxon signed-rank test was used to compare admission/before/after parameters.
Clinical and Paraclinical Data Collection
The Glasgow Outcome Scale-Extended score was recorded at the last medical examination before hospital discharge. The presence of cerebral infarction attributable to DCI was diagnosed by a senior neuroradiologist at the last CT or magnetic resonance imaging scan before discharge within 6 weeks after aSAH and was defined as infarction not present at the CT scan between 24 and 48 h after aneurysm embolization and not attributable to other causes, such as a ventricular catheter or intraparenchymal hematoma.
The hourly GCS score was obtained. The hourly PtiO2, intracranial pressure (ICP), and norepinephrine dose were obtained in those available from medical records 24 h before to 24 h after the end of levosimendan infusion. The Licox probe (Integra LifeSciences Corp., Plainsboro, NJ) is used to measure PtiO2. ICP is measured discontinuously in patients with external ventricular drainage.
Natremia, glycemia, arterial oxygen and carbon dioxide pressures, hemoglobinemia, oxygen saturation, and hemodynamic variables were also collected at the time point closest to the time of both CTP scans. Troponin, plasmatic creatinine, and lactatemia were collected the day before levosimendan infusion, the day of infusion, the day after infusion, and the third and the seventh days after the end of the infusion.
Clinical and paraclinical outcomes are presented as number (percentage) or median (IQR) as adjusted. The Wilcoxon signed-rank test was used to compare before/after parameters.
Results
Twenty-one patients received levosimendan for refractory CVS during the study period. Of these, only 16 had complete automated CTP analysis before and after levosimendan; perfusion analysis was not possible in one patient who was brain dead after treatment and in another who had a purely clinical evaluation without CTP after levosimendan. In the remaining three patients, technical difficulties in obtaining perfusion sequences (e.g., movement) made automated analysis impossible. A flow chart is provided in Online Appendix.
In the included patients, aSAH was severe, with a median WFNS score of 5 (IQR 2–5) and a Fisher score of 4 for all the patients. In all cases, the causative intracranial aneurysm was initially treated with embolization. Two patients (13%) died during the primary hospitalization, and ten (63%) had a cerebral infarction attributable to the DCI detected on final cerebral imaging before discharge. Good neurological outcome was observed in seven (44%) patients with a Glasgow Outcome Scale–Extended score > 3 at hospital discharge.
The first episode of CVS was diagnosed 5 days (IQR 4–7) after admission and was treated in all patients with hemodynamic optimization and intravenous milrinone treatment up to 2 µg/kg/min. Because of recurrent clinical signs, patients underwent two arterial angioplasties (IQR 1–2), including ten (63%) patients with at least one mechanical balloon dilatation in the arteries with the spasm.
Levosimendan was initiated 5 days (IQR 3–7) after the initial diagnosis of vasospasm, with a 24-h infusion and concomitant discontinuation of milrinone to avoid increasing cardiac side effects, and after the last attempt at arterial angioplasty, when clinical signs recurred. The first scan was performed 4.7 h (IQR 2.2–7.1) before the start of levosimendan infusion, and the second scan was performed 61.5 h (IQR 44.3–68.06) after the start of levosimendan infusion (see Fig. 1 for timeline). PtiO2 measurement was performed during levosimendan infusion in 8 of the 16 enrolled patients (50%).
Patient demographics, enrollment, treatment, and outcomes are shown in Table 1. Levosimendan globally improved Tmax by significantly reducing the mean delay within the initially hypoperfused regions (14.4 s [IQR 9.1–21] vs. 7.1 s [IQR 5.5–8.1] for pre-CTP and post-CTP, respectively; p < 0.001). Parametric Tmax maps for all patients are shown in Fig. 2. In the overall population, rCBV and rCBF were not statistically different before and after levosimendan treatment (rCBV was 94% [IQR 79–103] before treatment and 89% [IQR 72–103] after treatment, p = 0.63; rCBF was 85% [IQR 77–90] before treatment and 87% [IQR 73–98] after treatment, p = 0.98).
We found heterogeneity in the response to levosimendan, with seven patients completely normalizing their Tmax (mean Tmax < 6 s after levosimendan treatment). In these patients, we found increases in rCBF and rCBV of about 10%, although not quite at the threshold of statistical significance (rCBV 88% [IQR 80–97] vs. 99% [IQR 95–109], p = 0.47, and rCBF 84% [IQR 81–89] vs. 95% [IQR 89–101], p = 0.03, for pre-CTP and post-CTP, respectively). The subgroup of six patients who did not develop a cerebral infarction attributable to the DCI also showed an approximately 10% increase in rCBF and rCBV, but this was not statistically significant (rCBV 85% [IQR 79–99] vs. 95% [IQR 88–112], p = 0.21, and rCBF 81% [IQR 76–87] vs. 89% [IQR 84–99], p = 0.4, for pre-CTP and post-CTP, respectively; see Fig. 1 for all perfusion analysis results, differentiated between patients with and without DCI).
We observed diameters of the most spasmed artery (at the time of the first CTP) of 2.1 mm (IQR 1.9–2.2) on admission, 1.1 mm (IQR 1.0–1.3) prior to levosimendan administration, and 1.6 mm (IQR 1.5–1.8) post levosimendan treatment. There was a statistically significant difference in these diameters between admission and the final scan (p < 0.001) and between the scan conducted before levosimendan infusion and afterward (p < 0.001) (Fig. 3).
Diameter of the most spasmed artery (at the time of the first perfusion scan) in each patient. Values given in millimeters for the computed tomography (CT) scan on admission, for the CT scan just before levosimendan infusion, and for the CT scan just after levosimendan infusion. Values for each individual are connected by a dotted line. The Wilcoxon signed-rank test was used to compare admission/after and before/after parameters. Stars were used to show statistically significant comparisons
Median PtiO2 values for patients with this sensor were 13 mm Hg (IQR 10–18) on the day before levosimendan administration, 11 mm Hg (IQR 7–19) on the day of the levosimendan administration, and 12 mm Hg (IQR 6–21) on the day after levosimendan administration. Individual trajectories of PtiO2 are shown in Fig. 1. PtiO2 sensors were removed, on average, after the 48th hour following levosimendan infusion, and no further data were collected.
No significant differences were observed in physiological variables that might influence cerebral hemodynamics at the time of either CTP scan (Table 2). In particular, mean arterial pressure values were 108 mm Hg (IQR 106–110) at the pre-CTP scan and 105 mm Hg (IQR 96–115) at the post-CTP scan (p = 0.51), norepinephrine dosages were 0.28 µg/kg/min (IQR 0.06–0.82) at the pre-CTP scan and 0.15 µg/kg/min (IQR 0–0.83) at the post-CTP scan (p = 0.63), and PaCO2 values were 42 mm Hg (IQR 34–45) at the pre-CTP scan and 39 mm Hg (IQR 38–42) at the post-CTP scan (p = 0.94).
GCS scores were 10 (IQR 8–14) the day before levosimendan infusion, 12 (IQR 9–13) the day of infusion, 11 (IQR 9–13) the day after infusion, 11 (IQR 8–14) the third day after infusion, and 10 (IQR 8–14) the seventh day after infusion.
Tolerance data analysis was performed on the total 16 included patients. Median ICP values were 5 cm H2O (IQR 3–9) on the day before infusion, 8 cm H2O (IQR 4–11) on the day of the infusion, and 6 cm H2O (IQR 3–9) on the day after infusion. Two patients had ICP elevations above 20 mm Hg after levosimendan infusion. The norepinephrine dosage was stable in all patients (0.28 µg/kg/min [IQR 0.13–0.58] the day before infusion, 0.26 µg/kg/min [IQR 0.04–0.57] the day of the infusion, and 0.18 µg/kg/min [IQR 0.01–0.56] the day after infusion). No episodes of cardiac arrhythmias or troponin elevations were observed during the 7 days following infusion. These data are detailed in Table 3.
Discussion
Levosimendan is beginning to be recognized as a potential new treatment for CVS that could help prevent ischemic damage in cases of aSAH with DCI. However, although in vitro studies in animal models of CVS are promising, there is a significant gap in in vivo physiological data that would allow us to understand the mechanisms underlying treatment efficacy. This gap represents a major obstacle both to the clinical use of this new drug and to the design of randomized clinical trials to evaluate its efficacy. Our study attempts to fill this gap by using an imaging-based analysis that gives us access to cerebral hemodynamic data using CTP before and after levosimendan infusion while CVS recurs and thus the pathophysiological mechanism is present again. Paralleling these data with the few available PtiO2 measurements allows further physiological analysis.
The initial CTP scan was conducted when CVS clinical symptoms recurred, and a second CTP scan was conducted roughly 2.5 days afterward. This subsequent CT scan remains pertinent for evaluating the impact of levosimendan on cerebral hemodynamics, despite being completed outside the drug infusion time frame. This relevance derives from the drug’s distinct pharmacokinetics, with an active metabolite with a long elimination half-life allowing a persistent effect for up to a week [6, 7]. This active metabolite, OR-1896, reaches its peak concentration between 48 and 72 h after treatment initiation. In our study, use of levosimendan was linked to a reduction of Tmax by 50% in the oligemic regions impacted by CVS, with a trend of preserving rCBF and rCBV in these areas. Median Tmax after levosimendan infusion was improved but remained at 7.1 s, which is still abnormal and prolonged, indicating persistent cerebral oligemia and an ongoing CVS.
The evolution of PtiO2 should be approached with care because of the limited number of patients studied and the lack of precise statistical analysis of its progression. Individual trajectories appear to cease declining on introduction of levosimendan and slightly increase around the 24th hour, consistent with the pharmacokinetics of levosimendan, which achieves peak efficacy at this time [6]. However, median values remain stable, with a widening IQR suggesting heterogeneity in patient progression. No clinical changes in GCS scores were observed before or after treatment.
The improvement observed on CTP is consistent with the maintenance of a stable median PtiO2. The deterioration in cerebral hemodynamics due to CVS prior to levosimendan infusion appears to have stopped, suggesting at least partial efficacy of this drug. The concomitant discontinuation of milrinone is a potential bias in our analysis but should impact the analysis in the direction of worsening cerebral hemodynamics: the positive effect of this drug on CVS is indeed well documented [18, 19].
All of our patients demonstrated a notable enlargement in the diameter of the most constricted artery between the angiograms prior to and following administration of levosimendan. Consistent with these results in humans, experiments in animal models of aSAH in rats [11, 12] have shown that incubation of the basilar artery with levosimendan restores the vasodilatory effects of the altered endothelin B1 receptor and counteracts the vasoconstriction induced by inflammatory cytokines such as serotonin and prostaglandin F2-α. Therefore, we hypothesize from our analysis that levosimendan allows cerebral vasodilation in areas affected by the pathophysiological process of CVS.
Elevation of ICP is a major concern with any vasodilator drug. One patient who had experienced multiple episodes of high ICP on milrinone developed refractory intracranial hypertension after levosimendan treatment, leading to death. Levosimendan should be used with caution in this situation and only with continuous ICP monitoring.
Cardiac and hemodynamic safety may also be a concern. There was no episode of cardiac arrhythmia or an increase in the troponin, plasmatic creatinine, or lactatemia level up to day 7. We noticed that the norepinephrine dosage decreased as soon as levosimendan was introduced, and our patients did not experience hypotensive episodes, which would have been particularly dangerous given the altered cerebral hemodynamics. The simultaneous discontinuation of milrinone, which had previously been infused at a dosage of 2 µg/kg/min, could explain this relative decrease in norepinephrine because levosimendan may have a less vasoplegic effect than milrinone. We also note that the cardiac index stays steady, as the inotropic impact of milrinone is presumably transferred by that of levosimendan.
A major limitation of our work could be the cofactors of CBF evolution, which could strongly bias the analysis. We have tried to list them all to assess their respective importance, and comparison of their respective values at the time of both CTP scans shows no significant differences. First, systemic hemodynamics and amine dosage were similar at the time of both CTP scans. All other systemic cofactors (such as arterial CO2) were also similar. Finally, the timeline shows that no CTP was performed before complete cessation of other vasospasm treatments, and clinical signs of deterioration attributed to CVS recurred in all patients, making a delayed effect of these treatments implausible.
Our study has several limitations. We recognize that we have retrospectively included only a limited number of highly selected patients and over a long period of time, which may raise concerns about the generalizability of the results. However, the clinical situations are similar, and the management of vasospasm is nearly equivalent in all these patients, both in terms of the methods used and the chronology. It should be noted that not all patients had the same number of endovascular angioplasties. The long inclusion period is explained by the rarity of these catastrophic events, which are jointly judged by the intensive care and neuroradiology teams and justify the use of this off-label treatment. We attempted to search our cohort for similar patients who had not received levosimendan to assess clinical outcomes, but only few were found, making any attempt at comparison risky.
The lack of a control group in our study makes it difficult to distinguish the impact of levosimendan on CVS from the natural progression of the disease. Our analysis compares perfusion parameters between the first scan performed approximately 10 days after the initial aSAH and a second scan taken 2.5 days later, nearly 13 days after the hemorrhage. This comparison was indeed conducted after previous treatments had been attempted. The most common natural progression of CVS involves deterioration, peaking around days 7–10, and subsequent resolution over the following days. The measure of improvement between the two CTP scans could then only be the natural history of the disease. However, literature reports an average duration of approximately 21 days for the CVS [20, 21]. In the case of our patients, the continued clinical deterioration, despite using all available therapies, suggests a severe form of CVS with a potentially extended duration, possibly nearing the maximum reported one. In addition, arterial diameter analysis revealed vessels that were still significantly narrower on the second CTP scan than on the admission CT scan, suggesting an ongoing pathophysiologic process and therefore unresolved arterial spasm. If levosimendan is ineffective, a large infarct of the cerebral parenchyma should thus be expected after 2 days without treatment because milrinone and endovascular treatments have been discontinued. However, many patients do not develop infarction at discharge.
The comparison of pre- and posttreatment CTP already provides unique information about the potential effect of levosimendan on brain perfusion, paving the way for future prospective studies based on its use.
Conclusions
In the treatment of CVS refractory to induced hypertension, intravenous milrinone, and endovascular angioplasty, levosimendan administration was associated with a moderate improvement in cerebral hemodynamics, as assessed by perfusion imaging analysis, halving Tmax and preserving rCBF and rCBV. However, cautions should be exercised when using this drug in patients with intracranial hypertension, as one of our patients died of this complication during levosimendan treatment. Further studies are needed to validate this presumption of efficacy and to examine the clinical outcomes associated with the use of this treatment, as the design of this study does not allow the effect of levosimendan to be quantified from that of the natural history of CVS.
References
Dodd WS, Laurent D, Dumont AS, et al. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. J Am Heart Assoc. 2021;10(15):e021845. https://doi.org/10.1161/JAHA.121.021845.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
Brami J, Chousterman B, Boulouis G, et al. Delayed cerebral infarction is systematically associated with a cerebral vasospasm of large intracranial arteries. Neurosurgery. 2020;86(2):E175–83. https://doi.org/10.1093/neuros/nyz340.
Li K, Barras CD, Chandra RV, et al. A review of the management of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;126:513–27. https://doi.org/10.1016/j.wneu.2019.03.083.
Papp Z, Agostoni P, Alvarez J, et al. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. Card Fail Rev. 2020;6:e19. https://doi.org/10.15420/cfr.2020.03.
Antila S, Sundberg S, Lehtonen LA. Clinical pharmacology of levosimendan. Clin Pharmacokinet. 2007;46(7):535–52. https://doi.org/10.2165/00003088-200746070-00001.
Nieminen MS, Fruhwald S, Heunks LM, et al. Levosimendan: current data, clinical use and future development. Heart Lung Vessel. 2013;5(4):227–45.
Nieminen MS, Akkila J, Hasenfuss G, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36(6):1903–12. https://doi.org/10.1016/s0735-1097(00)00961-x.
Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978;48(2):173–8. https://doi.org/10.3171/jns.1978.48.2.0173.
Cengiz SL, Erdi MF, Tosun M, et al. Beneficial effects of levosimendan on cerebral vasospasm induced by subarachnoid haemorrhage: an experimental study. Brain Inj. 2010;24(6):877–85. https://doi.org/10.3109/02699051003789260.
Konczalla J, Wanderer S, Mrosek J, et al. Levosimendan, a new therapeutic approach to prevent delayed cerebral vasospasm after subarachnoid hemorrhage? Acta Neurochir (Wien). 2016;158(11):2075–83. https://doi.org/10.1007/s00701-016-2939-5.
Wanderer S, Andereggen L, Mrosek J, et al. Levosimendan as a therapeutic strategy to prevent neuroinflammation after aneurysmal subarachnoid hemorrhage? J Neurointerv Surg. 2022;14(4):408–12. https://doi.org/10.1136/neurintsurg-2021-017504.
Onichimowski D, Nosek K, Goraj R, et al. Use of levosimendan in the treatment of cerebral vascular vasospasm: a case study. Drug Des Devel Ther. 2018;12:1777–83. https://doi.org/10.2147/DDDT.S158237.
Cottenceau V, Poutier B, Gariel F, et al. May levosimendan be safe and effective in refractory vasospasm despite adequate treatment with repeated angiography and milrinone infusion after subarachnoid haemorrhage? Anaesth Crit Care Pain Med. 2019;38(6):665–7. https://doi.org/10.1016/j.accpm.2019.07.004.
Trinh-Duc A, Labeyrie MA, Caillard A, Ben Hassen W, Mebazaa A, Chousterman BG. Effects of levosimendan on occurrence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a case-control study. Crit Care. 2021;25(1):396. https://doi.org/10.1186/s13054-021-03824-x.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW. 720 Smith SM FSL. Neuroimage. 2012;62:782–90. https://doi.org/10.1016/j.neuroimage.2011.09.015.
Avants B, Tustison NJ, Song G. Advanced normalization tools: V1.0. Insight J. 2009. https://doi.org/10.54294/uvnhin.
Lakhal K, Hivert A, Alexandre PL, et al. Intravenous milrinone for cerebral vasospasm in subarachnoid hemorrhage: the MILRISPASM controlled before-after study. Neurocrit Care. 2021;35(3):669–79. https://doi.org/10.1007/s12028-021-01331-z.
Shankar JJ, dos Santos MP, Deus-Silva L, Lum C. Angiographic evaluation of the effect of intra-arterial milrinone therapy in patients with vasospasm from aneurysmal subarachnoid hemorrhage. Neuroradiology. 2011;53(2):123–8. https://doi.org/10.1007/s00234-010-0720-7.
Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage part I: incidence and effects. J Clin Neurosci. 1994;1(1):19–26. https://doi.org/10.1016/0967-5868(94)90005-1.
Harders AG, Gilsbach JM. Time course of blood velocity changes related to vasospasm in the circle of Willis measured by transcranial Doppler ultrasound. J Neurosurg. 1987;66(5):718–28. https://doi.org/10.3171/jns.1987.66.5.0718.
Funding
This study did not have funding.
Author information
Authors and Affiliations
Contributions
GC and CR collected clinical, biological, and radiological data to build the analyzed database. GC, HDC, and MB conceived and designed this study. GM and TT designed the analysis of the radiological perfusion data and reviewed all the images. HF performed the offline analysis of the perfusion CT scans, realigning the individual perfusion scans and propagating the ROIs of the oligemic regions from one CT scan to the other. GC reprocessed with Olea software all perfusion data. GC wrote the first manuscript. HDC, MB, GM, and TT contributed heavily to the review. All authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no competing interests.
Ethical Approval/Informed Consent
This study complies with national and European guidelines on the protection of personal health data (RGPD), as our clinical data warehouse is quality certified in EHDEN. Approval for this study was obtained from the local ethics committee (Comité d’Ethique de la Recherche du Centre Hospitalier Universitaire de Bordeaux). The nonopposition of patients to the use of their data was systematically sought, as required by national regulations for retrospective observational studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cane, G., de Courson, H., Robert, C. et al. Cerebral Hemodynamics and Levosimendan Use in Patients with Cerebral Vasospasm and Subarachnoid Hemorrhage: An Observational Perfusion CT-Based Imaging Study. Neurocrit Care 41, 174–184 (2024). https://doi.org/10.1007/s12028-023-01928-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01928-6