Abstract
Background
The monitoring of intracranial pressure (ICP) and detection of increased ICP are crucial because such increases may cause secondary brain injury and a poor prognosis. Although numerous ultrasound parameters, including optic nerve sheath diameter (ONSD), width of the crural cistern (WCC), and the flow velocities of the central retinal artery and middle cerebral artery, can be measured in patients after hemicraniectomy, researchers have yet to determine which of these is better for evaluating ICP. This study aimed to analyze the correlation between ICP and ultrasound parameters and investigate the best noninvasive estimator of ICP.
Methods
This observational study enrolled 50 patients with brain injury after hemicraniectomy from January 2021 to December 2021. All patients underwent invasive ICP monitoring with microsensor, transcranial, and ocular ultrasound postoperatively. We measured the ONSD including the dura mater (ONSDI), the ONSD excluding the dura mater, the optic nerve diameter (OND), the eyeball transverse diameter (ETD), the WCC, and the flow velocities in the central retinal artery and middle cerebral artery. Then, we calculated the ONSDI-OND (the difference between ONSDI and OND) and ONSDI/ETD (the ratio of ONSDI to ETD). Patients were divided into a normal ICP group (n = 35) and an increased ICP group (≥ 20 mm Hg, n = 15) according to the ICP measurements. Correlations were then assessed between the values of the ultrasound parameters and ICP.
Results
The ONSDI, ONSDI-OND, and ONSDI/ETD were positively associated with ICP (r = 0.455, 0.482, 0.423 and p = 0.001, < 0.001, 0.002, respectively), whereas the WCC was negatively associated with ICP (r = − 0.586, p < 0.001). The WCC showed the highest predictive power for increased ICP (area under the receiver operating characteristic curve [AUC] = 0.904), whereas the ONSDI-OND and ONSDI also presented with acceptable predictive power among the ONSD-related parameters (AUC = 0.831, 0.803, respectively). The cutoff values for increased ICP prediction for ONSDI, ONSDI-OND, and WCC were 6.29, 3.03, and 3.68 mm, respectively. The AUC of the combination of ONSDI-OND and WCC was 0.952 (95% confidence interval 0.896–1.0, p < 0.001).
Conclusions
The ONSDI, ONSDI-OND, and WCC were correlated with ICP and had acceptable accuracy levels in estimating ICP in patients after hemicraniectomy. Furthermore, WCC showed a higher diagnostic value than ONSD-related parameters, and the combination of ONSDI-OND and WCC was a satisfactory predictor of increased ICP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Decompressive craniectomy has become a standard option for patients with medically refractory intracranial pressure (ICP) [1, 2]. After the surgical procedure, it is imperative to monitor ICP and detect increased ICP, which may lead to secondary brain injury and a poor prognosis [3, 4]. The gold standards for ICP monitoring are invasive and include ICP monitor probe insertion in the brain parenchyma or ventricles and lumbar puncture. Despite their high accuracy and sensitivity, these invasive ICP measurements require advanced technical skills and risk causing hemorrhage and infection [5, 6]. Thus, finding a readily available and noninvasive monitoring method is necessary.
Recently, the optic nerve sheath diameter (ONSD) has become widely used as a surrogate parameter for the noninvasive estimation of ICP [7]. Anatomically, some of the space surrounding the optic nerve is a direct extension of the subarachnoid space containing cerebrospinal fluid (CSF). Thus, the ONSD will expand as ICP increases because of the compensatory redistribution of CSF [8]. However, for patients after hemicraniectomy, the CSF hydrodynamics may be altered [9], and thus whether ONSD can still be used as a proxy of ICP in these patients is unclear.
According to previous research, a complete or partially obliterated basal cisterns is considered a sign of increased ICP [10]. Given that the volume of brain matter, CSF, and intracranial blood is constant, any increase in one is compensated by a decrease in the volume of the other two. Acute brain injury often leads to compensatory displacement of the CSF into the lumbar cistern. As the mass lesion expands and the ICP increases, the basal cisterns are compressed by the medial temporal lobe [11]. Additionally, compression or effacement of the basal cisterns can impede CSF flow and increase the ICP even further. Additionally, as arteries and veins of the brain pass through the basal cisterns, compression of the basal cisterns may lead to blood vessel compression, which may aggravate brain ischemia and ICP elevation [12]. Some studies have also found that the flow velocities of the middle cerebral artery (MCA) as measured by transcranial color Doppler (TCD) sonography could also be useful for evaluating ICP [13]. For patients treated with hemicraniectomy, TCD sonography had an inbuilt advantage in observing the intracranial structures, such as the basal cistern and MCA. Because the borderline of the crural cistern (one part of basal cistern) was clearest among all the basal cistern parts, we hypothesized that either the width of the crural cistern (WCC) or the flow velocities of the MCA could reflect the changes in ICP.
Thus, we performed this study on Chinese patients who required invasive ICP monitoring after decompression craniectomy. We measured ONSD-related parameters and flow velocities and the resistance index of the central retinal artery (CRA) using transorbital ultrasound. At the same time, we measured the WCC, flow velocities, and the pulsatility index (PI) of the MCA using transcranial ultrasound. Until now, there has been no standard method for measuring ONSD. Some studies have defined ONSD as subarachnoid space excluding the dura mater around the optic nerve (ONSDE), whereas others have defined it as including the hypoechogenic strip of dura mater (ONSDI) [14]. Therefore, we wanted to compare which method was better. Because the optic nerve diameter (OND) and eyeball transverse diameter (ETD) are constant parameters, the difference between the ONSDI and OND (ONSDI-OND) and the ratio of the ONSDI to the ETD (ONSDI/ETD) may more accurately reflect the changes in the subarachnoid space and reduce individual differences. Thus, we measured the ONSD-related parameters including the ONSDI, ONSDE, ONSDI-OND, and ONSDI/ETD. This study aimed to determine the relationship between these ultrasonographic parameters and the directly measured ICP and explore the best surrogate parameters for the noninvasive estimation of ICP.
Methods
Study Population
This observational study was conducted on patients with brain injury who had undergone invasive ICP monitoring after hemicraniectomy in the neurosurgical intensive care unit of our institute from January 2021 to December 2021. The protocol was approved by the research ethics boards of Tongji Hospital, affiliated with Huazhong University of Science and Technology. According to the ethics standards of the institutional committee, the caregivers of all enrolled patients signed informed consent forms. Patients older than 18 years requiring hemicraniectomy and invasive ICP monitoring with a diagnosis of traumatic or nontraumatic brain hemorrhage or hydrocephalus were considered for inclusion. We enrolled 65 patients according to these inclusion criteria. The exclusion criteria were as follows: (1) a history of ocular diseases (such as glaucoma, optic neuritis, and optic nerve tumors) or optic trauma (n = 3); (2) unfeasibility of ultrasonic testing due to agitation (n = 5); (3) inability to measure parameters due to insufficient image quality (n = 2); (3) age younger than 18 years (n = 1); and (4) widely fluctuating ICP measurements due to unstable respiration (n = 4). The final 50 included patients were divided into the normal ICP group (n = 35, ICP < 20 mm Hg) and increased ICP group (n = 15, ICP ≥ 20 mm Hg). We conducted all examinations within 48 h after the surgery. To avoid operator differences, an experienced doctor (XX) performed all the transorbital and transcranial ultrasound procedures and measured the images while blinded to the results of ICP monitoring. A recorder (YL) collected other relevant data, including age, sex, body mass index, ICP, blood pressure, etc.
Measurement of ICP
ICP was measured through a microtransducer (Codman Micro-Sensor transducer; Johnson & Johnson) positioned at the decompressed side. Before collecting the data, the operator ensured that the ICP monitor (Codman ICP Express cranial monitor; Johnson & Johnson) had been zeroed. The ICP value was obtained from a reading of the monitor while the measured patient maintained stable respiration and relaxation. ICP greater than or equal to 20 mm Hg was defined as elevated ICP.
Transorbital Ultrasound Measurement
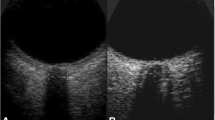
A Canon Aplio i800 (Japan) ultrasound device with an 18-MHz linear array transducer was used. Following the “as low as reasonably achievable” principle [15], the mechanical index and thermal index were adjusted to the requirements of orbital sonography. The patients were examined in a supine position with their heads elevated to 20–30°. The probe was gently placed on the closed left and right upper eyelids with coupling gel and adjusted to a suitable angle to show clear images with hyperechoic striped bands. Images in the transverse and sagittal sections of each eye were stored for measurement, and then we measured ONSD-related parameters at a depth of 3 mm behind the retina (Fig. 1). The ONSD-related parameters were the OND (measured as the distance inside the pia mater), ONSDI (measured as the distance between the external borders of the hypoechogenic dura mater), ONSDE (measured as the distance between the hyperechogenic subarachnoid diameter), ONSDI-OND, and ONSDI/ETD. All ONSD-related parameters were measured twice in the transverse and sagittal orientations for each eye, and then the final value was derived from the average of the eight values.
Image of optic nerve sheath with hyperechoic striped bands (left). The distance between the two yellow lines denotes ONSDI, the distance between the two blue line denotes ONSDE, and the distance between the two red line denotes OND (right). OND optic nerve diameter, ONSDE optic nerve sheath diameter excluding the dura mater, ONSDI optic nerve sheath diameter including the dura mater (Color figure online)
Transcranial Ultrasound Measurement
The transcranial ultrasound parameters were measured using a Canon Aplio i800 (Japan) ultrasound with a 2.5-MHz to 3.5-MHz convex array probe with the patient in a supine position. The probe was placed at the position of the skull defects, and the scan plane was consistent with that of the preauricular temporal acoustic bone window. Then, the probe was adjusted to an appropriate angle to show the circle of Willis in color Doppler flow imaging mode, and the butterfly-shaped mesencephalic brain stem surrounded by the hyperechogenic basal cistern was observed at the same time. Then, the color Doppler flow imaging mode was switched off, and the hyperechogenic basal cistern image at this angle was stored for measurement. The basal cistern consisted of the interpeduncular cistern, crural cistern, ambient cistern, and quadrigeminal cistern. Because the borderline of the crural cistern was the clearest among all parts of the basal cistern, we have chosen to measure the position of the crural cistern on ultrasonic imaging. We traced the outlines of the basal cistern and midbrain and then measured the WCC in the middle part of both sides twice each (Fig. 2). The WCC was defined as the average value of both sides.
Hyperechogenic basal cistern structure (left). The orange highlighted part stands for the interpeduncular cistern, the green highlighted part stands for the crural cistern, the blue highlighted part stands for the ambient cistern, and the yellow highlighted part stands for the quadrigeminal cistern. Measurement of the WCC on both sides (right). The red line stands for the outline of the midbrain, whereas the blue line stands for the borderline of the basal cistern. We have measured the middle part of the crural cistern. WCC, width of the crural cistern (Color figure online)
Measurement of Blood Flow Parameters
We examined the flow velocity of the CRA and MCA with pulsed-wave Doppler ultrasonography. We measured the peak systolic velocity (PSV), end-diastolic velocity (EDV), mean flow velocity (calculated as EDV + [PSV − EDV]/3), resistance index (calculated as PSV-EDV/PSV), and PI (calculated as PSV − EDV/mean flow velocity).
Statistical Analysis
The baseline characteristics and ultrasound parameters were presented as the means ± standard deviations (SDs) or medians with interquartile ranges. Student’s t-test and the Kolmogorov‒Smirnov test were used to compare data between patients in the increased and normal ICP groups. Spearman rank correlation was used to evaluate the association between the ultrasound parameters and ICP. The areas under the receiver operating characteristic curves (AUCs) were used to analyze predictors of increased ICP. The optimal cutoff value for each predictor was calculated. We used the distribution-based method to calculate the minimal detectable change (MDC). For a conventional confidence level of 95%, the MDC was defined as 1.96 × √2 × standard error of measurement. The standard error of measurement was calculated using the formula = SD × √(1 − ICC), where SD was the SD of two measurements and the intraclass correlation coefficient (ICC) was the test–retest reliability coefficient of intraobserver variability. All data were analyzed using IBM SPSS Version 21 (IBM Corp.), and p < 0.05 was considered statistically significant.
Results
A total of 50 patients were finally included in this research and divided into a normal ICP group (35 patients, ICP ranges from 2 to 19 mm Hg) and an increased ICP group (15 patients, ICP ranges from 20 to 42 mm Hg). Nineteen patients (38%) presented with cerebral contusion/laceration, 28 (56%) with acute intracerebral hematoma, and 3 (6%) with acute subdural hematoma. There were no significant differences between groups in age, sex, body mass index, mean artery pressure, or Glasgow Coma Scale score (all p > 0.05, Table 1).
Neither the OND nor ONSDE was different between the two groups (p > 0.05). The mean ONSDI, ONSDI-OND, and ONSDI/ETD of the increased ICP patients were 6.39 ± 0.41, 3.25 ± 0.33, and 0.27 ± 0.01 mm, respectively, and those of the normal ICP patients were 5.93 ± 0.41, 2.81 ± 0.30, and 0.26 ± 0.02 mm, respectively. The three ONSD-related parameters were significantly higher in the increased ICP group than in the normal ICP group (all p < 0.05). The mean WCC of the increased ICP group was 3.30 ± 0.65 mm, which was noticeably lower than that of the normal ICP group (4.28 ± 0.59 mm, p < 0.001). None of the Doppler ultrasound parameters related to the CRA and MCA were significantly different between the two groups (all p > 0.05, Table 1).
The intraclass correlation coefficient for intraobserver variability was 0.89 for OND, 0.9 for ONSDI, and 0.912 for WCC (all p < 0.001). The MDCs (with a 95% confidence interval) for OND, ONSDI and WCC were 0.25, 0.38, and 0.31 mm, respectively. The ONSDI, ONSDI-OND and ONSDI/ETD showed positive associations with ICP (r = 0.455, 0.482, and 0.423, respectively; all p < 0.05; Fig. 3). Particularly, for WCC, a negative relationship with ICP was observed (r = − 0.586, p < 0.001; Fig. 3). In addition, the Doppler ultrasonography parameters related to the CRA and MCA were not associated with ICP (all p > 0.05).
Scatterplots of the relationship between the ONSDI-OND and ICP (positively related, r = 0.482, left). Scatterplots of WCC vs. ICP (negatively related, r = − 0.586, right). ICP intracranial pressure, ONSDI-OND, the difference between the optic nerve sheath diameter including the dura mater and the optic nerve diameter, WCC width of the crural cistern
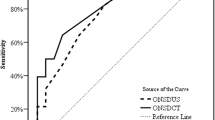
The receiver operating characteristic curve for the WCC showed that it remarkably differentiated increased ICP from normal ICP, with an AUC of 0.904 (sensitivity 85.7%, specificity 86.7%; Fig. 4, Table 2). Among the ONSD-related parameters, ONSDI and ONSDI-OND also presented great predictive power, with the latter (AUC = 0.831) slightly outperforming the former (AUC = 0.803). The combination of ONSDI-OND and WCC had the greatest discriminability between normal and elevated ICP, with an AUC of 0.952 (95% confidence interval 0.896–1.0, p < 0.001, Fig. 4, Table 2). The cutoff values for predicting increased ICP for ONSDI, ONSDI-OND, and WCC were 6.29, 3.03, and 3.68 mm, respectively.
ROC curve analysis of the predictive power of the WCC and combination (left). ROC curve analysis of the predictive power of the ONSD-related parameters including the ONSDI-OND, ONSDI, and ONSDI/ETD (right). AUC area under the receiver operating characteristic curve, ONSD optic nerve sheath diameter, ONSDI/ETD the ratio of the ONSD including the dura mater and the eyeball transverse diameter, ONSDI-OND the difference between the ONSD including the dura mater and the optic nerve diameter, ROC receiver operating characteristic, WCC width of the crural cistern
Discussion
Elevated ICP within 48 h following brain injury is known to be closely linked with a poor prognosis and higher mortality rates [16, 17]. Thus, monitoring ICP in patients with brain injury is crucial for recognizing elevated ICP and providing timely treatment. For patients after hemicraniectomy, because of skull defects, many ultrasound parameters, such as ONSD-related parameters, WCC, and Doppler ultrasonography parameters, can be obtained easily and accurately. Some ultrasound parameters have shown tremendous potential in detecting elevated ICP, but the most suitable parameter remains unknown. According to our research, ONSDI, ONSDI-OND, and WCC were well correlated with ICP, with WCC demonstrating the highest predictive power for increased ICP. Additionally, the combination of ONSDI-OND and WCC was the most precise predictor for this condition. None of the Doppler ultrasound parameters were correlated with ICP. To the best of our knowledge, this is the first study to show the correlation between WCC and invasive ICP measurements in patients after hemicraniectomy.
Many previous studies have shown that ultrasonographic ONSD is strongly correlated with ICP [18]. The study by Youm et al. [19] revealed that ONSDI, ONSDE, and ONSDI/ETD were all significantly associated with ICP, and the ONSDI/ETD showed the highest predictive power. In our study, ONSDE was not associated with ICP. In our observations, compared with the outer line of the dura mater, the outer line of the subarachnoid space was more likely to appear indistinct on ultrasonography. This might explain why ONSDE was not different between the two groups and not correlated with ICP in this study. Our results also showed that the ONSDI/ETD was weakly associated with ICP and was not the best predictor of elevated ICP. Youm et al. [19] measured the ETD from brain computed tomography (CT) with a clear eyeball outline, whereas we measured ETD through transorbital ultrasound. Because of the sidewall loss effect, our method might not have demonstrated the same accuracy as CT measurements, which may explain why our results were unlike those of the research done by Youm et al. [19]. We found that ONSDI-OND might provide a more reliable estimation of ICP than ONSDI, consistent with the study of Klinzing et al. [20]. When ICP increases, CSF passes through the optic foramen into the subarachnoid space surrounding the optic nerve. Because OND is a constant parameter, ONSDI-OND can more intuitively reflect the changes in the subarachnoid space and reduce individual differences.
Research by Wang et al. [21] found that ONSD was strongly related to the invasively measured ICP in patients with traumatic brain injury (TBI) after decompressive craniectomy. The ONSD of patients with elevated ICP was significantly higher than that of patients with normal ICP [21]. However, the study by Gao et al. [22] showed that ONSD measured on ultrasound was not significantly correlated with ICP after hemicraniectomy. Our study found that ONSDI had a weak correlation with ICP in patients after decompressive craniectomy. Several factors might explain the inconsistency in the findings. First, the altered CSF might affect the ONSD. Surgical craniectomy might reduce CSF secretion, affect CSF outflow, cause CSF redistribution, and prevent the ONSD from shrinking [23]. Second, because of the complex structural composition of the subarachnoid space, including trabeculae, septa, and stout pillars, the elastic fibers might not be able to rebound in time after a decrease in ICP, delaying reversal of the optic nerve sheath distention. Thus, when ICP drops to normal, the optic nerve sheath might still be wide [24, 25]. In this study, we also observed that postoperative ICP decreased to less than 15 mm Hg in some cases, but the ONSDI was still higher than 6.3 mm. Additionally, the standard range of ONSD is inconsistent [26]. Therefore, the ONSD might be inaccurate in some cases and prone to providing a false positive result. To solve this problem, we measured the WCC through transcranial ultrasound, hoping to improve ultrasound accuracy in detecting ICP.
Previous studies have shown that the basal cistern narrows or even disappears when ICP increases [27,28,29]. However, this change has rarely been quantified; the status of the basal cistern has only been divided into normal, compressed, or absent according to CT or magnetic resonance images in most previous studies, which is not sufficiently accurate for analysis. According to previous research, patients with severe TBI still had open basal cisterns on head CT despite an elevated ICP [30]. We supposed that the width of the basal cistern might be a more sensitive and accurate indicator for detecting ICP changes. Skull defects in patients who have undergone bone flap removal allow the possibility for ultrasonic observation of the basal cistern. This study conducted a correlation analysis between WCC and ICP to determine whether WCC could detect increased ICP. Our study provided evidence that WCC was significantly negatively correlated with ICP and, compared with ONSD-related parameters, had a higher diagnostic value in evaluating ICP elevation. Our results suggested that this crural cistern quantifying method, measuring WCC by transcranial ultrasound, might yield a lower false positive rate for increased ICP than ONSD-related parameters. The combination of ONSDI-OND and WCC achieved a satisfactory result as a predictor of increased ICP.
Although some researchers have demonstrated that the MCA PI is significantly correlated with ICP, more studies have revealed that TCD parameters were not significantly correlated with ICP, a recent systematic review demonstrated that the TCD-PI was insufficiently accurate in detecting elevated ICP, suggesting that it might not be helpful for evaluating this parameter [31, 32]. The study by Youm et al. [19] showed that the PSV or PI of the CRA was not related to the ICP in patients with TBI treated without hemicraniectomy. Gao et al. [33] also found that the MCA PI value was ineffective in quantifying the ICP in patients after decompressive craniectomy. In this study, we found no correlation between ICP and the Doppler ultrasonography parameters related to the CRA and MCA, consistent with earlier findings. There are many possible reasons why Doppler ultrasonography parameters cannot accurately evaluate the ICP, such as the diversity of brain injury mechanisms, cerebral resistance and compliance, physiological variations, and interobserver variability [19, 34]. Therefore, the use of Doppler ultrasonography parameters for the diagnosis of elevated ICP should be considered with caution.
Limitations
Our study also has several limitations. First, this study was limited by its relatively small size; studies with larger samples could include more patients with increased ICP and should be conducted in the future. Second, we measured the WCC in the middle point and defined the WCC as the average value of both sides. With this method, it may have side-to-side variability and variability of width on the same side, which might affect the accuracy of the results. In the future, we may measure several points around the middle position of both sides to obtain the average value or trace the area of the crural cistern region to reduce the variability. Third, the exact trajectory of how the sheath diameter or individual components at the root of the subarachnoid space change over time is unknown, so we should pay attention to the possibility of delaying reversal of the optic nerve sheath distention, which may lead to false positive results. Fourth, the ultrasound parameters could only predict the probability of a high or normal ICP and could not provide the exact value of the ICP, so they cannot replace the direct and invasive ICP measurements.
Conclusions
Reasonable ultrasonic parameters for evaluating ICP in patients after hemicraniectomy included the ONSDI, ONSDI-OND, and WCC. Furthermore, WCC showed a higher diagnostic value than ONSD-related parameters, and the combination of ONSDI-OND and WCC was a satisfactory predictor of increased ICP. This ICP evaluation method has good application prospects for severe neurosurgical patients because of the ability to perform the measurements at bedside examination and their noninvasive nature. More studies should be conducted in the future to examine the validity of this method.
Abbreviations
- ICP:
-
Intracranial pressure
- ONSD:
-
Optic nerve sheath diameter
- WCC:
-
Width of crural cistern
- CRA:
-
Central retinal artery
- MCA:
-
Middle cerebral artery
- ONSDI:
-
ONSD including the dura mater
- ONSDE:
-
ONSD excluding the dura mater
- OND:
-
Optic nerve diameter
- ETD:
-
Eyeball transverse diameter
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- PSV:
-
Peak systolic velocity
- EDV:
-
End-diastolic velocity
- MFV:
-
Mean flow velocity
- RI:
-
Resistance index
- PI:
-
Pulsatility index
- MDC:
-
Minimal detectable changes
- ICC:
-
Intraclass correlation coefficient
References
Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Datab Syst Rev. 2019;12(12):003983.
Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neursurg. 2006;104(4):469–79.
Vik A, Nag T, Fredriksli OA, et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neursurg. 2008;109(4):678–84.
Hawryluk GWJ, Rubiano AM, Totten AM, et al. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020;87(3):427–34.
Walek KW, Leary OP, Sastry R, et al. Risk factors and outcomes associated with external ventricular drain infections. Infect Control Hosp Epidemiol. 2022;43(12):1859–66.
Dasic D, Hanna SJ, Bojanic S, Kerr RSC. External ventricular drain infection: the effect of a strict protocol on infection rates and a review of the literature. Br J Neurosurg. 2006;20(5):296–300.
Robba C, Santori G, Czosnyka M, et al. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2018;44(8):1284–94.
Jeub M, Schlapakow E, Ratz M, et al. Sonographic assessment of the optic nerve and the central retinal artery in idiopathic intracranial hypertension. J Clin Neurosci. 2020;72:292–7.
Czosnyka M. Post-traumatic hydrocephalus: influence of craniectomy on the CSF circulation. J Neurol Neurosurg Psychiatry. 2000;68(2):246–8.
Jacobs B, Beems T, van der Vliet TM, Borm GF, Vos PE. The status of the fourth ventricle and ambient cisterns predict outcome in moderate and severe traumatic brain injury. J Neurotrauma. 2010;27(2):331–40.
Mancall EL, Brock DG, Gray H. Gray’s clinical neuroanatomy: the anatomic basis WAfor clinical neuroscience. Philadelphia, PA: Elsevier; 2011.
Hong JH, Jeon I, Seo Y, Kim SH, Yu D. Radiographic predictors of clinical outcome in traumatic brain injury after decompressive craniectomy. Acta Neurochir (Wien). 2021;163(5):1371–81.
Wang Y, Duan Y-Y, Zhou H-Y, et al. Middle cerebral arterial flow changes on transcranial color and spectral Doppler sonography in patients with increased intracranial pressure. J Ultrasound Med. 2014;33(12):2131–6.
Stevens RRF, Gommer ED, Aries MJH, et al. Optic nerve sheath diameter assessment by neurosonology: a review of methodologic discrepancies. J Neuroimaging. 2021;31(5):814–25.
Toms DA. The mechanical index, ultrasound practices, and the ALARA principle. J Ultrasound Med. 2006;25(4):560–1.
Badri S, Chen J, Barber J, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38(11):1800–9.
Calviello L, Donnelly J, Cardim D, et al. Compensatory-reserve-weighted intracranial pressure and its association with outcome after traumatic brain injury. Neurocrit Care. 2018;28(2):212–20.
Lochner P, Czosnyka M, Naldi A, et al. Optic nerve sheath diameter: present and future perspectives for neurologists and critical care physicians. Neurol Sci. 2019;40(12):2447–57.
Youm JY, Lee JH, Park HS. Comparison of transorbital ultrasound measurements to predict intracranial pressure in brain-injured patients requiring external ventricular drainage. J Neurosurg. 2022;136(1):257–63.
Klinzing S, Hilty MP, Bechtel-Grosch U, Schuepbach RA, Bühler P, Brandi G. Dynamic optic nerve sheath diameter changes upon moderate hyperventilation in patients with traumatic brain injury. J Crit Care. 2020;56:229–35.
Wang J, Li K, Li H, et al. Ultrasonographic optic nerve sheath diameter correlation with ICP and accuracy as a tool for noninvasive surrogate ICP measurement in patients with decompressive craniotomy. J Neurosurg. 2020;133(2):514–20.
Gao Y, Li Q, Wu C, Liu S, Zhang M. Diagnostic and prognostic value of the optic nerve sheath diameter with respect to the intracranial pressure and neurological outcome of patients following hemicraniectomy. BMC Neurol. 2018;18(1):199.
Shapiro K, Fried A, Takei F, Kohn I. Effect of the skull and dura on neural axis pressure-volume relationships and CSF hydrodynamics. J Neurosurg. 1985;63(1):76–81.
Hansen H-C, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalmol. 2011;89(6):e528–32.
Rajajee V, Fletcher J, Rochlen L, Jacobs T. Comparison of accuracy of optic nerve ultrasound for the detection of intracranial hypertension in the setting of acutely fluctuating vs stable intracranial pressure: post-hoc analysis of data from a prospective, blinded single center study. Crit Care. 2012;16(3):R79.
Chen H, Ding G-S, Zhao Y-C, Yu R-G, Zhou J-X. Ultrasound measurement of optic nerve diameter and optic nerve sheath diameter in healthy Chinese adults. BMC Neurol. 2015;15(1):106.
Maas AIR, Steyerberg EW, Butcher I, et al. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):303–14.
Miller MT, Pasquale M, Kurek S, et al. Initial head computed tomographic scan characteristics have a linear relationship with initial intracranial pressure after trauma. J Trauma. 2004;56(5):967–73.
Avanali R, Bhadran B, Panchal S, et al. Formulation of a three-tier cisternal grade as a predictor of in-hospital outcome from a prospective study of patients with traumatic intracranial hematoma. World Neurosurg. 2017;104:848–55.
Kouvarellis AJ, Rohlwink UK, Sood V, Van Breda D, Gowen MJ, Figaji AA. The relationship between basal cisterns on CT and time-linked intracranial pressure in paediatric head injury. Childs Nerv Syst. 2011;27(7):1139–44.
Zhang X, Medow JE, Iskandar BJ, et al. Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol Meas. 2017;38(8):R143–82.
Fernando SM, Tran A, Cheng W, et al. Diagnosis of elevated intracranial pressure in critically ill adults: systematic review and meta-analysis. BMJ. 2019;366:l4225.
Gao Y, Li Q, Wu C, Liu S, Zhang M. Use of a Doppler-based pulsatility index to evaluate cerebral hemodynamics in neurocritical patients after hemicraniectomy. J Ultrasound Med. 2019;38(9):2469–75.
Ahmad M, Legrand M, Lukaszewicz A-C, Charlier P, Mateo J, Payen D. Transcranial Doppler monitoring may be misleading in prediction of elevated ICP in brain-injured patients. Intensive Care Med. 2013;39(6):1150–1.
Funding
There was no funding associated with this study.
Author information
Authors and Affiliations
Contributions
XX, KZ, and AT conceived and designed the study. YL and JL performed the experiments and analyzed the data. XX wrote the article. XX, RX, and AT reviewed and edited the manuscript. All authors discussed the results and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest to this work.
Ethical Approval
This study was approved by the research ethics boards of Tongji Hospital Affiliated with Huazhong University of Science and Technology. According to the ethics standards of the institutional committee, the caregivers of all enrolled patients signed informed consent forms.
Informed Consent
We confirmed that this manuscript complies with all instructions to authors. All authors discussed the results and approved the manuscript. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously and not under consideration for publication elsewhere, in whole or in part.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Lu, Y., Liu, J. et al. Diagnostic Value of the Combination of Ultrasonographic Optic Nerve Sheath Diameter and Width of Crural Cistern with Respect to the Intracranial Pressure in Patients Treated with Decompressive Craniotomy. Neurocrit Care 39, 436–444 (2023). https://doi.org/10.1007/s12028-023-01711-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01711-7