Abstract
Background
Brain oxygenation improvement is a sought-after goal in neurocritical care patients. Previously, we have shown that cerebral blood flow improvement by cardiac-gated intracranial pressure (ICP) modulation using an intracranial pulsating balloon is feasible in a swine model. We sought to explore specific ICP modulation protocols to assess the feasibility of influencing brain oxygenation.
Methods
A previously presented electrocardiogram (ECG)-gated intracranial balloon pump in which volume, timing, and duty cycle of balloon inflation could be altered was used. Different protocols were tested in a swine model of normal and elevated ICP attained by intracranial fluid infusion with continuous monitoring of physiological parameters, and brain tissue oxygen tension (PbtO2) was measured at baseline and after device activation.
Results
We studied five swine, subjected to two main protocols differing in their phase relative to the cardiac cycle. In reduced brain perfusion status (ICP > 20 mm Hg, PbtO2 < 15 mm Hg), the late-diastolic-early-systolic (Inflation/deflation) protocol showed consistent elevation in PbtO2 (+ 9%, p < 0.01), coupled with ICP reduction (− 12%, p < 0.01), whereas the early-systolic-late-diastolic (inflation/deflation) protocol resulted in PbtO2 reduction (− 4%, p < 0.01), coupled with ICP increase (+ 5% above baseline, p < 0.01). No significant changes in brain oxygenation or ICP were observed at normal perfusion status (ICP < 20 mm Hg, PbtO2 > 15 mm Hg).
Conclusions
Intracranial cardiac-gated balloon pump activation can influence cerebral oxygenation and raise PbtO2 above threshold values. This study supports the concept of late-diastolic pressure rise, coupled with early-systolic pressure drop, as a potential effector of flow augmentation leading to improve brain tissue oxygenation. Further studies are warranted to assess the translational potential of using an intracranial cardiac-gated balloon pump device to improve brain tissue oxygenation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral oxygenation improvement is an important goal in the care of patients with severe brain injury. Numerous studies have demonstrated a potential importance of maintaining brain tissue oxygen tension (PbtO2) above threshold levels and that doing so may influence outcome [1,2,3], leading to current ongoing trails [4] to further establish brain perfusion management. The recent algorithm of the Seattle International Brain Injury Consensus Conference (SIBICC) puts forth specific treatment recommendations regarding the treatment of low PbtO2 [5]. These treatment recommendations are generally based on well-accepted interventions that increase systemic oxygenation, lower intracranial pressure (ICP), or improve blood flow to the brain. The intracranial cardiac-gated balloon pump (ICBP) [6] is a novel experimental device composed of a balloon connected to a cardiac-gated pump, which is programmed to both inflate and deflate the balloon rapidly in specific time points within the cardiac cycle. When implanted intracranially and using specific defined protocols, ICP can be modulated because balloon volume directly changes the pressure–volume relationship within the cranium. In a previous study, we demonstrated that specific activation protocols that modulate ICP can improve cerebral blood flow (CBF) and reduce ICP in a swine model [6]. Although CBF is an important physiological parameter, it is less often measured in clinical practice in patients with brain injury. PbtO2 is more commonly measured in patients with severe brain injury in the intensive care unit setting. In this study, we sought to explore whether the intracranial pulsating balloon could be used to influence brain tissue oxygenation at baseline and under conditions of raised ICP.
Methods
Intracranial cardiac-gated balloon pump (ICBP)
The experimental system was designed and constructed in our laboratory and is composed of a linear motor that drives a syringe pump connected to a balloon via a high-pressure catheter (Fig. 1). The pump, FRLS-AC servo motor (HIWIN Technologies Corp., Huntley, IL), is controlled by a computer. The computer receives signals from a data acquisition system (USB-6002 16-Bit; National Instruments, Austin, TX) using a digital control card designed to acquire data from several sensors: (1) two Millar pressure probes (3.5F Millar Mikro-Tip pressure catheter; Houston, TX), one for measurement of ICP and the other for arterial carotid pressure monitoring; (2) an electrocardiogram (ECG) monitor (Philips, Amsterdam, Netherlands); and (3) a tissue oxygenation monitoring device (Licox probe and monitor; Integra Lifesciences Inc., Plainsboro, NJ).
The software synchronizes the forward and backward motion of the piston to the cardiac cycle on a beat-to-beat basis at specified timings. The motor was preset to inflate and deflate the balloon at a specific delay time relative to the ECG R-wave, which was detected by a real-time R-wave detection algorithm. The prototype balloons were made from a flexible, elastic, biocompatible polyurethane modified from commercial parenchymal ICP probes (Spiegelberg GmbH & Co. KG, Hamburg, Germany), allowing for small (up to 2.0 mL) nontraumatic inflation and deflation, designed for intracranial insertion into the ventricular space via burr hole. The balloons were connected to a rigid catheter that was attached to an all-glass 10-mL syringe with luer-lock tip (Poulten & Graf GmbH, Wertheim, Germany) driven by the motor. The system was described in detail in previous works [6, 7].
The system parameters that were altered between different intervention protocols were inflation time delay from the R-wave (at the millisecond level), deflation time delay from the R-wave, piston displacement velocity (determining the profile of volume change with a speed of up to 4,000 cm/second), and balloon volume. By changing these parameters, different profiles of balloon inflation and deflation relative to the cardiac cycle, as well as the percentage of the cardiac cycle through which the balloon is deflated or inflated, could be manipulated, and theoretically a multitude of ICP waveforms could be generated. The acquisition system enabled us to continuously measure the brain tissue oxygenation level, carotid arterial pressure, and ICP (at 16-bit resolution and a sampling rate of 200 Hz) synchronous to any volumetric change induced by the pump.
Preparation of the Animal
We studied five female Yorkshire swine weighing from 41 to 50 kg in the large animal sterile surgical facility available in our institution. All swine were obtained from licensed suppliers (Lahav, Israel), quarantined for a minimum of 7 days before study entry, and maintained in an accredited animal care facility under the guidelines of the Guide for the Care and Use of Laboratory Animals following an institutional review board approval.
Anesthesia was induced using sodium pentothal (20 mg/kg, intravenously), and the animals were then intubated and placed on a ventilator with isoflurane gas (1.0–1.5%) used to maintain anesthesia throughout the study. Animals were placed in a supine position immediately after intubation, and a carotid 5F arterial line (micropuncture access kit; Cook Medical, Bloomington, IN) was inserted under ultrasound guidance. ECG, carotid arterial blood pressure, respiration, temperature, arterial blood gases, and pH were monitored. All swine had a Foley catheter inserted. Blood gases and pH were maintained within normal ranges (pH 7.4, partial pressure of carbon dioxide 35–45 mm Hg, partial pressure of oxygen 95–100 mm Hg) mechanically by controlling the ventilation volume and frequency, and body temperature was maintained with a thermal blanket (37 ± 1 °C) and intravenous infusion of a saline solution of Ringer’s lactate at a rate of 25 mL/hour throughout unless otherwise dictated by protocol as described below.
The animals were then carefully rolled into a prone position. Transverse and sagittal incisions were made posterior to the eye and at the midline, and the skin, fascia, and temporalis muscles were retracted to expose the skull. Three separate burr holes were drilled using an automatic drill (Summex; Stryker, Portage, MI) 1 cm right off the midline and 1 cm posterior to the coronal suture, 1 cm left off the midline and 1 cm posterior to the coronal suture, and 1 cm right off the midline and 1 cm anterior to the coronal suture. The balloon catheter, Licox probe, and ICP sensors were then implanted under sterile conditions (Fig. 1). The ICP probe was inserted into the parenchyma (at a depth of about 1 cm). The balloon was inserted into the ventricle under ultrasound guidance. The burr holes were closed with bone wax (Bonewax; Aesculap, Center Valley, PA).
Experimental Protocol
To create a gradual elevation in ICP, we used a slow intraventricular NaCl 0.9% infusion, mimicking hydrocephalus [8]. An additional burr hole was drilled contralateral to the device-instrumented hemisphere, 1.5 cm left off the midline and 1.5 cm anterior to the coronal suture. A 16-gauge continuous-drainage tube (lumbar external drainage catheter; Codman Neuro, Raynham, MA) was inserted into the ventricle until clear cerebrospinal fluid (CSF) was seen in the lumen. This tube was connected to an automatic syringe programmable pump (KDS 510 Dual Syringe Pump System; KS Scientific, Holliston, MA) that infused saline at a slow fixed rate (between 0.02 and 0.05 mL/minute, depending on the tested protocol and stage of experiment), gradually raising ICP. All probes were kept in position throughout the experiment. PbtO2 was measured continuously by assessing perfusion in a volume < 0.3 mm3 at a sampling frequency of 200 Hz for brain tissue oxygenation (partial oxygen pressure measured as mm Hg).
ICP was raised in a graded fashion, as described above, to test the device’s function and efficacy at both normal and pathological PbtO2 and ICP levels [9,10,11].
Importantly, serial arterial blood gas, pH, and hemoglobin levels were measured and animal’s temperature tests were performed every 5 to 10 min to ensure all parameters remained normal and stable throughout the PbtO2 monitoring period to ensure no systemic changes would alter the PbtO2 level.
The balloon was inserted in a partially inflated form and was left inflated for a few minutes until a new equilibrium was reached and ICP returned to its preinsertion level. The device was then tested, first at baseline normal ICP and PbtO2 levels and later, sequentially, with increases in ICP and at varying levels of PbtO2.
Activation protocols were divided to “in-phase” and “out of phase” according to previous knowledge gained by experiments using a CBF probe [6].
Prior to device activation, recorded ICP and PbtO2 levels were verified as stable. Specifically, after balloon introduction with an initial minimal volume, as well as before every activation protocol in which the balloon volume was changed, a 10-min stabilization period was allowed. Data analyzed in every measurement included 10 min preactivation, followed by 10 min of device activation and 10 more minutes post activation. ICP and PbtO2 measurements were averaged over the selected time periods. The postactivation period was compared with the preactivation period to assess whether parameters returned to baseline following activation. At every activation, ICP and PbtO2 changes before and after device activation relative to baseline were recorded and percentage change was calculated.
At the end of the experimental protocol, animals were killed by injection of potassium chloride.
Methods of Device Activation
Based on our past experience, we employed two methods of activation to assess the efficacy of ICP modulation in oxygenation augmentation or reduction. For both methods, a volume of 2 mL was set for balloon deflation and inflation. Piston speed was set to 2,000 cm/second. Timing was set according to the protocol used.
The first method (protocol A), described earlier, consisted of balloon inflation being performed during diastole to elicit a temporary pressure rise, which in theory might augment the exit of venous blood as well as CSF from the cranium. We speculated that this pressure rise was not high enough to affect the arterial vasculature. The balloon was then deflated just prior to systole in an attempt to decrease resistance to incoming arterial blood and potentially allow greater arterial inflow to enter the cranium (Fig. 2a). This method was termed “late diastole-early systole” (LaDES). For LaDES, inflation at an average of 650 milliseconds delay from R-wave identified by ECG (approximately as 80% of the cardiac cycle went by), with deflation occurring after 10% of the next cardiac cycle. Because this was shown to improve CBF [6], the current target was to examine how significant the effect of this would be on brain tissue oxygenation, specifically at normal and reduced PbtO2 levels.
a, Impact of the late-diastolic-early-systolic activation protocol on intracranial pressure (ICP) waveform and brain tissue oxygen tension (PbtO2) level. Balloon volume (Bal. Vol.) (inflation/deflation) is left inflated for a longer duration relative to the cardiac cycle. b, Impact of the early-systolic-late-diastolic activation protocol on ICP waveform and PbtO2 level. Arterial blood pressure (ABP) remained constant and the balloon activation was relative to the electrocardiogram (ECG) R-wave detection
The second method (protocol B), in which balloon inflation was performed in systole and deflation was performed in early diastole, was termed “early systole-late diastole” (ESLaD). This was tested to further elucidate the mechanism behind flow augmentation as a methodological step and to assess the importance of arterial inflow disruption by ICP increase (Fig. 2b). For ESLaD, inflation at an average of 100 milliseconds delay from R-wave identified by ECG (approximately as 15% of the cardiac cycle went by), with deflation occurring when approximately reaching 80% of the cardiac cycle, in late diastole.
In all swine that were studied after protocols A and B were defined, each swine underwent sequential activation of protocol LaDES, followed by a time period in which all parameters returned to baseline (prior to LaDES) before the ESLaD protocol was tested. This was repeated three times at each ICP level studied (i.e., n = 15 as each data set was analyzed independently).
Data Processing
Data were analyzed using an automated algorithm using MATLAB (R2014b; MathWorks, Inc., Natick, MA). For waveform analysis, ICP, arterial carotid pressure, and thermal blood flow signals were high pass filtered (second-order High Pass (HP) Butterworth filter with cut-off frequency of Fc = 30 Hz) to eliminate power line noise effects. Single ICP and CBF values, as well as mean values, were determined from unfiltered data.
All estimations of ICP and PbtO2 parameters were performed on nonaveraged data. Comparisons were made between data segments immediately before and after pump activation and deactivation, and the 10-min segments of continuous activity were analyzed for trends. Segments in which the balloon cycle delay or missed beats occurred in more than 10% of the points due to R-wave detection failure, or heart rate differed more than 10% from the median for more than 25% of the cardiac cycles were excluded.
To assess significant changes in PbtO2 and ICP levels, physiological parameters during the device activation period were compared with physiological parameters preactivation as well as post activation. An analysis of variance test was used to assess whether the changes were significant. Cases in which partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (Paco2), mean arterial pressure (MAP), hemoglobin (Hb), or pH levels changed by more than 5% compared with preactivation levels, analyzed over a 10-min period, were excluded.
Results
Baseline Systemic and Cerebral Physiological Parameters
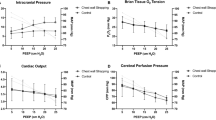
ICP was increased in a graded fashion over the experimental protocol (Table 1). PbtO2 levels were monitored and showed gradual reduction as ICP rose beyond a level of 20 mm Hg. No significant changes were observed either in ECG signal, heart rate, arterial blood pressure, PaO2, Paco2, and pH levels because all were kept within the normal range as ICP and PbtO2 varied.
Effect on ICP Waveform
In accordance with previous studies, ICP waveform changes were evident immediately with device activation and reverted to their baseline with termination of ICBP activation. In all animals, balloon-induced ICP changes had several characteristic features. Characteristic ICP patterns were elicited for LaDES and ESLaD (Fig. 2).
Effect on PbtO2
We identified two main baseline states in which device effect was tested: normal ICP combined with normal PbtO2 levels (normal physiology) and increased ICP combined with reduced PbtO2 levels (reduced brain perfusion). Data were analyzed according to the combination of PbtO2 and ICP levels, whereas other parameters remained stable.
The LaDES activation protocol led, on average, to a significant improvement in the PbtO2 level (Table 2) of 9.18 ± 9.97% (p < 0.01) if the preactivation baseline PbtO2 level was less than 15 mm Hg and ICP was beyond 20 mm Hg (reduced brain perfusion status). Importantly, there was a lasting effect after pump termination before an eventual return to baseline after approximately 10 min (Fig. 3a).
Data over minutes from a single swine. The top panel shows the measured arterial blood pressure (ABP) waveform in the carotid artery. The middle panel shows the measured intracranial pressure (ICP), with the first blue arrow denoting initial balloon activation and the second blue arrow denoting balloon deactivation. The bottom panel shows the measured brain tissue oxygen tension (PbtO2), with the first blue arrow denoting initial balloon activation and the second blue arrow denoting balloon deactivation. a, Impact of the late-diastolic-early-systolic activation protocol over minutes in a low perfusion state showing a rise in PbtO2. b, Impact of the early-systolic-late-diastolic activation protocol over minutes in a low perfusion state showing a reduction in PbtO2
During the activation period, as well as after balloon activation, ICP dropped significantly, on average, by − 12.31 ± 6.25% (p < 0.01) compared with its baseline level (Table 2).
In contrast, When the preactivation PbtO2 baseline level was greater than 15 mm Hg with ICP levels less than 20 mm Hg (normal physiology status), no significant PbtO2 or ICP level changes were observed.
In comparison, the ESLaD activation protocol under baseline reduced brain perfusion status demonstrated a significant reduction in PbtO2 during device activation (Fig. 3b) across baseline PbtO2 levels, with a return to baseline following deactivation (Table 3). ICP levels increased concomitantly with activation, returning to baseline afterward. Activating the device in normal physiology status did not yield any significant changes in ICP or PbtO2 levels.
Effect on Systemic Physiological Parameters
Balloon activation did not lead to significant changes in the ECG signal, heart rate, arterial blood pressure (Tables 2 and 3), or pH or Hb levels.
Discussion
Previous studies have explored the effect of ICP modulation on ICP morphology, ICP dose, and CBF [6, 12, 13]. In a swine model with elevated ICP, cardiac-gated ICP modulation with specific activation protocols was shown to be effective in reducing ICP dose and improving CBF. In the current study, we demonstrate that ICP modulation can also improve PbtO2. This is important because PbtO2 is a parameter that is often measured in patients with severe brain injury, and low PbtO2 is known to be associated with poor outcome in patients with traumatic brain injury. The recently published algorithm of the SIBICC recommends treatment interventions based on maintaining ICP below threshold values and keeping PbtO2 above threshold values [3, 4, 14, 15], while acknowledging an equipoise as ongoing trials attempt to resolve this and give a clearer indication of the role of PbtO2 as a clinical parameter. Our study attempted to take an approach concordant with both the SIBICC algorithm and the Brain Oxygen Optimization in Severe TBI (BOOST) studies of brain tissue oxygenation guided therapy. Both the SIBICC algorithm and the BOOST study involve a paradigm in which specific intervention strategies are guided not only by the PbtO2 value as a unitary parameter but also by the state of both ICP and PbtO2 at a given time. In this regard, we studied the effect of ICBP activation both at baseline when both ICP and PbtO2 were normal and under conditions of elevated ICP and low PbtO2, accounting for two of the four clinical scenarios described in the SIBICC algorithm and BOOST study. Interestingly, we found that under conditions of normal ICP and normal PbtO2, ICBP activation did not lead to a significant rise in PbtO2.
This supports the concept of oxygen tension within the brain tissue representing excess oxygen, which, in nonischemic conditions, is not correlated with on CBF [4, 16]. In contrast, when ICP was elevated and PbtO2 was low, ICBP activation with the LaDES protocol significantly improved PbtO2 and reduced ICP. This reinforces the conception that specific treatment interventions may only prove efficacious under particular physiological and clinical conditions. Further studies of ICBP and specific activation protocols will need to account for the conditions under which device activation is performed, with the goal of identifying defined states in which its use may be beneficial. Novel therapeutic approaches that can help improve brain tissue oxygenation and reduce ICP when both are abnormal have the potential to offer benefit if they can be translated into clinical care.
In this study, we also aimed to reinforce the principles that underlie the mechanisms at work in ICBP activation by taking a counter-efficacious approach. That is, we developed an activation protocol for the ICBP that would oppose optimal cerebrovascular flow, the ESLaD protocol. Our results demonstrate that employing the counter-efficacious ESLaD protocol does indeed impair brain tissue oxygenation and increased ICP dose. Because other model parameters, such as fraction of inspired oxygen (FiO2), PaO2, Hb, and MAP, were kept constant in the experimental protocol, it is reasonable to conclude that the primary determinant of a decreasing PbtO2 during the ESLaD protocol is modulated by its detrimental effect on CBF, which creates a transmural pressure rise interfering with systolic inflow. Depressed levels of PbtO2, although minimal, were also observed to some extent in the postactivation period, possibly suggesting a longer stabilization time required because it took 15 min for oxygenation levels to return to baseline. The experimental evidence that different ICBP activation protocols have distinct effects on cerebral oxygenation lend support to the postulate that the observed effects reflect a significant influence on intracranial physiology. In addition, these data also emphasize the importance of clearly defining ICBP activation protocols that improve cerebral physiological parameters. In any possible future translational studies of an ICBP-like therapeutic device, “getting it right” regarding proper activation protocols will be critical to potential efficacy and will be critical to ensure patient safety. Lastly, there are clinical scenarios in which a moderate reduction in CBF may be beneficial, such as in young patients following traumatic brain injury in whom hyperemia may cause elevated ICP. In such clinical scenarios, a moderate reduction in CBF without crossing thresholds for tissue hypoxia may aid in meeting treatment goals.
The close relationship between CBF and PbtO2 has been demonstrated previously [16]. As such, it is perhaps not surprising that ICBP activation improves brain tissue oxygenation, because a previous study with the device demonstrated improved CBF [6]. Our current study supports these previous findings and may add clinical significance by allowing utility of ICBP activation to be gauged by an oft-use measurement parameter. This may facilitate translational studies in patients with severe brain injury in whom poor brain tissue oxygenation occurs.
Our study has several limitations. First, like all studies using a parenchymal probe of brain tissue oxygenation, the data only reflect local physiological conditions and not hemispheric measurements. Second, we assessed ICBP activation protocols only under baseline conditions and with high ICP and low PbtO2. We did not simulate conditions of normal ICP with low PbtO2 or those of high ICP and normal PbtO2. Further studies will be needed to establish whether specific activation protocols are efficacious under these conditions. In addition, a better understanding of the dose response to ICBP activation will need to be assessed in future investigations, and ideally the LaDES and ESLaD protocols should be tested in separate animals because there may be a potential effect of one protocol testing to interact with and affect the other protocol testing outcome on the same pig. This was mitigated to some extent by allowing stabilization time and return to baseline between activations.
Lastly, the study did not investigate the effects of longer-term activation of the ICBP device and the potential safety issues related to long-term activation. Further investigations regarding the safety of long-term use will be needed if the device is to be trialed in translational studies.
Conclusions
In this preliminary large animal study, we demonstrate that the use of a ICBP with a specific activation protocol can improve brain tissue oxygenation as measured under conditions of elevated ICP and low PbtO2. Further studies are warranted to assess the safety and efficacy of ICP modulation technology to evaluate its potential for translation to the care of patients with severe brain injury.
References
Oddo M, Bösel J, Le Roux P, et al. Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocrit Care. 2014. https://doi.org/10.1007/s12028-014-0024-6.
Oddo M, Levine JM, MacKenzie L, et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery. 2011. https://doi.org/10.1227/NEU.0b013e3182287ca7.
Diaz-Arrastia R, LeRoux P, Bullock R, et al. Management strategies for reduced brain oxygen in severe traumatic brain injury: the BOOST-2 trial. J Neurotrauma. Published online 2012.
Leach MR, Shutter LA. How much oxygen for the injured brain - can invasive parenchymal catheters help? Curr Opin Crit Care. 2021. https://doi.org/10.1097/MCC.0000000000000810.
Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International severe traumatic brain injury consensus conference (SIBICC). Intens Care Med Publish Online. 2020. https://doi.org/10.1007/s00134-019-05900-x.
Doron O, Or T, Battino L, Rosenthal G, Barnea O. Cerebral blood flow augmentation using a cardiac-gated intracranial pulsating balloon pump in a swine model of elevated ICP. J Neurosurg. 2020. https://doi.org/10.3171/2019.1.JNS182864.
Doron O, Barnea O, Stocchetti N, Or T, Nossek E, Rosenthal G. Cardiac-gated intracranial elastance in a swine model of raised intracranial pressure: a novel method to assess intracranial pressure–volume dynamics. J Neurosurg. 2021. https://doi.org/10.3171/2020.3.JNS193262.
Krishnamurthy S, Li J, Schultz L, McAllister JP. Intraventricular infusion of hyperosmolar dextran induces hydrocephalus: a novel animal model of hydrocephalus. Cerebrospinal Fluid Res. 2009. https://doi.org/10.1186/1743-8454-6-16.
Bohman LE, Heuer GG, MacYszyn L, et al. Medical management of compromised brain oxygen in patients with severe traumatic brain injury. Neurocrit Care. 2011. https://doi.org/10.1007/s12028-011-9526-7.
Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury: clinical article. J Neurosurg. 2010. https://doi.org/10.3171/2010.1.JNS09506.
Ramirez de Noriega F, Manley GT, Moscovici S, et al. A swine model of intracellular cerebral edema – Cerebral physiology and intracranial compliance. J Clin Neurosci. 2018. https://doi.org/10.1016/j.jocn.2018.10.051.
Di Rocco C, Pettorossi VE, Caldarelli M, Mancinelli R, Velardi F. Experimental hydrocephalus following mechanical increment of intraventricular pulse pressure. Experientia Published online. 1977. https://doi.org/10.1007/BF01918814.
Luciano MG, Dombrowski SM, Qvarlander S, et al. Novel method for dynamic control of intracranial pressure. J Neurosurg Publis Online. 2017. https://doi.org/10.3171/2016.4.JNS152457.
Puccio A, Moore C, Fetzick A, et al. Brain oxygen optimization in severe traumatic brain injury-phase 3 (boost-3) trial. J Neurotrauma. 2018;35(16).
A. P, C. M, A. F, N.R. T, R. D-A, D.O. O. Brain tissue oxygen monitoring and management in severe traumatic brain injury-(boost-2) trial: a secondary analysis. J Neurotrauma. 2018;35(16).
Rosenthal G, Sanchez-Mejia RO, Phan N, Hemphill JC, Martin C, Manley GT. Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury: clinical article. J Neurosurg Published online. 2011. https://doi.org/10.3171/2010.6.JNS091360.
Author information
Authors and Affiliations
Contributions
OD: experiment design, analysis, and writing; YZ: experiment design, analysis, and writing; GR: experiment design and review; OB: experiment design.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval/informed consent
The authors confirm adherence to ethical guidelines and indicate that ethical approvals (institutional review board) were met.
Source of support
This study was funded by a KAMIN research grant, issued by the Israeli Innovation Authority, awarded to Dr. Doron and Professor Barnea (KAMIN grant 56,350).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doron, O., Zadka, Y., Rosenthal, G. et al. Intracranial Pulsating Balloon-Based Cardiac-Gated ICP Modulation Impact on Brain Oxygenation: A Proof-of-Concept Study in a Swine Model. Neurocrit Care 37, 689–696 (2022). https://doi.org/10.1007/s12028-022-01541-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01541-z