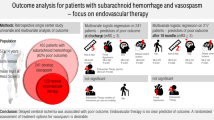
Abstract
Background
The present study explores the frequency, diagnostic approach, and therapeutic management of cerebral vasospasm in a cohort of children with moderate-to-severe traumatic and nontraumatic subarachnoid hemorrhage (SAH).
Methods
This was a single-center retrospective study performed over a 10-year period, from January 2010 to December 2019. Children aged from one month to 18 years who were admitted to the pediatric or adult intensive care unit with a diagnosis of SAH were eligible. Cerebral vasospasm could be suspected by clinical signs or transcranial Doppler (TCD) criteria (mean blood flow velocity > 120 cm/s or an increase in mean blood flow velocity by > 50 cm/s within 24 h) and then confirmed on cerebral imaging (with a reduction to less than 50% of the caliber of the cerebral artery).
Results
Eighty patients aged 8.6 years (3.3–14.8 years, 25–75th centiles) were admitted with an initial Glasgow Coma Scale score of 8 (4–12). SAH was nontraumatic in 21 (26%) patients. A total of 14/80 patients (18%) developed cerebral vasospasm on brain imaging on day 6 (5–10) after admission, with a predominance of nontraumatic SAH (12/14). The diagnosis of cerebral vasospasm was suspected on clinical signs and/or significant temporal changes in TCD monitoring (7 patients) and then confirmed on cerebral imaging. Thirteen of 14 patients with vasospasm were successfully treated using a continuous intravenous infusion of milrinone. The Pediatric Cerebral Performance Category score at discharge from the intensive care unit was comparable between children with vasospasm (score of 2 [1–4]) vs. children without vasospasm (score of 4 [2–4]) (p = 0.09).
Conclusions
These findings indicate that cerebral vasospasm exists in pediatrics, particularly after nontraumatic SAH. The use of TCD and milrinone may help in the diagnostic and therapeutic management of cerebral vasospasm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) is a rare but life-threatening neurological disease in children. In this population, mortality can reach 25% within the first hours, and in the survivors, SAH may result in long-term neurological deficits [1]. Compared with adults, SAH is mostly related to severe traumatic brain injury in children [2]. Otherwise, SAH can be caused by arteriovenous malformations, vascular aneurysms, cerebral tumors, and hematologic diseases [1]. Indeed, nontraumatic SAH represents 16% of strokes in the pediatric population, with an incidence of 0.4 per 100,000 person-years [3].
Cerebral vasospasm is a common complication of SAH and a leading cause of mortality and morbidity through the development of delayed cerebral ischemia and cerebral infarction [4]. Its pathophysiology is not completely understood, and different mechanisms have been proposed [5, 6]. In children, the frequency of cerebral vasospasm ranges from 21 to 40% of patients, according to the cause of SAH [2, 7]. Cerebral vasospasm is usually defined as a reduction in arterial diameter of more than 50% relative to admission diameter on cerebral imaging [8]. Because neurological clinical evaluation is limited in patients receiving sedative drugs, transcranial Doppler (TCD) monitoring could be used to detect vasospasm at the bedside [9]. However, no reference values have been established in children until recently [10], and TCD recordings can be influenced by several factors, including age, hematocrit, gender, fever, and metabolic disturbances [11].
Cerebral vasospasm prevention and treatment are based on oral or intravenous administration of nimodipine (i.e., a calcium channel blocker) [12, 13]. In the pediatric population, data about nimodipine are limited [14, 15], and the American and European guidelines have not made a statement for the pediatric population [12, 13]. In addition, intraarterial or intravenous administration of milrinone, a selective phosphodiesterase 3 inhibitor, has emerged as a promising option to treat vasospasm in adults [16,17,18,19]. Surprisingly, the use of milrinone is scarcely reported in pediatrics.
In this context, the present study aims to report the frequency of cerebral vasospasm in a cohort of children with moderate-to-severe SAH and describe the diagnostic approach and the therapeutic management of patients who subsequently developed cerebral vasospasm.
Methods
Design and setting
This single-center retrospective study was performed over a 10-year period, from January 2010 to December 2019, at the Grenoble-Alpes University Hospital. We analyzed the medical records of consecutive children aged one month to 18-years-old admitted to the pediatric or adult intensive care unit (ICU) with a diagnosis of SAH. Data acquired during the study period were extracted from an ICU information management system (Centricity High Acuity Critical Care; GE Healthcare, Vélizy, France). The local ethics committee of the Grenoble-Alpes University Hospital and the national data protection commission approved the study according to MR‐004 (Méthodologie de Référence) reference methodology (ref. 2205066v0, June 29th, 2020). According to French legislation, patients were informed and nonopposition was checked, but written consent was not required. We followed STROBE guidelines for this observational study [20].
Therapeutic management before cerebral vasospasm diagnosis
All patients were managed according to the most recent guidelines [13, 14]. In the case of ruptured aneurism, patients underwent securing of the aneurism within 48 h of admission, either by clipping or coiling. Maintenance of euvolemia, normothermia, electrolytes, and metabolic balance were targeted. In patients with alteration of consciousness, sedation (with midazolam and opioids) and mechanical ventilation in normoxia and normocapnia were required. In addition, these patients had external ventricular drainage to treat hydrocephalus and maintain cerebral perfusion pressure as appropriate for the neurological condition by vasoactive support with norepinephrine and, if needed, plasma volume expansion with crystalloids. Nimodipine was given orally or in the gastric tube every 4 h (0.5 mg/kg).
Data collection
Demographic, clinical, biological, TCD, and imaging data were collected at admission in the ICU. Clinical data included Glasgow Coma Score, neurological symptoms, blood pressure, intracranial pressure, and temperature. Additionally, the World Federation of Neurological Surgeons score [21] and the Pediatric Logistic Organ Dysfunction [22] score were calculated. Invasive intracranial pressure could be measured through an intraparenchymal probe and/or an external ventricular drain. Imaging data included the modified Fisher score from computed tomography scan or magnetic resonance imaging findings at admission [23]. Biological data included blood concentrations of lactate, magnesium, glucose, and hemoglobin content.
Diagnosis of cerebral vasospasm
The development of a new focal neurologic deficit was an indication of cerebral imaging. In our unit, patients are clinically screened at least every 8 h. Patients were treated for cerebral vasospasm on the basis of cerebral imaging only. As well, TCD monitoring was performed. A 4-h persistent elevation in TCD cerebral blood flow velocities that met adult criteria for vasospasm (see below) systematically triggers a recommendation for imaging. TCD data included blood flow velocities (systolic, mean, and diastolic) and pulsatility index in the middle cerebral artery at both sides. During the study period, we used criteria described in adults to suspect vasospasm [24, 25]. These criteria included (1) a mean blood flow velocity higher than 120 cm/s or (2) a change in mean blood flow velocity by more than 50 cm/s in 24 h in the middle cerebral artery.
Cerebral imaging was performed in case of new clinical events, such as focal deficit, drowsiness, confusion, or recurrence of headache. The decision was made by the in-charge physician. The choice between computed tomography angiography (CTA) and magnetic resonance angiography (MRA) was based on patient stability and access to the machine. Cerebral vasospasm was defined as a reduction to less than 50% of the caliber of the cerebral artery on cerebral imaging.
Diagnostic and therapeutic management of vasospasm
Children who developed cerebral vasospasm received treatment including the elevation of mean arterial blood pressure to 20 mm Hg higher than baseline values. Continuous intravenous infusion of milrinone (Corotrope; Sanofi-Adventis, Gentilly, France) was introduced at a dosage of 1 µg/kg/min as soon as vasospasm was confirmed by cerebral imaging. Doses of nimodipine were not modified when initiating milrinone, and both treatments could be started and used at the same time. Norepinephrine support and/or reduction by 50% of initial continuous rate of milrinone were considered if the patient had poor hemodynamic tolerance to milrinone. In case of persistent arterial hypotension, nimodipine or milrinone treatment was discontinued.
End points
The primary end point was the frequency of cerebral vasospasm after SAH in children diagnosed by using CTA or MRA. Secondary end points were TCD values in patients with cerebral vasospasm, tolerance to milrinone, outcome of patients with SAH (including the measurement of the Pediatric Cerebral Performance Category score [26] at discharge from ICU), ICU and hospital lengths of stay, duration of mechanical ventilation, and hospital mortality.
Statistical analysis
Continuous variables were expressed as median (interquartile range) and compared by using the nonparametric Mann–Whitney U-test. Categorical variables were expressed as number (%) and compared by using the χ2 or Fisher’s exact test, as appropriate. All tests were two-sided, and all p values were considered significant if they were less than 0.05. Statistical analysis was performed by using SPSS (SPSS 26.0, Chicago, IL).
Results
Study population
During the study period, 81 patients with SAH were eligible, and data were available in 80 (Fig. 1). Baseline characteristics are presented in Table 1. SAH was primarily related to traumatic brain injury (n = 59/80) (details in the Supplementary Material). There were 14 patients (18%) who developed subsequent cerebral vasospasm according to CTA or MRA findings, and in 7 of them, the diagnosis of vasospasm was suspected from changes in TCD recordings. The frequency of cerebral vasospasm was higher in patients with nontraumatic SAH (12/21) versus 2/59 patients with traumatic SAH (p < 0.001). Patients who developed a vasospasm had a lower Glasgow Coma Score (6 [6–9] vs. 14 [3–15], p = 0.04) and a higher World Federation of Neurological Surgeons score (5 [4, 5] vs. 4 [2–5], p < 0.001) on admission. They also required a higher level of care during the first 24 h, as reflected by more use of osmotherapy, external ventricular drains, mechanical ventilation, and vasopressor. The median delay between admission and cerebral vasospasm diagnosis and duration of vasospasm were 6 days (5–10) and 9 days (8–17), respectively.
TCD
TCD data were available in 44/80 patients (55%) (Table 1). Cerebral blood flow velocities at admission or during vasospasm did not differ significantly according to the age and gender. There were seven patients who had TCD findings compatible with vasospasm. Systolic, mean and diastolic blood flow velocities were 191 (190–213), 121 (111–131), and 70 (59–85) cm/s, respectively, and were confirmed with brain imaging.
ICU management
The management of the 14 patients who had cerebral vasospasm is detailed in Table 2. Thirteen of 14 children were treated with continuous intravenous infusion of milrinone at a dosage of 1.0 (0.6–1.0) µg/kg/minute for a median duration of treatment of 14 (8–19) days. Despite the use of norepinephrine to restore arterial blood pressure, medical treatment was discontinued in two patients (nimodipine) and one patient (milrinone). Rescue procedures for persistence or recurrence of vasospasm, that is, mechanical angioplasty and/or intraarterial administration of milrinone/nimodipine, were not required.
Outcome
There were 12/80 patients who died in the ICU with no difference between patients who had vasospasm and the others. Deaths of patients with traumatic brain injury were not due to vasospasm. In nontrauma-related SAH the mortality was high (7/21 patients, and no difference in those who has and did not have vasospasm).
The Pediatric Cerebral Performance Category score at ICU discharge was comparable between the two groups: 2 (1–4) with vasospasm versus 4 (2–4) without vasospasm, respectively (p = 0.09). Children with vasospasm had a longer stay in the ICU (7 [2–16] vs. 22 [20–26] days, p < 0.005) and in the hospital (15 [7–29] vs. 30 [26–32] days, p = 0.05) compared with children without vasospasm (Table 1).
Discussion
The present study indicates that cerebral vasospasm can be found in a significant proportion of children with moderate-to-severe SAH, particularly after nontraumatic SAH. Children with nontraumatic SAH should be carefully monitored, given the risk of cerebral vasospasm, especially between day 5 and day 10.
The diagnosis of cerebral vasospasm can be difficult in children. The neurological examination is not always reliable in young children, and not possible in sedated patients. In addition, the decision of brain imaging to explore cerebral arteries using CTA or MRA should be balanced with the transfer to radiological facility in patients who are unstable, and to the risk of radiation exposure (CTA) in children. In this context, the use of TCD to detect signs of cerebral vasospasm may help physicians to suspect cerebral vasospasm as suggested previously [10, 12]. A multidisciplinary expert consensus have recently formulated 34 recommendations in four domains regarding the TCD use in pediatrics and provided normal TCD values by age [10]. Of note was that most of TCD studies included children with traumatic brain injury [4, 27] or brain malaria [9, 28]. However, there was no validated cut-offs for diagnosing vasospasm in the mean cerebral artery in children. TCD has a pretest probability for detecting vasospasm of 32% and posttest probability of 45% when adult criteria are used for children [29]. In our study, TCD data were missing in a significant number of patients with vasospasm. Our TCD findings should be thus interpreted with caution.
Literature regarding the treatment of cerebral vasospasm after SAH in pediatrics is limited. Oral nimodipine was associated with no adverse event or episodes of arterial hypotension using 1 mg/kg every 4 h [14]. In our study, nimodipine was discontinued in two patients with episodes of arterial hypotension. Milrinone has been successfully used in adults with documented vasospasm [16, 18, 30]. Surprisingly, its use was reported in one postoperative pediatric case only [31]. In the present study, one patient had arterial hypotension that needed norepinephrine.
In the present study, we found that the cause of SAH had a significant impact on frequency of vasospasm and on patient outcome. The amount of blood in subarachnoid spaces—as reflected by the Fisher score—might explain the difference in the frequency of vasospasm between traumatic and nontraumatic SAH [2, 7]. Outcomes of patients with traumatic brain injury, especially, are heavily influenced by the extent of traumatic brain injury, more than that of vasospasm, despite traumatic SAH is a prognostic criteria in adult cohorts [32]. Arteriovenous malformation outcomes are far more dependent upon the location of the malformation than the presence of vasospasm. Importantly, our findings regarding the outcomes of patients should be interpreted with caution since the groups presented in Table 1 are not strictly exchangeable.
Limitations
The present study has several limitations. Firstly, this is a single-center retrospective study. We studied all children consecutively admitted to the ICU during the study period, which should have limited any attrition bias. However, there were missing data regarding TCD recordings. In addition, because of large variations in SAH care management [19], our findings cannot be transposed to all patients and centers. Secondly, we used TCD criteria for vasospasm derived from those found in adults in the absence of criteria validated in children [11]. Thirdly, there were three patients who developed arterial hypotension within 2 h of the initiation of medical treatment for vasospasm. Because nimodipine and milrinone were given at the same time, it was not possible to distinguish which drug caused hypotension. Finally, other therapeutic interventions for cerebral vasospasm, including plasma volume expansion and crystalloids were not collected in our study due to the retrospective design. We acknowledge that this may result in incomplete exploration of therapeutic management.
Conclusions
A significant proportion of pediatric patients developed cerebral vasospasm after SAH according to brain imaging. Based on these findings, we recommend aggressive neuromonitoring, especially in patients with nontraumatic-related SAH. Future studies are warranted to establish TCD reference values and to evaluate preventative or therapeutic options for cerebral vasospasm in these children.
References
Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25:416–21.
Krishna H, Wani AA, Behari S, Banerji D, Chhabra DK, Jain VK. Intracranial aneurysms in patients 18 years of age or under, are they different from aneurysms in adult population? Acta Neurochir (Wien). 2005;147:469–76;discussion 476.
Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–94.
O’Brien NF, Maa T, Yeates KO. The epidemiology of vasospasm in children with moderate-to-severe traumatic brain injury. Crit Care Med. 2015;43:674–85.
Ciurea AV, Palade C, Voinescu D, Nica DA. Subarachnoid hemorrhage and cerebral vasospasm—literature review. J Med Life. 2013;6:120–5.
Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2014;85:1343–53.
Garg K, Singh PK, Sharma BS, Chandra PS, Suri A, Singh M, et al. Pediatric intracranial aneurysms–our experience and review of literature. Childs Nerv Syst. 2014;30:873–83.
Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
LaRovere KL, O’Brien NF. Transcranial Doppler sonography in pediatric neurocritical care: a review of clinical applications and case illustrations in the pediatric intensive care unit. J Ultrasound Med. 2015;34:2121–32.
O’Brien N, Wainwright M, Kaplan S, Appavu B, Erklauer J, Ghosh S, et al. Practice recommendations for transcranial doppler ultrasonography in critically ill children in the pediatric intensive care unit: a multidisciplinary expert consensus statement. J Pediatr Intensive Care. 2021;10:133–42.
O’Brien NF. Reference values for cerebral blood flow velocities in critically ill, sedated children. Childs Nerv Syst. 2015;31:2269–76.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT; American Heart Association Stroke C et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–37.
Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, European SO. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112.
Heffren J, McIntosh AM, Reiter PD. Nimodipine for the prevention of cerebral vasospasm after subarachnoid hemorrhage in 12 children. Pediatr Neurol. 2015;52:356–60.
Song Y, Qian SY, Li Y, Liu J, Li Z, Jia XL, et al. [Effectiveness and safety of nimodipine in preventing cerebral vasospasm after subarachnoid hemorrhage in children]. Zhonghua Er Ke Za Zhi. 2019;57:338–43.
Crespy T, Heintzelmann M, Chiron C, Vinclair M, Tahon F, Francony G, et al. Which protocol for milrinone to treat cerebral vasospasm associated with subarachnoid hemorrhage? J Neurosurg Anesthesiol. 2019;31:323–9.
Lannes M, Zeiler F, Guichon C, Teitelbaum J. The use of milrinone in patients with delayed cerebral ischemia following subarachnoid hemorrhage: a systematic review. Can J Neurol Sci. 2017;44:152–60.
Velly LJ, Bilotta F, Fabregas N, Soehle M, Bruder NJ, Nathanson MH; European N; Critical Care Interest G. Anaesthetic and ICU management of aneurysmal subarachnoid haemorrhage: a survey of European practice. Eur J Anaesthesiol. 2015;32:168–76.
Arakawa Y, Kikuta K, Hojo M, Goto Y, Ishii A, Yamagata S. Milrinone for the treatment of cerebral vasospasm after subarachnoid hemorrhage: report of seven cases. Neurosurgery 2001;48:723–8; discussion 728–30.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.
Report of World Federation of Neurological Surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985–6.
Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–7.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–27. discussion 21–7.
Mascia L, Fedorko L, terBrugge K, Filippini C, Pizzio M, Ranieri VM, et al. The accuracy of transcranial Doppler to detect vasospasm in patients with aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2003;29:1088–94.
Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA. Vasospasm probability index: a combination of transcranial doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:1101–12.
Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74.
O’Brien NF, Reuter-Rice KE, Khanna S, Peterson BM, Quinto KB. Vasospasm in children with traumatic brain injury. Intensive Care Med. 2010;36:680–7.
O'Brien NF, Mutatshi Taty T, Moore-Clingenpeel M, Bodi Mabiala J, Mbaka Pongo J, Ambitapio Musungufu D, et al. Transcranial Doppler ultrasonography provides insights into neurovascular changes in children with cerebral malaria. J Pediatr. 2018;203:116–24.
Moftakhar P, Cooke DL, Fullerton HJ, Ko NU, Amans MR, Narvid JA, et al. Extent of collateralization predicting symptomatic cerebral vasospasm among pediatric patients: correlations among angiography, transcranial Doppler ultrasonography, and clinical findings. J Neurosurg Pediatr. 2015;15:282–90.
Lannes M, Teitelbaum J, del Pilar CM, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care. 2012;16:354–62.
Afshari FT, Fitzgerald JJ, Higgins JN, Garnett MR, Fernandes HM, Santarius T. Diffuse cerebral vasospasm following resection of a hypoglossal schwannoma in a child. Br J Neurosurg. 2014;28:541–3.
Roozenbeek B, Lingsma HF, Lecky FE, Lu J, Weir J, Butcher I, et al. Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit Care Med. 2012;40:1609–17.
Acknowledgements
None.
Funding
This work received no funding.
Author information
Authors and Affiliations
Contributions
CI, AM, and AD designed the study. CI, GF, and CB collected and analyzed the data. CI, GF, CB, IW, JFP, and GM wrote the article. All authors approve of the final manuscript.
Corresponding author
Ethics declarations
Ethical approval/informed consent
Our study adheres with ethical guidelines (informed consent was not required according to the design of the study). The local ethics committee of the Grenoble-Alpes University Hospital and the national data protection commission approved the study according to MR‐004 reference methodology (ref. 2205066v0, June 29th, 2020).
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Isola, C., Evain, JN., Francony, G. et al. Cerebral vasospasm in children with subarachnoid hemorrhage: frequency, diagnosis, and therapeutic management. Neurocrit Care 36, 868–875 (2022). https://doi.org/10.1007/s12028-021-01388-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01388-w