Abstract
Background
A significant proportion of patients with subarachnoid hemorrhage have a normal cerebral angiogram. Patients with angiographically negative subarachnoid hemorrhage (anSAH) with either perimesencephalic- (panSAH) or aneurysmal-pattern hemorrhage (aanSAH, also known as diffuse anSAH) have an excellent prognosis, but only if underlying vascular abnormalities are completely excluded. The rate of occult aneurysms in patients with aanSAH varies widely across studies. The purpose of this study was to quantify the value of repeat DSA in these patients.
Methods
We reviewed the records of all patients initially diagnosed with aanSAH after a screening DSA at a single tertiary neurovascular referral center from January 2006–April 2018. Patients with panSAH and traumatic SAH were excluded. We also performed a systematic review and meta-analysis of positive second DSAs in previously published case series of patients with aanSAH who underwent two serial DSAs. For meta-analysis, PubMed Central, MEDLINE and Cochrane Library databases were searched for pertinent studies up to November 2019. The rate of aneurysm detection on repeat angiography was extracted from each study. Pooled rates for positive second angiogram were calculated as untransformed proportions in a binary random-effects model meta-analysis. Inter-study heterogeneity was calculated using an I2 statistic.
Results
Three of 27 patients (11.1%) with aanSAH and at least two DSAs were subsequently found to have a cerebral aneurysm in our institutional dataset. Twenty-six studies in our systematic review met inclusion criteria, and the pooled rate of positive second angiogram was 10.4% (95% CI 7.3%—13.5%, P < 0.001). Substantial inter-study heterogeneity was observed in the meta-analysis (I2 = 61.7%, P < 0.001).
Conclusions
One in 10 patients with aanSAH has an occult ruptured aneurysm. A second-look DSA should be strongly considered in these cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 10–20% of patients with subarachnoid hemorrhage (SAH) will have a normal cerebral angiogram. Compared to patients with aneurysmal SAH (aSAH), those with angiographically negative subarachnoid hemorrhage (anSAH; also called cryptogenic SAH or non-aneurysmal SAH) have a good prognosis with low risk of delayed cerebral ischemia (DCI), infarction, poor functional outcome and death [1,2,3].This observation was initially described in patients with perimesencephalic-pattern anSAH hemorrhage (panSAH; also termed pretruncal hemorrhage). Recent studies have shown that patients with aneurysmal-pattern anSAH (aanSAH; also known as diffuse-pattern anSAH) may have a more severe presentation and a greater risk for hydrocephalus than patients with panSAH but have a good functional prognosis relative to patients with aSAH [1,2,3]. Given that aSAH is associated with higher rates of DCI, in-hospital mortality and poorer functional outcome than aanSAH, confirming the absence of an underlying aneurysm is helpful in prognosticating these patients [1].
Numerous studies have demonstrated that the rate of a positive findings upon repeat DSA in cases of perimesencephalic anSAH is 1% or less [4,5,6]. However, few studies have addressed the utility of repeat angiography in patients with aneurysmal-pattern anSAH, in whom the likelihood of an occult aneurysm is much higher. In the literature, different approaches to ruling out occult pathology in these patients with various combinations of repeat DSA, CT angiography (CTA), magnetic resonance angiography (MRA) and magnetic resonance imaging (MRI) of the brain or spine have been described [7]. In addition to the variable utilization of these imaging modalities, the optimal timing of repeat imaging is unknown. Studies vary widely in the timing of repeat imaging with reported intervals ranging between a few days and several months [e.g., refs 8, 9].
The purpose of this study was to determine the utility and optimal timing of repeat DSA in patients with aanSAH. We present a case series of patients with aanSAH at a major neurovascular referral center as well as a systematic review and meta-analysis of the rate of positive findings on repeat DSA in these patients. Furthermore, we present a systematic review of the timing of repeat DSA to determine whether early and late repeat angiography differ in detecting occult aneurysms.
Methods
Patient Selection
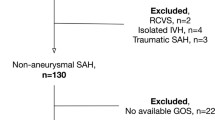
This study was approved by the Institutional Review Board at Mayo Clinic, Rochester. Due to the observational design of the study, consent was waived for study participants. All patients have the option to opt out of observational research upon initiation of care, and these patients were not included in the study. Clinical records from our institution January 2006–April 2018 were reviewed for all patients diagnosed with aneurysmal or angiographically negative SAH. Patients with any form of trauma associated with symptom onset were excluded from this study. Only patients with aanSAH, or diffuse-pattern anSAH, were included in this study; patients with panSAH, peripheral or sulcal SAH only were excluded. In our practice, all patients with aneurysmal-pattern SAH are admitted to the neurointensive care unit for treatment. Following initial head CT and DSA, additional imaging was performed at the discretion of a multidisciplinary team of neurointensivists, neuroradiologists and neurosurgeons. Upon admission, all patients with non-traumatic SAH were treated according to well-accepted guidelines for management of aneurysmal SAH [10]. All patients were presumed to have aneurysmal etiology upon presentation and were administered standard-dose tranexamic acid and nimodipine. Once aneurysmal etiology was considered “ruled out” by DSA, these interventions were stopped.
Imaging Definitions
In accordance with the original descriptions of anSAH, patients with focal hemorrhage in front of the brainstem with extension into the perimesencephalic cisterns and with or without limited superior extension into the suprasellar cistern or medial third of the Sylvian fissure were diagnosed as having perimesencephalic-pattern anSAH [2, 3, 11]. Patients with aneurysmal-pattern hemorrhage included both patients with diffuse SAH extending beyond the medial third of the medial Sylvian fissure as well as more focal areas of hemorrhage consistent with rupture of an aneurysm of the peripheral anterior, middle or posterior cerebral arteries (e.g., with extension into the lateral Sylvian or interhemispheric fissure). Only patients with aneurysmal-pattern SAH were included for analysis in this study. As the goal of this study was to determine the utility of repeat angiography, we included only patients with at least two DSAs in our analysis.
Systematic Review of the Literature
This systematic review was performed in accordance with PRISMA guidelines [12] and is registered with the PROSPERO international prospective register of systematic reviews (ID 143,351; https://www.crd.york.ac.uk/prospero/). A schematic of the review protocol is presented in Fig. 2. PubMed Central, MEDLINE and Cochrane Library databases were searched in duplicate (CN, KR) for all studies with the terms, “angiographically negative subarachnoid hemorrhage,” “perimesencephalic hemorrhage,” “pretruncal hemorrhage,” or “non-aneurysmal subarachnoid hemorrhage” in the title, abstract or keywords. All included studies were required to define patients as having aneurysmal-pattern SAH, report the number of patients with aanSAH with at least two DSAs and also report the number of patients with aanSAH who had a second DSA that identified the causative lesion. Studies that did not distinguish patients based on hemorrhage pattern, presented only data on patients with panSAH or did not present data on the number of patients with aanSAH and a positive second DSA were excluded. Only English-language manuscripts were considered for review. The bibliographies of included studies as well as pertinent review articles were screened for additional studies that met inclusion criteria. The title screen (CN, KR) and data extraction (CN, SO) were performed in duplicate; any discrepancies were resolved by consensus. The strength of study quality was assessed using the Oxford Centre for Evidence-Based Medicine (OCEBM) grading system (https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/).
Statistical Comparisons
Pooled rates for positive second angiogram were calculated as untransformed proportions in a binary random-effects model meta-analysis using weights according to study size, and inter-study heterogeneity was calculated with an I2 statistic using OpenMetaAnalyst (Brown University, Providence, RI; http://www.cebm.brown.edu/openmeta/#).
Data Availability
Raw data extracted for meta-analysis may be accessed directly through the Dryad Data Portal (DOI pending).
Results
Incidence of Occult Cerebral Aneurysms and Utility of Digital Subtraction Angiogram
Between January 2006 and April 2018, 563 patients were admitted to our institution for treatment of SAH. Initial DSA was negative in 103 patients (18.3%), of which 36 (35.0%) displayed aneurysmal-pattern SAH. Digital subtraction angiography was repeated in 27 patients with aanSAH (75%) at a median of seven days after the initial DSA (mean 7.8 days, range 4–15 days). In seven patients in whom the clinical suspicion of aSAH was low, the initial DSA was followed by CTA (N = 4), MRA (N = 2) or observation (N = 1). Two patients with aanSAH were scheduled for follow-up DSA following discharge and were lost to follow-up.
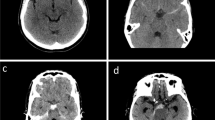
Three patients who underwent repeat DSA were found to harbor cerebral aneurysms that were not detected on the initial DSA (11.1% false-negative rate on first DSA; Fig. 1). In all instances, the aneurysm was 2 mm or smaller in maximum diameter. One patient was found to have a left superior cerebellar artery pseudoaneurysm, which was treated by embolization of the parent vessel. An additional patient presented with a 2-mm aneurysm associated with a perforating artery from the first segment of the posterior cerebral artery. This aneurysm was observed and spontaneously thrombosed at three-month follow-up. The third patient was found to have a 2-mm aneurysm arising from the callosomarginal artery (Fig. 1). The patient was taken to surgery, which was complicated by intraoperative rupture not controlled with clip grafting, and the artery was sacrificed. In both instances following intervention, the patients recovered without neurologic deficit. On retrospective review of the initial DSAs, the aneurysm was not visible in two cases and equivocally visible in one. The final rate of aanSAH was 5.7%.
Case example. A 53-year-old female presented with thunderclap headache, and a CT scan demonstrated diffuse subarachnoid hemorrhage without intraventricular hemorrhage. On the night of admission, she became progressively more somnolent with expansion of her ventricular system, and an external ventricular drain (EVD) was placed. Followed EVD placement, an angiogram was performed which demonstrated no clear evidence of underlying aneurysm. (bottom left). On post-bleed day (PBD) six, her EVD was removed without issue, and she was transferred out of the ICU. On PBD 12, repeat angiography demonstrated a 2-mm left pericallosal artery aneurysm which was not discernible on the original DSA. The patient subsequently underwent craniotomy for clipping of the aneurysm and was dismissed home without complication on post-operative day five
The overall complication rate for DSAs performed in this study was 1/63 (1.6%). The complication of this study was a post-procedural groin hematoma in a patient who underwent repeat angiography which did not require operative intervention. When considering only second angiograms, this translates to a repeat-angiogram complication rate of 3.7%.
Systematic Review and Meta-Analysis
The literature search returned 555 records. Twenty-six pertinent studies published between 1996 and 2019 were detected (Fig. 2, Table 1). [36 , 8, 9 , 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] All data were extracted from case series of patients with aanSAH who were followed through time with serial DSAs (OCEBM evidence level 4). Among all patients with non-traumatic SAH, the pooled incidence of aanSAH upon presentation was 6.9% (95% CI 5.4–8.4%; P < 0.001; I2 = 95.3%, P < 0.001). Among 896 patients with aanSAH and a repeat DSA, repeat DSA revealed an occult lesion in 10.4% (95% CI 7.3%—13.5%, P < 0.001), although there was substantial inter-study heterogeneity (I2 = 61.7%, P < 0.001; Fig. 3). In serial leave-one-out analyses, this result remained significant with a pooled rate range of 9.0–10.9%; (P < 0.001); inter-study heterogeneity remained substantial (I2 ≥ 42.8%). The final incidence of aanSAH following any screening paradigm was 6.1% (95% CI 4.7%—7.5%’ P < 0.001; I2 = 95.7%, P < 0.001).
Ten studies performed repeat angiography within two weeks of presentation, two studies performed repeat angiography 6 weeks or later. The remaining studies either performed repeat angiography over a broad time window or did not present data on timing of repeat angiography. Studies that repeated a DSA within two weeks of presentation had a pooled detection rate of 8.1% (95% CI 4.1–12.1%; P < 0.001; I2 = 58.4%, P = 0.010), and those that repeated it six weeks or later after presentation had a pooled detection rate of 5.7% (P = 0.056; I2 = 0, P = 0.400).
Discussion
About one in 10 patients who present with aneurysmal-pattern SAH and a negative initial DSA will have an occult aneurysm on repeat DSA. The yield of the second DSA does not appear to increase beyond 2 weeks, and therefore, performing a repeat DSA within this early interval is reasonable. Although heterogeneity across studies limits the reliability of the combined estimates, there is enough information to conclude that a second DSA should be strongly considered in patients with aneurysmal-pattern anSAH.
Based on the results of this study, about 6% of patients with a pattern of hemorrhage compatible with a ruptured aneurysm will have no identifiable abnormality on imaging. The etiology of anSAH is not clear, but the most widely accepted hypothesis is that it represents a venous hemorrhage. This is supported by the observation that about half of patients with perimesencephalic anSAH have “primitive” drainage of the basal vein of Rosenthal directly to a dural venous sinus on at least one side, about twice as frequently as patients with aSAH [36]. Similar findings have not been specifically documented in aneurysmal-pattern anSAH, however.
Since about 10% of patients with aanSAH will have a detectable aneurysm on repeat DSA, it is possible that a proportion of these patients harbor a ruptured aneurysm that obliterates upon rupture or remains occult by conventional imaging techniques. However, indirect evidence for a venous etiology comes from the low risk of DCI in aanSAH, which is triggered by adventitial exposure to oxyhemoglobin after rupture of arterial aneurysms [1, 37,38,39]. Additionally, the functional outcomes and mortality risk in aneurysmal-pattern anSAH parallel those of perimesencephalic anSAH much more closely than aSAH, which might indicate a shared etiology [1]. Further imaging studies are necessary to further elucidate specific etiologies of aanSAH, and prior studies of patients with panSAH offer clues of potential pathologies in patients with more diffuse hemorrhage. Specifically, intramural arterial dissection has been observed in patients with perimesencephalic anSAH [40]. Similarly, arteriovenous malformations, aneurysms and pseudoaneurysms of the spine may initially present as anSAH, but this is a very uncommon, and most patients have a physical exam and presentation consistent with a spinal lesion, such as sudden-onset pain localized to the level of the lesion [7, 41]. The most common definitive etiology for anSAH is an occult cerebral aneurysm, and a repeat DSA remains the gold standard for diagnosis. One prior systematic review and meta-analysis estimated the rate of positive findings on repeat DSA in patients with diffuse-pattern anSAH to be 10.0% [15]. However, that study captured significantly fewer patients than the one presented here, did not account for inter-study heterogeneity and included one study that reported no data on the pattern of hemorrhage of patients with anSAH [42]. Additionally, although hampered by inter-study heterogeneity, our study was able to capture additional data on the optimal timing of repeat DSA. The initial DSA may be negative for a variety of reasons, including errors in interpretation, insufficient vessel imaging (e.g., three- versus four-vessel angiography) and the innately transient nature of imaging features of blister aneurysms. While limited, the available data suggest that early repeat DSA is at least non-inferior to delayed DSA, but several data-specific factors limit the scope of any further conclusions on this issue. Only two studies specifically presented data on more delayed imaging (i.e., 6 weeks), and seven studies presented no data on timing of repeat DSA at all (Table 1). Several studies presented a wide range of timing for repeat DSA, but none of these presented data on the aneurysm detection rate between subgroups based on timing. Therefore, it is currently not possible to quantify the benefit of one time point over another, and further study is needed to address this important question. Additionally, timing of a repeat study may simply capture aneurysms that are distinctly visible at different time points [5].
Our study has several limitations. First, there is significant inter-study heterogeneity. The observed rates of both aneurysmal-pattern anSAH prevalence and positive second DSA vary across studies, even amongst more recent ones, suggesting that imaging quality and the definition of aanSAH differ substantially between studies. Inclusion criteria based on hemorrhage pattern likely varied between studies as evidenced by the numerous terms used to describe these patients in the literature. Our own case series is limited by its retrospective nature and relatively small cohort size. Nevertheless, the consistency of our results with those reported elsewhere in the literature lends external validity to the original data presented here. Our study does not allow us to estimate the yield of other diagnostic modalities (notably, non-invasive angiography), but given the very small size and difficult visualization of these occult aneurysms, it is highly unlikely that they could be detected with imaging of lower sensitivity than DSA.
Conclusions
Our institutional experience and meta-analysis of available data demonstrate about one in ten patients with aneurysmal-pattern anSAH will have a causative lesion identified on repeat DSA. A second DSA should strongly be considered in patients with a diffuse pattern of hemorrhage. Limited data do not suggest a benefit to delaying the repeat study beyond two weeks from ictus.
Abbreviations
- anSAH:
-
Angiographically negative subarachnoid hemorrhage
- DSA:
-
Digital subtraction angiography
- SAH:
-
Subarachnoid hemorrhage
References
Nesvick CL, Oushy S, Rinaldo L, et al. Clinical complications and outcomes of angiographically negative subarachnoid hemorrhage. Neurology. 2019;92:e2385-94.
Rinkel GJ, Wijdicks EF, Hasan D, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet. 1991;338:964–8.
Rinkel GJ, Wijdicks EF, Vermeulen M, et al. The clinical course of perimesencephalic nonaneurysmal subarachnoid hemorrhage. Ann Neurol. 1991;29:463–8.
Mensing LA, Vergouwen MDI, Laban KG, et al. Perimesencephalic hemorrhage: a review of epidemiology, risk factors, presumed cause, clinical course, and outcome. Stroke. 2018;49:1363–70.
Kalra VB, Wu X, Matouk CC, et al. Use of follow-up imaging in isolated perimesencephalic subarachnoid hemorrhage: a meta-analysis. Stroke. 2015;46:401–6.
Geng B, Wu X, Brackett A, et al. Meta-analysis of recent literature on utility of follow-up imaging in isolated perimesencephalic hemorrhage. Clin Neurol Neurosurg. 2019;180:111–6.
Germans MR, Coert BA, Majoie CB, et al. Yield of spinal imaging in nonaneurysmal, nonperimesencephalic subarachnoid hemorrhage. Neurology. 2015;84:1337–40.
Khan AA, Smith JD, Kirkman MA, et al. Angiogram negative subarachnoid haemorrhage: outcomes and the role of repeat angiography. Clin Neurol Neurosurg. 2013;115:1470–5.
Kaim A, Proske M, Kirsch E, et al. Value of repeat-angiography in cases of unexplained subarachnoid hemorrhage (SAH). Acta Neurol Scand. 1996;93:366–73.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2012;43:1711–37.
van Gijn J, van Dongen KJ, Vermeulen M, et al. Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35:493–7.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Gupta SK, Gupta R, Khosla VK, et al. Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: is it a benign entity? Surg Neurol. 2009;71:566–71 (discussion 571, 571–562, 572).
Fontanella M, Rainero I, Panciani PP, et al. Subarachnoid hemorrhage and negative angiography: clinical course and long-term follow-up. Neurosurg Rev. 2011;34:477–84.
Bakker NA, Groen RJ, Foumani M, et al. Repeat digital subtraction angiography after a negative baseline assessment in nonperimesencephalic subarachnoid hemorrhage: a pooled data meta-analysis. J Neurosurg. 2014;120:99–103.
Andaluz N, Zuccarello M. Yield of further diagnostic work-up of cryptogenic subarachnoid hemorrhage based on bleeding patterns on computed tomographic scans. Neurosurgery. 2008;62:1040–6 (discussion 1047).
Delgado Almandoz JE, Jagadeesan BD, Refai D, et al. Diagnostic yield of repeat catheter angiography in patients with catheter and computed tomography angiography negative subarachnoid hemorrhage. Neurosurgery. 2012;70:1135–42.
Yu DW, Jung YJ, Choi BY, et al. Subarachnoid hemorrhage with negative baseline digital subtraction angiography: is repeat digital subtraction angiography necessary? J Cerebrovasc Endovasc Neurosurg. 2012;14:210–5.
Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: When is digital subtraction angiography still needed? AJNR Am J Neuroradiol. 2010;31:696–705.
Little AS, Garrett M, Germain R, et al. Evaluation of patients with spontaneous subarachnoid hemorrhage and negative angiography. Neurosurgery. 2007;61:1139–50 (discussion 1150–1131).
Dalyai R, Chalouhi N, Theofanis T, et al. Subarachnoid hemorrhage with negative initial catheter angiography: a review of 254 cases evaluating patient clinical outcome and efficacy of short- and long-term repeat angiography. Neurosurgery. 2013;72:646–52 (discussion 651–642).
Topcuoglu MA, Ogilvy CS, Carter BS, et al. Subarachnoid hemorrhage without evident cause on initial angiography studies: diagnostic yield of subsequent angiography and other neuroimaging tests. J Neurosurg. 2003;98:1235–40.
Rogg JM, Smeaton S, Doberstein C, et al. Assessment of the value of MR imaging for examining patients with angiographically negative subarachnoid hemorrhage. AJR Am J Roentgenol. 1999;172:201–6.
Jung JY, Kim YB, Lee JW, et al. Spontaneous subarachnoid haemorrhage with negative initial angiography: a review of 143 cases. J Clin Neurosci. 2006;13:1011–7.
Kumar R, Das KK, Sahu RK, et al. Angio negative spontaneous subarachnoid hemorrhage: Is repeat angiogram required in all cases? Surg Neurol Int. 2014;5:125.
Moscovici S, Fraifeld S, Ramirez-de-Noriega F, et al. Clinical relevance of negative initial angiogram in spontaneous subarachnoid hemorrhage. Neurol Res. 2013;35:117–22.
Maslehaty H, Petridis AK, Barth H, et al. Diagnostic value of magnetic resonance imaging in perimesencephalic and nonperimesencephalic subarachnoid hemorrhage of unknown origin. J Neurosurg. 2011;114:1003–7.
Khan N, Schuknecht B, Yonekawa Y. Presentation and management of patients with initial negative 4-vessel cerebral angiography in subarachnoid hemorrhage. Acta Neurochir Suppl. 2002;82:71–81.
Hashimoto H, Iida J, Hironaka Y, et al. Use of spiral computerized tomography angiography in patients with subarachnoid hemorrhage in whom subtraction angiography did not reveal cerebral aneurysms. J Neurosurg. 2000;92:278–83.
Canhao P, Ferro JM, Pinto AN, et al. Perimesencephalic and nonperimesencephalic subarachnoid haemorrhages with negative angiograms. Acta Neurochir (Wien). 1995;132:14–9.
Zhong W, Zhao P, Wang D, et al. Different clinical characteristics between perimesencephalic subarachnoid hemorrhage and diffuse subarachnoid hemorrhage with negative initial angiography. Turk Neurosurg. 2014;24:327–32.
Berdoz D, Uske A, de Tribolet N. Subarachnoid haemorrhage of unknown cause: clinical, neuroradiological and evolutive aspects. J Clin Neurosci. 1998;5:274–82.
Delgado Almandoz JE, Crandall BM, Fease JL, et al. Diagnostic yield of catheter angiography in patients with subarachnoid hemorrhage and negative initial noninvasive neurovascular examinations. AJNR Am J Neuroradiol. 2013;34:833–9.
Bashir A, Mikkelsen R, Sorensen L, et al. Non-aneurysmal subarachnoid hemorrhage: when is a second angiography indicated? Neuroradiol J. 2018;31:244–52.
Yap L, Dyde RA, Hodgson TJ, et al. Spontaneous subarachnoid hemorrhage and negative initial vascular imaging–should further investigation depend upon the pattern of hemorrhage on the presenting CT? Acta Neurochir (Wien). 2015;157:1477–84.
Rouchaud A, Lehman VT, Murad MH, et al. Nonaneurysmal perimesencephalic hemorrhage is associated with deep cerebral venous drainage anomalies: a systematic literature review and meta-analysis. AJNR Am J Neuroradiol. 2016;37:1657–63.
Dietrich HH, Dacey RG Jr. Molecular keys to the problems of cerebral vasospasm. Neurosurgery. 2000;46:517–30.
Nishizawa S, Laher I. Signaling mechanisms in cerebral vasospasm. Trends Cardiovasc Med. 2005;15:24–34.
Ishiguro M, Morielli AD, Zvarova K, et al. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–60.
Schievink WI, Wijdicks EF. Origin of pretruncal nonaneurysmal subarachnoid hemorrhage: ruptured vein, perforating artery, or intramural hematoma? Mayo Clin Proc. 2000;75:1169–73.
Yue H, Ling W, Ou Y, et al. Intracranial subarachnoid hemorrhage resulting from non-cervical spinal arteriovenous lesions: analysis of possible cause of bleeding and literature review. Clin Neurol Neurosurg. 2019;184:105371.
Prestigiacomo CJ, Sabit A, He W, et al. Three dimensional CT angiography versus digital subtraction angiography in the detection of intracranial aneurysms in subarachnoid hemorrhage. J Neurointerv Surg. 2010;2:385–9.
Funding
None.
Author information
Authors and Affiliations
Contributions
CLN, EFW, GL and AAR conceived the study and contributed to its design. CLN, SO, KR and LR collected the data. CLN and PK performed statistical analyses. CLN drafted the manuscript. All authors discussed the results and provided critical feedback on the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rightsHuman and animal rights
This study was performed with Institutional Review Board (IRB) approval for minimal-risk human studies research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nesvick, C.L., Oushy, S., Ravindran, K. et al. Repeat Catheter Angiography in Patients with Aneurysmal-Pattern Angiographically Negative Subarachnoid Hemorrhage. Neurocrit Care 36, 52–60 (2022). https://doi.org/10.1007/s12028-021-01247-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01247-8