Abstract
Background
In aneurysmal subarachnoid hemorrhage (SAH), clot volume has been shown to correlate with the development of radiographic vasospasm (VS), while the role of cerebrospinal fluid (CSF) volume remains largely elusive in the literature. We evaluated CSF volume as a potential surrogate for VS in addition to SAH volume in this retrospective series.
Patients and Methods
From a consecutive cohort of aneurysmal SAH (n= 320), cases were included when angiographic evaluation for VS was performed (n= 125). SAH and CSF volumes were volumetrically quantified using an algorithm-assisted segmentation approach on initial computed tomography after ictus. Association with VS was analyzed using regression analysis. Receiver operating characteristic (ROC) curves were used to evaluate predictive accuracy of volumetric measures for VS and to identify cutoffs for risk stratification.
Results
Among 125 included cases, angiography showed VS in 101 (VS+), while no VS was observed in 24 (VS−) cases. In volumetric analysis, mean SAH volume was significantly larger (26.8 ± 21.1 ml vs. 12.6 ± 12.2 ml, p= 0.001), while mean CSF volume was significantly smaller (63.0 ± 31.2 ml vs. 85.7 ± 62.8, p= 0.03) in VS+ compared to VS− cases, respectively. The absence of correlation for SAH and CSF volumes (Pearson R − 0.05, p= 0.58) indicated independence of both measures of the subarachnoid compartment, which was a prerequisite for CSF to act as a new surrogate for VS not related to SAH. Regression analysis confirmed an increased risk of VS with increasing SAH (OR 1.06, 95% CI 1.02–1.11, p= 0.006), while CSF had a protective effect toward VS (OR 0.99, 95% CI 0.98–0.99, p= 0.02). SAH/CSF ratio was also associated with VS (OR 1.03, 95% CI 1.01–1.05, p= 0.015). ROC curves suggested cutoffs at 120 ml CSF and 20 ml SAH for VS stratification. Combination of variables improved stratification accuracy compared to use of SAH alone.
Conclusion
This study provides a proof of concept for CSF correlating with angiographic VS after aneurysmal SAH. Quantification of CSF in conjunction with SAH might enhance risk stratification and exhibit advantages over traditional scores. The association of CSF has to be corroborated for delayed cerebral ischemia to further establish CSF as a surrogate parameter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) is a neurological emergency commonly caused by rupture of an intracranial aneurysm, which leads to bleeding into the subarachnoid space usually filled with cerebrospinal fluid (CSF) [1]. Although improvements in intensive care medicine and diligent aneurysm treatment have led to a decrease in overall mortality, SAH remains a highly perilous condition with frequent neurologic disability [2]. In particular, delayed cerebral ischemia (DCI) is a source of severe additional neurologic morbidity and a significant determinator of outcome. Nowadays, DCI is primarily used to describe a clinical deterioration of SAH patients with a typical timely delay after initial bleeding or aneurysm treatment. Critical vessel narrowing, originally referred to as vasospasm (VS), is frequently observed as the radiographic correlate of DCI potentially leading to ischemic stroke. In contrast, diagnosis of VS without a clinical correlate of DCI has been reported not to correlate with outcome [3]. While VS can be diagnosed by various imaging modalities, angiography is regarded the diagnostic gold standard to detect arterial vessel narrowing [4,5,6].
Although there was progress in understanding the pathophysiology of DCI, the frequent clinical dilemma of initializing diagnostic measures after signs of DCI or potential tissue damage has occurred remained in the absence of reliable predictive markers or surrogates for DCI or VS.
Since the 1980s, the Fisher grade has been established to appraise the risk of VS based on the extent of subarachnoid bleeding based on computed tomography (CT) imaging [7]. Including later modifications of the original score, the (modified) Fisher grade is in widespread clinical use until today, despite its limitations and inaccuracies that have been described in the literature [8, 9].
As a main shortcoming, (modified) Fisher accounts for differences in subarachnoid blood by approximation of clot diameter while emphasizing intraventricular or intracerebral extension. However, modern image processing and segmentation can provide more accurate estimations of SAH volume, which has been shown to correlate well with observation of DCI and VS [10,11,12].
Until now, the role of CSF volume as a surrogate for the development of DCI or VS remains elusive. In hypothesis, larger amounts of physiologic CSF space could trigger amounts of SAH on the one hand, but could also provide larger space for dilution of blood metabolites on the other. This could explain a decreased risk of VS in older patients and account for toxic pathogenesis for development of VS discussed in the literature [13, 14]. Before evaluating CSF as a potential surrogate in SAH, however, it is a prerequisite to rule out a correlation of CSF space and size of blood clot. Only if CSF and SAH volumes act as independent measures, CSF volume could hold additional predictive value for VS compared to Fisher grade or blood volume alone.
In this study, we performed volumetric analysis to quantify subarachnoid blood and particularly CSF in order to evaluate their impact upon the development of radiographic vasospasm in a retrospective series of aneurysmal SAH. Moreover, we sought to evaluate whether CSF quantification or the SAH/CSF ratio would allow for a more accurate prediction of VS compared to blood volume alone.
Patients and Methods
Patient Selection
Our institutional review board approved this retrospective study, and the requirement for patient informed consent was waived (S-540/2016).
For this assessment, all consecutive cases treated for aneurysmal SAH at departments of neurosurgery and neurology at an academic hospital between 2010 and 2014 were retrospectively reviewed. Eligible cases for quantitative analysis were selected according to the following criteria: age above 18 years, evidence for SAH on CT within 24 h of symptom onset, detection of an intracranial aneurysm as the cause of SAH.
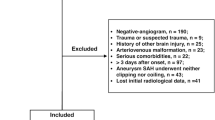
Additional conventional angiography was liberally performed at our institution for detection and treatment of VS when clinical DCI was observed. Only cases with an additional angiography during day 0–21 after ictus were included into the analysis. Patients with a preexisting VP shunt or an external ventricular drainage (EVD) prior to the initial CT scan were excluded to prevent bias for CSF quantification. See Fig. 1 for a flowchart of patient selection.
Treatment Concept in Aneurysmal SAH
During intensive care unit (ICU) stay, all patients were routinely monitored for signs of DCI including hourly clinical examination and transcranial Doppler sonography (twice per day). Any new neurologic deficit, any reduction of > 1 point in Glasgow Coma Scale (GCS), or peak systolic flow-velocities > 200 cm/s was regarded clinically significant surrogates for DCI. Invasive neuromonitoring of intracranial pressure and brain tissue oxygenation was applied on an individual basis. CT with the addition of CT perfusion was performed to corroborate suspected DCI followed by conventional angiography to detect or rule out VS as the underlying cause. Angiography was regularly performed after first detection of perfusion deficits or if no cause for neuroworsening was found in the prior diagnostic steps. Evaluation for VS was performed by neuroradiologists based on established criteria defining VS when artery diameter was reduced by > 34% [15]. Endovascular treatment for VS was applied by intra-arterial nimodipine or balloon angioplasty on an individual basis (Table 1).
CSF drainage was achieved exclusively by EVD if hydrocephalus was diagnosed on initial CT. CSF was drained continuously at 15 cm above ear level. No forced drainage was applied. All patients received oral nimodipine (6 × 60 mg/day if tolerable for blood pressure). Best medical care after detection of VS consisted of basic intensive care measures including hypertension and hemodilution.
Clinical Parameters
Clinical parameters were gathered from patient records involving age, sex, neurological status on admission according to the GCS and the modified Rankin Scale (mRS). Severity and extension of SAH were evaluated by the World Federation of Neurological Societies (WFNS) grade and the conventional Fisher grade. Outcome was assessed by GCS and mRS at discharge, by mRS at follow-up of 3–6 months after SAH and by 30-day mortality, according to availability. Furthermore, we assessed the length of ICU stay and the time when angiography was performed for evaluation of VS. Details about aneurysm side, localization, and method of repair (surgical clipping or interventional occlusion) were collected as well as measures for VS treatment after additional angiography. Additional surgical interventions for CSF diversion, permanent CSF shunting, tracheostomy, or decompressive hemicraniectomy were also assessed. Favorable outcome was considered in mRS 0–2 and unfavorable outcome in mRS 3–5.
Image Processing and Volumetric Analysis
Volumetric analysis was performed on routine clinical CT scans acquired within 24 h after onset of symptoms. Image analysis was performed using The Medical Imaging Interaction Toolkit, a free, open-source research platform for medical image analysis (www.mitk.org). We used a machine-learning-based algorithm that has been previously described for automatic segmentation of intracerebral hemorrhage, which is also capable of extracting CSF besides hemorrhage quantifications [16]. The segmentation approach was performed as a voxel-wise random forest classification task for either hemorrhage, CSF, or an undefined class. Classification was based on local descriptive gray value image features including first- and second-order statistics and texture features describing the neighborhood properties of a voxel, which produced prediction maps for the target classes. Postprocessing of prediction maps included removal of isolated voxels not assigned or different to its neighbors (remove/smooth island effect) and Gaussian smoothing of volume boundaries [16].
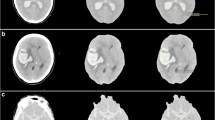
After the algorithm was applied to each respective CT scan, an initial segmentation result was proposed for the desired classes hemorrhage, CSF, and undefined voxels. All segmentations were checked for accuracy in conjunction by two authors (M.S. and J.J.) blinded to clinical data. Further manual postprocessing was performed in order to minimize misclassification and to accurately capture the amount of hemorrhage and CSF volume. In cases where SAH was associated with significant ICH, hemorrhage segmentation was subdivided into pure SAH and total blood (SAH + ICH) volumes, respectively. All volumes were read out in milliliters (ml). An example for the segmentation process is illustrated in Fig. 2.
Segmentation of SAH and CSF volume. Representative slices of unenhanced brain CT (a, b) with corresponding segmentation results (a*, b*). The intracranial compartment (red outline) was evaluated for segments corresponding to SAH (red filling) and CSF (green filling) using an automatic segmentation algorithm with subsequent manual verification and correction (Color figure online)
Statistical Analysis
Demographic data were compared using the Chi-squared test for contingency analysis. The Mann–Whitney U test was used to compare ordinal data and nonparametric SAH and CSF quantifications. Student’s t test was used to compare other continuous variables. Volumetric measures were evaluated for correlation by Pearson correlation coefficients followed by linear regression. Radiographic VS defined by critical vessel narrowing on conventional angiography was chosen as the primary outcome variable in this study. Univariate binary logistic regression analysis was calculated to evaluate variables of VS. Volumetric measures were included as continuous variables. Additionally, the Boruta algorithm implemented in “R” was used for identification of important variables for VS. Boruta compares the importance of real variables with the importance of random features created from the same data set (shadow features) by statistical testing in several runs of a random forest and enables a stable and unbiased selection of important from unimportant variables from an information system in an iterative approach [17]. Receiver operating characteristic (ROC) curves and area under the curve (AUC) analysis were performed to compare the value of different volumetric measures to predict the primary outcome according to their sensitivity and specificity. Youden’s index (sensitivity + specificity − 1) was used to identify appropriate cutoff values of volumetric measures for outcome prediction [18]. p values < 0.05 were regarded statistically significant. Statistical analysis was performed using SPSS v24, GraphPad Prism v5 and R (“Boruta” package).
Results
Demographics
A total of 125 cases were included into volumetric analysis. Median age was 57 years (range 19–85 years) with a 3:1 preponderance of females. On admission, median WFNS grade was 3 suggesting intermediate affected SAH patients (interquartile range [IQR] 2–4). Median Fisher grade was 4, while no patient was Fisher grade 1, according to the inclusion criteria. The anterior and middle cerebral arteries were the predominant aneurysm locations in 57.6% of cases. Aneurysm repair was achieved by surgical clipping in 54 cases (42%) and by interventional coiling in 71 cases (58%).
Median stay on ICU was 21 days reflecting the standard treatment regimen at our center keeping SAH patients under close surveillance during the first 3 weeks after SAH. Additional angiographic examination showed vasospasm (VS+) in 104 cases in this study (83%). No vasospasm was found despite suspected clinical DCI in 21 cases (17%) (VS−). VS was diagnosed on median day 8 after SAH. Demographic data are summarized in Table 1.
Treatment Details
Among VS+ and VS− subgroups, no statistically significant differences were found for sex, age, and different modalities for aneurysm repair. VS+ cases presented with higher median WFNS scores compared to VS− cases, respectively (3, IQR 2–4 vs. 3, IQR 1–4, p= 0.049). Median Fisher grade failed to stratify subgroups for their risk of VS+ versus VS− (4, IQR 3–4 vs. 4 IQR 3–4, p= 0.49), respectively. The VS+ group was more clinically effected requiring significantly longer ICU stay (p= 0.0003), more extensive interventional VS treatment (p < 0.0001), and more frequent temporary (p= 0.018) or permanent (p= 0.0014) CSF diversion and tracheostomy (p= 0.003) (see Table 1 for details).
Outcome Assessment
In our cohort that included patients showing clinical signs of DCI, new cerebral infarctions were significantly more frequent in VS+ compared to VS− cases (59% vs. 33% p= 0.03). At discharge, median GCS (9 vs. 13, p= 0.021) and mRS (5 vs. 4, p= 0.043) indicated significantly worse outcome in VS+ compared to VS− cases. At discharge, favorable outcome was observed significantly more often in VS− (29%) compared to VS+ (12%, p= 0.04). Further follow-up was available for a subset of 93 cases (74.4%) at median of 3 months after SAH (IQR 3–6 m, range 1–12 m). During follow-up, both subgroups showed an improvement in median mRS (VS+: 4 and VS−: 2), reflecting persistent severe disability in VS+ cases. A 30-d mortality was comparable in both subgroups (p= 0.34) (Table 1).
Volumetric Analysis
Volumetric analysis showed mean SAH volume of 24.4 ± 20.6 ml and mean total blood of 33.1 ± 30.6 ml when including associated ICH which was found in 24/125 cases (19%). Quantification of CSF on initial CT in the absence of any CSF diversion was a focus of this study. We found mean CSF volume of 66.8 ± 38.9 ml. The mean SAH/CSF ratio was 50.5% in this study but showed a wide range (0.1–541%), which illustrates a heterogeneous association of both measures after aneurysmal SAH.
While Fisher grade was comparable in VS+ and VS− cases (median grade 4 vs. 4, p= 0.49), we found significantly larger mean amounts of SAH (26.8 ± 21.1 ml vs. 12.6 ± 12.2 ml, p= 0.001) and total blood volume (35.9 ± 31.2 ml vs. 19.2 ± 22.8 ml, p= 0.002) in VS+ versus VS− cases, respectively. On the contrary, CSF volume was significantly lower in VS+ compared to VS− cases (63.0 ± 31.2 ml vs. 85.7 ± 62.8 ml, p= 0.03). SAH/CSF ratio was significantly higher in VS+ cases (55.8 ± 67.4 ml vs. 24.5 ± 34.2, p= 0.02). See Table 2 and Fig. 3 for details.
Volumetric analysis. Box plots of volumetric analysis for a subarachnoid hemorrhage volume, b total blood volume (including intraparenchymal blood clots, if applicable), c cerebrospinal fluid (CSF) volume, d SAH/CSF ratio in patients with vasospasm (VS+) and without vasospasm (VS−). Volumes are indicated in ml. p values according to Mann–Whitney U test
Correlation of Volumetric Measures
As a core finding, neither SAH (Pearson r = − 0.05, p= 0.58) nor total blood volume (Pearson r = − 0.18, p= 0.051) was significantly correlated to CSF volume. Furthermore, linear regression was inconclusive for SAH and CSF (β = − 0.03 95% CI − 0.12 to 0.07, r2 = 0.002) as well as for total blood volume and CSF (β = − 0.14 95% CI − 0.27 to 0.001, r2 = 0.031). This indicated that blood and CSF quantifications were independent parameters on initial CT supporting our hypothesis that CSF could serve as a surrogate parameter to predict VS after aneurysmal SAH.
Fisher grade had a positive correlation with total blood volume (i.e., SAH + ICH) (Pearson r = 0.20, p= 0.02) but was not significantly correlated with SAH volume per se (Pearson r = 0.073, p= 0.42). This reflects a known shortcoming of the Fisher score with constraints in stratifying SAH cases without ICH or ventricular hemorrhage for VS risk.
Moreover, we found that age was positively correlated with SAH (Pearson r = 0.28, p= 0.001) and CSF (Pearson r = 36, p < 0.0001) suggesting a larger total subarachnoid space but also larger SAH clots in older patients. WFNS grade was negatively associated with CSF (Pearson r = − 0.30, p= 0.001) illustrating less CSF present in poor-grade SAH cases. See Table 3 for detailed correlation analysis.
Variables Associated with the Development of Vasospasm
In univariate binary logistic regression for variables of VS, only volumetric parameters and Fisher grade had a significant association in this study (Table 4). Age, sex, and aneurysm treatment had no influence on the development of VS. Aneurysm location was not significantly associated with VS per se. Anterior cerebral artery aneurysms, however, had a significantly higher risk of VS compared to all other vascular territories in our cohort. Regarding volumetric measures, higher volumes of SAH (HR 1.06, p= 0.006) and total blood (HR 1.03, p= 0.02) significantly increased, while higher CSF volumes (HR 0.99, p= 0.02) decreased the risk of VS, respectively. SAH/CSF ratio was identified as a significant risk factor for VS (HR 1.03, p= 0.015) (Table 4).
Since options for multivariate regression were limited considering the small number of VS− cases in our cohort, we used the Boruta approach to identify important variables under consideration of potential interactive variables. Iterative Boruta runs consecutively rejected age, WFNS grade, EVD placement, Fisher grade, sex, and aneurysm treatment as important variables. Aneurysm location was classified as potentially important, while all volumetric measures were confirmed as important variables for VS, respectively (Fig. 4).
Boruta analysis of variables for vasospasm. Selection of variables correlating to vasospasm according to their importance (z-score). Green columns indicate “important” variables, yellow columns indicate potentially important variables, red columns indicate “rejected” variables. Blue columns represent “shadow attributes” created as random features from the same data set to allow for comparison. CSF cerebrospinal fluid, EVD placement of extraventricular CSF drainage, SAH subarachnoid hemorrhage (Color figure online)
Value of Volumetric Measures as Outcome Predictors
ROC curves were used to compare the discriminative capacity of different volumetric measures for VS according to their respective sensitivity and specificity (Fig. 5). The value of SAH volume was confirmed by an AUC of 0.73 (95% CI 0.61–0.85, p= 0.001). Comparably, the SAH/CSF ratio achieved an AUC of 0.74 (95% CI 0.63–0.86, p < 0.0001). Even though regression and Boruta analysis suggested a significant association with VS for CSF, AUC was inconclusive at 0.56 (95% CI 0.40–0.72, p= 0.39) (Fig. 5). For Fisher grade, area under ROC curve was 0.50 (95% CI 0.36–0.65, p= 0.97).
Identification of Cutoffs for Clinical Decision Guidance
Youden’s index was used to identify cutoff values from volumetric measures [18]. Youden’s index peaked at 39.6% for 19.80 ml SAH suggesting a clinically suitable cutoff at 20 ml SAH. For CSF, the index peaked at 24.7% for 121.9 ml suggesting a cutoff at 120 ml CSF. Youden’s was 38.8% for a SAH/CSF ratio of 21.2% suggesting a cutoff at 20% SAH/CSF ratio.
Table 5 shows test statistics for the selected cutoffs with regard to VS. For SAH, the 20 ml cutoff could serve as a confirmatory test given its high specificity but had limited value for screening. Cases with CSF ≥ 120 ml appeared to suffer from VS less frequently, which was pointed out with high sensitivity. However, 60% negative predictive value (NPV) illustrates that 4 out of 10 cases still developed VS despite high values of CSF. However, combined use of both volumetric surrogates illustrated potential benefits for prediction of VS: The 20% cutoff for SAH/CSF ratio improved sensitivity compared to SAH volume alone. Particularly by sequenced use of CSF followed by SAH cutoff values illustrated in Fig. 6, all cases with beneficial prognosticators (CSF ≥ 120 ml, SAH < 20 ml) were identified as VS− (6/6, 100%). Cases with adverse markers (CSF < 120 ml, SAH ≥ 20 ml) were identified as VS+ with 94% accuracy (50/53).
Discussion
In this volumetric analysis, we evaluated the role of subarachnoid blood and particularly CSF for risk assessment to develop radiographic VS in a retrospective series of aneurysmal SAH of 125 cases. SAH and CSF volumes were volumetrically quantified, and their association with VS was statistically analyzed. ROC curves were used to define and evaluate cutoffs with regard to their accuracy to stratify patients for their risk of VS.
For the first time in the literature, we describe the association of CSF with development of VS, while SAH volume and total blood volume were confirmed as known predictors, which has been the cornerstone for the Fisher grade for many years [7]. Moreover, we illustrate that CSF and SAH volumes act as independent measures which triggered the evaluation of CSF as an additional clinical surrogate parameter for VS in this study.
Quantifications of Intracranial Volumes
Quantifications of SAH and total blood volume in our retrospective sample both showed a significant association to our primary outcome parameter radiographic VS, which supports our cohort to be representative for aneurysmal SAH cases. In the literature, multiple approaches have been sought in order to improve predictive value of the Fisher grade or to individualize VS risk by using scores incorporating clot volume and additional clinical data [8, 9, 19, 20]. We observed a correlation for total blood volume but not pure SAH volume with traditional Fisher grade (Table 3), which reflects a common shortcoming of the traditional Fisher grade regarding its discrimination of cases with less extended SAH.
Our approach was to evaluate the role of CSF volume in this regard. As a prerequisite for CSF to act as an independent surrogate for VS, it was important to exclude an association for CSF with SAH volume. This would otherwise render CSF similar to a negative image of SAH volume within a fixed subarachnoid space with limited additional value for VS prediction.
In this regard, we could neither find a correlation for SAH and CSF (Table 3), nor could CSF amount be derived from SAH volumes via linear regression. This underscores that both variables are to be regarded independently rather than interchangeably, and in other words, an increase in one does not implicate a decrease in the other volume, respectively.
This proposed CSF as a new surrogate and harbors potential synergistic effects for VS prediction when information of SAH and CSF variables is combined.
Impact of Volumetric Measures as Variables of Radiographic VS
We composed this study to deliver a proof of concept for the association of CSF with VS and performed binary regression analysis to evaluate the impact of volumetric measures in comparison with other clinical parameters. In regression analysis, we identified CSF as a protective variable (HR 0.99) implicating a decreasing risk of VS with increasing CSF. Increasing SAH (HR 1.06) and total blood volumes (HR 1.03) were confirmed as known risk factors [20,21,22].
Among clinical variables, only Fisher grade and aneurysm location had noticeable associations with VS (Table 4) as expected from comparable literature [21, 23]. Accordingly, regression for sex, age, or modality of aneurysm repair was inconclusive with regard to risk of VS [4, 24].
Since the limited number of VS− cases in our cohort precluded proper multivariate regression analysis, we chose the established Boruta algorithm to evaluate variables under consideration of their respective interaction. Boruta is a machine-learning-based algorithm assessing features for their importance regarding a chosen outcome. During multiple iterations, underperforming features are recursively excluded to yield a minimal optimal variable subset that holds the strongest impact on outcome [17, 25]. Boruta analysis (Fig. 4) illustrates a superior importance of volumetric measures, including CSF, for development of VS and precludes that volumetric measures are under influence of relevant interaction by the clinical variables analyzed.
In particular, we found a significant association with VS when SAH and CSF were combined in their respective SAH/CSF ratio (HR 1.03). This new information could help to stratify subgroups of patients suffering from comparable clot sizes: Younger patients with less CSF (i.e., narrow sulci and ventricles) would exhibit high SAH/CSF ratios corresponding to higher VS risk, while older patients with an enlarged CSF system could be stratified for lower VS risk according to their respective lower SAH/CSF ratio [6, 24].
However, VS has been associated with acute hydrocephalus and ventricular enlargement in the literature, which appears contradictory to our findings [24]. In fact, VS+ cases were significantly more likely to receive CSF drainage as a surrogate for hydrocephalus in our series (Table 1), while VS− cases exhibited larger absolute CSF amounts. We assume differences CSF distribution with variation of extraventricular and cisternal CSF space as a likely explanation for this finding. However, separate evaluation of intra- and extraventricular CSF was not yet feasible with our segmentation methodology to further support this hypothesis.
Clinical Decision Guidance Using Volumetric Measures
While traditional or modified Fisher grades are frequently used tools in clinical practice and research, superior discriminative capacity for quantifications of SAH volume has been illustrated in the literature using ROC analysis. We replicated these findings reflected by AUC for SAH volume (0.73), while the Fisher grade (AUC 0.50) had limited predictive value in our study [10, 12, 22]. Moreover, our 20 ml cutoff for SAH to identify high-risk patients was in strong accordance with literature suggesting SAH volumes of 15–20 ml for stratification of patients for DCI or 30-d mortality [12, 26].
When evaluating ROC curves for CSF, however, discriminative capacity was inconclusive (AUC 0.56) most likely due to the broad range of observed CSF volumes (range 7–232 ml) and the heterogeneity of CSF volumes among VS+ and VS− subgroups. Nevertheless, the vast majority of VS+ cases had CSF volumes below 120 ml suggesting a potential cutoff for screening (Table 5). Initially, this cutoff appeared markedly high, considering that total CSF amount has traditionally been estimated between 90 and 150 ml. However, recent imaging studies quantified significantly larger mean CSF volumes in normal volunteers (180–264 ml), hydrocephalus, or neurodegenerative and psychiatric diseases, where volumes exceeding 400 ml have been reported [27,28,29].
In our evaluation, we could illustrate the potential of combining SAH and CSF thresholds for prediction of VS. For SAH/CSF ratio, putting clot burden in relation to spare CSF was able to improve sensitivity with acceptable specificity in comparison with using SAH volume alone (Table 5). Particularly, sequenced use of CSF followed by SAH cutoffs, bringing together high sensitivity from CSF and specificity from SAH (Table 5), allowed for improved stratification for VS risk in our cohort (Fig. 6).
After identifying CSF as a potential surrogate for VS, these findings support our proof of concept and illustrate how this could translate into more accurate identification of patients at risk of VS in clinical practice.
As a main drawback to our findings, the required elaborate image processing precludes on-time volumetric analysis in clinical practice to date. This makes volumetric associations or cutoffs difficult to conceive in practice particularly since quick estimation tools, comparable to the Broderick method for ICH, are absent for SAH or CSF [30]. However, algorithm-based, automatic image segmentation known from glioma surveillance will offer real-time volumetric data for clinical decision support also in SAH in the near future [31]. The evaluation of SAH/CSF ratio and our illustration of sequenced use of SAH and CSF cutoffs reflect our effort to underscore the potential and utility of new volumetric outcome surrogates that will be provided by technical advancements.
Limitations
Our study has several limitations that are related to its retrospective nature. First, we report results from a selected cohort of patients with aneurysmal SAH. Since a proof of concept for the role of CSF volume in SAH was the aim of this study, we chose radiographic VS as a robust primary outcome parameter for statistical analysis. Consequently, all cases in this study had signs of clinical DCI which triggered additional angiography during initial treatment. However, this decision was not controlled for in our retrospective setting, which implies a negative selection of patients regarding their initial SAH grade, observation of new cerebral infractions, and overall outcome leading to imbalance of subgroups. Considering the total number of screened cases, however (n= 320), inclusion of n= 125 cases that were examined for VS (39%) corresponds with the expected frequency of DCI in aneurysmal SAH [20,21,22].
Further studies are necessary to evaluate the role of CSF beyond this selected cohort of patients in SAH in general. This will show whether the association for CSF described in this proof-of-concept study holds true also in clinical deteriorations not related to critical vessel narrowing and in comparison with cases not suffering from DCI [32].
Moreover, application of CSF drainage was not controlled in our retrospective study. Hence, bias on the development of VS by alteration of CSF volume cannot be precluded, even though EVD placement had no relevant effect on VS in Boruta analysis [33]. All CSF measurements were taken prior to application of drainage, however.
Comprehensive outcome assessment was not available for all patients in this study and follow-up periods were heterogeneous, which limited detailed outcome analysis. So far, mRS at discharge and follow-up expectedly shows worse outcome in VS+ cases, but the effect of CSF on outcome could not be analyzed in further detail. Since CSF volume was significantly correlated with WFNS grade, however, this implies a potential association also with outcome. The role of volumetric measures and particularly CSF for outcome prediction has to be evaluated in future studies.
Summary and Conclusion
Quantification of SAH and CSF can provide useful information for decision guidance in initial treatment of aneurysmal SAH and might exhibit advantages over traditional scores. While the association of SAH volume with VS was corroborated, we identified CSF volume as a supplementary surrogate parameter. Higher amounts of CSF might indicate a reduced risk of development of VS particularly when exceeding 120 ml on initial CT after ictus. Moreover, the combination of volumetric values in the SAH/CSF ratio and sequenced stratification by CSF followed by SAH cutoffs could improve prediction accuracy for VS in clinical practice.
References
Rabinstein AA, Lanzino G, Wijdicks EFM. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2010;9:504–19.
Rinkel GJE, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349–56.
Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Defining vasospasm after subarachnoid hemorrhage. Stroke. 2009;40:1963–8.
Harrod CG, Bendok BR, Batjer HH. Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage: a review. Neurosurgery. 2005;56:633–54.
Le Roux PD, Elliott JP, Eskridge JM, Cohen W, Winn HR. Risks and benefits of diagnostic angiography after aneurysm surgery: a retrospective analysis of 597 studies. Neurosurgery. 1998;42:1245–8.
Li K, Barras CD, Chandra RV, Kok HK, Maingard JT, Carter NS, et al. A review of the management of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;126:513–27.
Fisher CM, Kistler JP, Davis JM, et al. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9.
Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2001;32:2012–21.
Kramer AH, Hehir M, Nathan B, Gress D, Dumont AS, Kassell NF, et al. A comparison of 3 radiographic scales for the prediction of delayed ischemia and prognosis following subarachnoid hemorrhage. J Neurosurg. 2008;109:199–207.
Jiménez-Roldán L, Alén JF, Gómez PA, Lobato RD, Ramos A, Munarriz PM, et al. Volumetric analysis of subarachnoid hemorrhage: assessment of the reliability of two computerized methods and their comparison with other radiographic scales. J Neurosurg. 2012;118:84–93.
Zijlstra IA, Gathier CS, Boers AM, Marquering HA, Slooter AJ, Velthuis BK, et al. Association of automatically quantified total blood volume after aneurysmal subarachnoid hemorrhage with delayed cerebral ischemia. Am J Neuroradiol. 2016;37:1588–93.
Ko S-BB, Choi HA, Carpenter AM, Helbok R, Schmidt JM, Badjatia N, et al. Quantitative analysis of hemorrhage volume for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2011;42:669–74.
Torbey MT, Hauser TK, Bhardwaj A, Williams MA, Ulatowski JA, Mirski MA, et al. Effect of age on cerebral blood flow velocity and incidence of vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2001;32:2005–11.
Weir B, Macdonald RL, Stoodley M. Etiology of cerebral vasospasm. Acta Neurochir Suppl. 1999;72:27–46.
Fujimura M, Joo J-Y, Kim J-S, Hatta M, Yokoyama Y, Tominaga T. Preventive effect of clazosentan against cerebral vasospasm after clipping surgery for aneurysmal subarachnoid hemorrhage in Japanese and Korean patients. Cerebrovasc Dis. 2017;44:59–67.
Scherer M, Cordes J, Younsi A, Sahin Y-A, Götz M, Möhlenbruch M, et al. Development and validation of an automatic segmentation algorithm for quantification of intracerebral hemorrhage. Stroke. 2016;47:2776–82.
Kursa MB, Rudnicki WR. Feature selection with the Boruta package. J Stat Softw. 2010;36:1–3.
Reibnegger G, Schrabmair W. Optimum binary cut-off threshold of a diagnostic test: comparison of different methods using Monte Carlo technique. BMC Med Inform Decis Mak. 2014;14:99.
Kistler JP, Crowell RM, Davis KR, Heros R, Ojemann RG, Zervas T, et al. The relation of cembral vasospasrn to the extent and location of subarachnoid blood visualized by CT scan: a prospective study. Neurology. 1983;33:424.
Lee H, Perry JJ, English SW, Alkherayf F, Joseph J, Nobile S, et al. Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019;130:1914–21.
de Oliveira Manoel AL, Jaja BN, Germans MR, Yan H, Qian W, Kouzmina E, et al. The VASOGRADE. Stroke. 2015;46:1826–31.
de Rooij NK, Greving JP, Rinkel GJE, Frijns CJM. Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2013;44:1288–94.
Abla AA, Wilson DA, Williamson RW, Nakaji P, McDougall CG, Zabramski JM, et al. The relationship between ruptured aneurysm location, subarachnoid hemorrhage clot thickness, and incidence of radiographic or symptomatic vasospasm in patients enrolled in a prospective randomized controlled trial. J Neurosurg. 2014;120:391–7.
Inagawa T. Risk factors for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a review of the literature. World Neurosurg. 2016;85:56–76.
Kursa MB. Robustness of Random Forest-based gene selection methods. BMC Bioinform. 2014;15:8.
Friedman JA, Goerss SJ, Meyer FB, Piepgras DG, Pichelmann MA, McIver JI, et al. Volumetric quantification of Fisher Grade 3 aneurysmal subarachnoid hemorrhage: a novel method to predict symptomatic vasospasm on admission computerized tomography scans. J Neurosurg. 2002;97:401–7.
Hodel J, Lebret A, Petit E, Leclerc X, Zins M, Vignaud A, et al. Imaging of the entire cerebrospinal fluid volume with a multistation 3D SPACE MR sequence: feasibility study in patients with hydrocephalus. Eur Radiol. 2013;23:1450–8.
Yamada S, Ishikawa M, Yamamoto K. Comparison of CSF distribution between idiopathic normal pressure hydrocephalus and Alzheimer’s disease. AJNR Am J Neuroradiol. 2016;37:1249–55.
Alperin N, Bagci AM, Lee SH, Lam BL. Automated quantitation of spinal CSF volume and measurement of craniospinal CSF redistribution following lumbar withdrawal in idiopathic intracranial hypertension. Am J Neuroradiol. 2016;37:1957–63.
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93.
Kickingereder P, Isensee F, Tursunova I, Petersen J, Neuberger U, Bonekamp D, et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 2019;20:728–40.
Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2014;85:1343–53.
Reilly C, Amidei C, Tolentino J, Jahromi BS, Macdonald RL, Health P. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:255–61.
Acknowledgements
We thank Dr. Tom Bruckner for statistic counseling.
Funding
All authors declare that there is no financial support to disclose.
Author information
Authors and Affiliations
Contributions
MS contributed in Design of Study, Data Collection, Data Analysis and Writing of Paper. JOJ contributed in Data Collection and Data Analysis. JC contributed in Data Collection and Image Analysis. LW contributed in Data Collection and Writing of Paper. AY, SS, MAM, KMH, AU contributed in Data Analysis and Writing of Paper. KZ contributed in Design of Study, Data Analysis and Writing of Paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest to disclose.
Ethical approval
Ethics approval was granted by the insitutional review board of Heidelberg University (ref. S-540/2016).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scherer, M., Jung, JO., Cordes, J. et al. Association of Cerebrospinal Fluid Volume with Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage: A Retrospective Volumetric Analysis. Neurocrit Care 33, 152–164 (2020). https://doi.org/10.1007/s12028-019-00878-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00878-2