Abstract
Background/Objective
Posterior reversible encephalopathy syndrome (PRES) is a clinical and radiologic entity, typically manifesting as reversible neurological symptoms and signs of white matter edema on magnetic resonance imaging. PRES has been widely described in adults. Studies of PRES in children are mostly limited to case series and case controls.
Methods
Retrospective chart review of patients under 21 years with PRES admitted at a tertiary children’s hospital from 2011 to 2016. They were compared to controls matched for age and mortality risk using the Pediatric Index of Mortality-2 score.
Results
Sixteen cases of PRES were identified in 13 patients (ages 5–17 years, 46% male). PRES presented with altered mental status (75%), seizures (77%), headache (31%), and vision changes (23%). In patients who recovered (n = 11), median days to symptom resolution was three (range 1–8). PRES patients had a higher mortality rate (15% vs. 5%, p < 0.05) and higher mean length of stay (13.1 vs. 4.6 days) and were more likely to have autoimmune disease (p < 0.05), immunosuppression (p < 0.05), and anemia (p < 0.05). No PRES patients were diagnosed with epilepsy by last known follow-up, and all of whom had been started on an antiepileptic drug were discontinued within 13 months. Sepsis was suspected in 53% of PRES patients and 59% of controls (p = 1.00). All PRES patients had stage II hypertension, versus 41% of controls (p < 0.05). Average creatinine in PRES was 2.35 mg/dL compared to 0.90 mg/dL in controls (p < 0.05). PRES patients had lower serum calcium (p < 0.05). After correcting for albumin, no association between PRES and hypocalcemia remained. PRES patients had a higher length of stay (13.1 vs. 4.6 days, p < 0.05) and mortality rate (15% vs. 3%, p < 0.05).
Conclusions
Immunosuppression, autoimmune disease, renal insufficiency, anemia, and hypertension are associated with PRES after controlling for mortality risk in critically ill children. There was no association between corrected serum calcium and sepsis with PRES.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
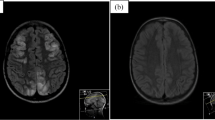
Posterior reversible encephalopathy syndrome (PRES) was first described in 1996 as a clinical and radiologic diagnosis infrequently encountered in the pediatric population [1]. Since the first description, its incidence has increased, although the exact global incidence in both pediatric and adult populations remains unknown. Although termed “posterior,” imaging abnormalities can occur in any brain region [2]. Radiographic correlates are best seen as white matter hyperintensities on T2 and fluid-attenuated inversion recovery magnetic resonance imaging (MRI) sequences [1]. The disease classically presents with a combination of altered mental status (AMS), seizures, headache, and visual disturbances and is often precipitated by a hypertensive crisis. Both clinical and radiographic findings are typically, but not always, reversible. Postulated pathophysiologic mechanisms include the development of vasogenic edema secondary to disordered cerebral autoregulation and endothelial injury [1, 3,4,5,6,7,8]. Most cases of PRES are isolated; however, there are case reports of both adult and pediatric patients with recurrences of PRES [9,10,11,12].
The disease typically occurs in patients with renal disease, autoimmune disorders, leukemia, and other malignancies. PRES can be precipitated by chemotherapy, hematopoietic stem cell transplant, or immunosuppressive agents, most commonly cyclosporine and tacrolimus [4, 10, 13,14,15,16,17]. In addition to the aforementioned conditions, PRES has been described in the setting of severe sepsis in the absence of hypertensive crisis in adults [18]. This finding has not been replicated in children.
Although PRES has been widely described in the adult population, the pediatric literature is less robust. A recent comparative study between children and adults found that affected children were more likely to have multi-organ failure than affected adults [19]. A second study found that hypertension, cytotoxic drug use, and anemia were independently associated with PRES in ten children being treated in an intensive care setting [20]. These patients, however, were compared to all other pediatric intensive care unit (PICU) patients without controlling for disease severity.
In this study, we performed a retrospective chart review for patients ages 21 and under who had MRI findings suggestive of PRES and whose medical record supported this diagnosis. We compared these patients to PICU patients matched for age and risk of mortality to determine if these and other characteristics are correlated with the development of PRES [21].
Materials and Methods
This is a retrospective-matched case-control study of patients treated at Cohen Children’s Medical Center of New York between August 2011 and April 2016. The study was approved by the Northwell Health System Institutional Review Board and granted a waiver of informed consent.
Sixteen cases of PRES were identified in 13 patients over a five-year period. Cases were found by searching all MRI reports in the study period for “PRES,” “posterior reversible encephalopathy syndrome,” and “posterior reversible leukoencephalopathy.” Review of the MRIs by an independent pediatric neuroradiologist confirmed findings consistent with PRES and identified affected neurologic regions. Review of magnetic resonance venogram (MRV) data and any follow-up MRI was also performed, when available. We excluded cases where the diagnosis was suspected but not confirmed. We selected three control patients for each PRES patient, matched for both age and risk of mortality at admission based on the Pediatric Index of Mortality-2 (PIM-2) score [21]. We accepted a PIM-2 score within ten percent as acceptable matching. We then reviewed the medical records for clinical and laboratory data for patients diagnosed with PRES and a total of 39 matched controls. We excluded scheduled admissions from our control group. Demographic data collected included age, race, and PIM-2 score. The two highest consecutive blood pressures (BP) in the 48 h prior to the development of PRES were collected. We further classified BP into the absence of hypertension, stage I hypertension (BP >=95th percentile), or stage II hypertension (BP > 99th percentile plus 5 mmHg) based on age-related percentiles per CDC guidelines. These definitions of hypertension have been accepted by the American Academy of Pediatrics and the American Society of Pediatric Nephrology [22]. Collected laboratory values included highest and lowest serum sodium, glucose, calcium, magnesium, and platelet count during the admission. We also collected the highest serum creatinine, blood urea nitrogen, and white blood cell count, as well as serum albumin within 24 h of lowest calcium value. Clinical data collected included mortality, length of PICU stay, comorbidities, clinical suspicion of sepsis, which we defined as whether bacterial cultures were sent, and any identified infectious agents. For PRES patients, the presence of AMS, seizures, headache, visual changes, future development of epilepsy, cause of death, and duration from PRES onset to death (when applicable), and whether or not there was a recurrence of PRES were also collected. We evaluated functional status at admission and discharge for PRES patients using the validated Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scales [23]. Antiepileptic drug use, agent, and duration of therapy were reported when information was available.
Statistical Analysis
For each factor, exact conditional logistic regression was used to examine the association between PRES and that factor. Due to the small sample size, only univariate analyses were carried out. For hypertension, it was not possible to obtain stable estimates using conditional logistic regression, due to the pattern of the data. Therefore, the Cochran–Mantel–Haenszel test was used. For all calculations, differences were considered statistically significant at a two-tailed p < 0.05 level. No adjustment for multiple comparisons was made, due to the exploratory nature of the study. All data analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Sixteen cases of PRES were identified in 13 patients (ages three to 17 years, 46% male). There was no difference in gender or race between the PRES and control groups. Baseline characteristics of PRES and control patients can be found in Table 1. All comparisons between patients with PRES and their matched controls can be found in Table 2. Mean PICU length of stay was higher in PRES patients (13.1 vs. 4.6 days, p = 0.017). Patients with PRES were more likely to have autoimmune disease (p = 0.03), anemia (p = 0.0002), or be on immunosuppressive therapy or otherwise immunocompromised (p = 0.002). Sepsis was suspected in 53% of PRES patients and 59% of controls (p = 1.00). There was no difference in rates of malignancy between PRES and control patients.
For the first admission, PRES presented with seizures (77%), altered mental status (75%), headache (31%), and vision changes (23%). Two PRES patients (15%) died while hospitalized as compared with one (3%) control (p < 0.05). In the PRES patients discharged alive (n = 11), median days to symptom resolution was three (range 1–8). One patient with class IV lupus nephritis had two recurrences of PRES within 1 year. POPC and PCPC scores were unchanged from admission to discharge for all but one surviving PRES patient; patient 12 had a POPC of 2 on admission and 3 at discharge and an unchanged PCPC. Seven of the eleven PRES patients were discharged on an antiepileptic drug (AED); six were treated with levetiracetam (dosing ranged from 20–30 mg/kg/day) and one was treated with phenytoin 5 mg/kg/day. Duration of AED therapy ranged from 3 to 25 months. Surviving PRES patients were followed for median of 3.0 years (range 17 days–8.4 years). No patients developed epilepsy at last known follow-up. See Table 3 for clinical features of PRES patients. Affected brain regions, MRV results, and follow-up MRI data (when available) are noted in Table 4.
Patients with PRES were more likely than matched controls to have a higher systolic (150 mmHg vs. 128 mmHg, p = 0.0002), diastolic (96 mmHg vs. 74 mmHg, p < 0.001), and mean arterial BP (114 mmHg vs. 91 mmHg, p < 0.0001) during their admission. All PRES patients had stage II hypertension, whereas 41% of controls had stage II hypertension, and 28.2% had stage 1 (p = 0.002). Average serum creatinine in PRES was 2.35 compared to 0.90 in controls (p = 0.04), indicating higher rate of renal disease in PRES patients. Patients with PRES had lower serum calcium as compared to controls (p = 0.019); however, after correcting for serum albumin, no association between PRES and hypocalcemia was found. There was no association between alterations in serum sodium, glucose, or magnesium, and PRES. There was no difference in the incidence of leukocytosis, thrombocytopenia, or thrombocytosis between the two groups. PRES patients had a higher mortality rate (15% vs. 3%, p < 0.05). The two mortalities in the PRES cohort were secondary to viral sepsis, pulmonary hemorrhage, and withdrawal of care in a patient status post-bone marrow transplant (patient 3) and viral sepsis, renal failure and withdrawal of care in a patient with medulloblastoma (patient 4).
Discussion
PRES was first described in a case series over 20 years ago, yet implications of the disease in children have not yet been fully elucidated [1]. Our study identifies clinical characteristics and outcomes of children with PRES. This design is unique in that PRES patients are matched to controls by risk of mortality at ICU admission. Previous studies that have compared patients with and without PRES did not control for disease severity or risk of mortality. As we know PRES patients typically have significant comorbidities, matching for risk of mortality may help differentiate which factors are specifically related to PRES and not simply secondary to the critically ill state.
Many explanations for the pathogenesis of PRES have been described; however, its exact origin remains unknown. It is believed that some form of cerebral vasculature endothelial cell dysfunction occurs in the setting of a chronic insult, such as hypertension or the use of cytotoxic agents. This leads to the development of cerebral vasogenic edema and results in the constellation of symptoms known as PRES [24]. PRES typically presents with a combination of seizures, altered mental status, visual disturbances, and headaches. Seizures are almost universally generalized tonic–clonic in nature, although other types, such as focal seizures, have been reported [25].
Manifestations of PRES in our population were similar to those described in other studies, including alterations in mental status, seizures, headaches, and vision changes [2, 3, 20]. Symptom resolution occurred at a median of 3 days after onset in the 85% of patients who survived, which is consistent with previously published data. Half of the patients in our cohort were managed with an AED at hospital discharge. In all cases, the AED was discontinued at last known follow-up. None of our patients went on to develop epilepsy. Additionally, PRES patients had a 15% mortality rate as compared to a three percent mortality rate in the control group, who had an equal risk of mortality on PICU admission. This is higher than reported in other studies, possibly secondary to our low sample size. The cause of death in both cases was due to withdrawal of care in the setting of multi-system organ failure and was temporally distant from the development of PRES. Still, this high mortality rate is interesting given that controls were matched for risk of mortality on PICU admission.
A recent retrospective study by Raj et al. looked at the relationship between hypertension, anemia, and PRES. These authors found hypertension and anemia to be associated with the development of PRES [20]. Ten patients with PRES were compared with all other PICU patients in the same time period. A limitation with this design, where patients were not matched with those of similar illness severity or risk of mortality, could allow for patient differences that are confounded by illness severity rather than the studied disease process. Our study design differs in that we used a control cohort matched for age and risk of mortality. With this design change, we replicated some of the findings of Raj et al. Both anemia and stage II hypertension continued to be independently associated with PRES. Additionally, PRES patients in our study had a higher incidence of pharmacologic immunosuppression and autoimmune disease than our control cohort. In contrast to their study, we found renal disease to be more prevalent in patients with PRES. This could provide an explanation for the association between anemia and PRES, as anemia is a consequence of long-standing renal impairment.
Additionally, we examined the relationship between PRES and electrolyte disturbances. No association was found with serum sodium, glucose, or magnesium. We did, however, find an association with hypocalcemia and PRES. As many of the diseases known to be associated with PRES, including renal disease and malignancies, can be associated with hypoalbuminemia, we felt it was important to examine if this association remained after correcting serum calcium for serum albumin. After correcting for albumin, there was no longer an association between corrected calcium levels and PRES.
In adults, PRES occurs in the settings of hypertension, renal disease, dialysis, malignancy, autoimmune diseases, eclampsia, and organ transplantation [26, 27] and is self-limiting in 35–100% of cases [1, 28]. When comparing our findings to the adult literature, there are some key differences. Bartynski et al. described vessel irregularity consistent with vasospasm in their hypertensive and septic adult patients with PRES. Approximately, half of our PRES cohort had MRV data at diagnosis, all of which were within normal limits with the exception of a filling defect in the MRI of one patient that is thought to be congenital. In further contrast to the adult literature, we did not find an association between suspected infection and the onset of PRES. A lack of difference in white blood cell counts between patients with and without PRES also supports this finding. We found no differences in rates of malignancy and transplantation between the two groups, while adults with PRES had higher rates of both. Similar to findings in adult studies, we found that patients with PRES are more likely to carry the diagnosis of an autoimmune disease.
Two patients included in our study had recurrent episodes of PRES. There are multiple case reports of recurrence of PRES in the pediatric literature. One case report described a 14-year-old female with end-stage renal disease who presented with multiple episodes of PRES in a 5-month period who underwent renal transplantation with no further episodes of PRES [11]. Our group included one teenage patient with class IV lupus nephritis on multiple antihypertensives who presented with PRES three times, always precipitated by medication non-adherence. Her mental status returned to baseline after each episode, and she had no seizure activity in between episodes. The second patient had microscopic polyangiitis. He presented with PRES at age ten with headaches and seizures, and again 18 months later, with seizures and altered mental status. He made a full recovery after each hospitalization.
Limitations
Limitations to our study include its retrospective design and small sample size. As such, we were unable to carry out multivariable analyses due to the low incidence of PRES, limiting our ability to draw conclusions related to the interplay of different factors associated with PRES. The small sample size may also lead to a lack of precision in the univariate analysis. Additionally, all patients were at a single center. This may introduce bias based on our specific patient population and treatment strategies. We used the PIM-2 score to compare patients of similar illness severity. The PIM-2 score has been validated as a measure of mortality risk. As such, it can be used to compare groups of patients based on their assigned scores.
Conclusion
Our study finds PRES to be associated with hypertension, renal disease, immunosuppression, autoimmune disease, and anemia in children after controlling for mortality risk. PRES patients had a higher mortality rate and LOS than the control cohort. We hope that continued research in this area will provide the medical community with a better understanding of this entity, as patients with PRES continue to have a high incidence of mortality.
Abbreviations
- AED:
-
Antiepileptic drug
- AMS:
-
Altered mental status
- DBP:
-
Diastolic blood pressure
- LEV:
-
Levetiracetam
- MAP:
-
Mean arterial pressure
- MRI:
-
Magnetic resonance imaging
- MRV:
-
Magnetic resonance venogram
- PICU:
-
Pediatric intensive care unit
- PIM-2:
-
Pediatric Index of Mortality-2
- PRES:
-
Posterior reversible encephalopathy syndrome
- SBP:
-
Systolic blood pressure
- SLE:
-
Systemic lupus erythematous
- PCPC:
-
Pediatric cerebral performance category
- POPC:
-
Pediatric overall performance category
References
Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500.
Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85:1566–75.
Yamamoto H, Natsume J, Kidokoro H, et al. Clinical and neuroimaging findings in children with posterior reversible encephalopathy syndrome. Eur J Paediatr Neurol. 2015;19:672–8.
Akin F, Kilicaslan C, Solak ES, Uzun M, Aygun S, Arslan S. Posterior reversible encephalopathy syndrome in children: report of three cases. Childs Nerv Syst. 2014;30:535–40.
Endo A, Fuchigami T, Hasegawa M, et al. Posterior reversible encephalopathy syndrome in childhood: report of four cases and review of the literature. Pediatr Emerg Care. 2012;28:153–7.
Gupta V, Bhatia V, Khandelwal N, Singh P, Singhi P. Imaging findings in pediatric posterior reversible encephalopathy syndrome (PRES): 5 years of experience from a tertiary care center in India. J Child Neurol. 2016;31:1166–73.
Muscal E, Traipe E, de Guzman MM, Myones BL, Brey RL, Hunter JV. MR imaging findings suggestive of posterior reversible encephalopathy syndrome in adolescents with systemic lupus erythematosus. Pediatr Radiol. 2010;40:1241–5.
Yamada A, Ueda N. Age and gender may affect posterior reversible encephalopathy syndrome in renal disease. Pediatr Nephrol. 2012;27:277–83.
Ergun T, Lakadamyali H, Yilmaz A. Recurrent posterior reversible encephalopathy syndrome in a hypertensive patient with end-stage renal disease. Diagn Interv Radiol. 2008;14:182–5.
Onder AM, Lopez R, Teomete U, et al. Posterior reversible encephalopathy syndrome in the pediatric renal population. Pediatr Nephrol. 2007;22:1921–9.
Daniel NJ, Hernandez CL, Walker RA. Recurrent posterior reversible encephalopathy syndrome in a pediatric patient with end-stage renal disease. J Emerg Med. 2014;46:e39–42.
Hagemann G, Ugur T, Witte OW, Fitzek C. Recurrent posterior reversible encephalopathy syndrome (PRES). J Hum Hypertens. 2004;18:287–9.
Won SC, Kwon SY, Han JW, Choi SY, Lyu CJ. Posterior reversible encephalopathy syndrome in childhood with hematologic/oncologic diseases. J Pediatr Hematol Oncol. 2009;31:505–8.
Khan RB, Sadighi ZS, Zabrowski J, Gajjar A, Jeha S. Imaging patterns and outcome of posterior reversible encephalopathy syndrome during childhood cancer treatment. Pediatr Blood Cancer. 2016;63:523–6.
Kim SJ, Im SA, Lee JW, et al. Predisposing factors of posterior reversible encephalopathy syndrome in acute childhood leukemia. Pediatr Neurol. 2012;47:436–42.
Panis B, Vlaar AM, van Well GT, et al. Posterior reversible encephalopathy syndrome in paediatric leukaemia. Eur J Paediatr Neurol. 2010;14:539–45.
Tang JH, Tian JM, Sheng M, et al. Study of posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia after induction chemotherapy. J Child Neurol. 2016;31:279–84.
Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179–90.
Habetz K, Ramakrishnaiah R, Raina SK, Fitzgerald RT, Hinduja A. Posterior reversible encephalopathy syndrome: a comparative study of pediatric versus adult patients. Pediatr Neurol. 2016;65:45–51.
Raj S, Overby P, Erdfarb A, Ushay HM. Posterior reversible encephalopathy syndrome: incidence and associated factors in a pediatric critical care population. Pediatr Neurol. 2013;49:335–9.
Slater A, Shann F, Pearson G. Paediatric Index of Mortality Study G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–85.
National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76.
Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74.
Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–25.
Kastrup O, Gerwig M, Frings M, Diener HC. Posterior reversible encephalopathy syndrome (PRES): electroencephalographic findings and seizure patterns. J Neurol. 2012;259:1383–9.
Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–10.
Hobson EV, Craven I, Blank SC. Posterior reversible encephalopathy syndrome: a truly treatable neurologic illness. Perit Dial Int. 2012;32:590–4.
Burnett MM, Hess CP, Roberts JP, Bass NM, Douglas VC, Josephson SA. Presentation of reversible posterior leukoencephalopathy syndrome in patients on calcineurin inhibitors. Clin Neurol Neurosurg. 2010;112:886–91.
Author information
Authors and Affiliations
Contributions
GF contributed to study design, data collection, and manuscript preparation; MAM involved in data collection and manuscript preparation; NK took part in study design and statistical analysis; PA contributed to MRI review and manuscript preparation; RT involved in study design and manuscript preparation; and AK took part in principle investigator, study design, and manuscript preparation.
Corresponding author
Ethics declarations
Conflicts of interest
All authors have no conflicts of interest.
Ethical Approval/Informed Consent
This study was approved by the Northwell Health System Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fisler, G., Monty, M.A., Kohn, N. et al. Characteristics and Outcomes of Critically Ill Pediatric Patients with Posterior Reversible Encephalopathy Syndrome. Neurocrit Care 32, 145–151 (2020). https://doi.org/10.1007/s12028-019-00720-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00720-9