Abstract
This historical vignette revisits the main contributions by Nils Lundberg, a neurosurgeon, that were published in the late 1950s and early 1960s. The Lundberg studies also definitively established that symptoms of abnormal brainstem function resulted from abnormal intracranial pressure (ICP), and moreover, even variations in ICP could produce clinical symptoms. The most innovative result of continuous monitoring was the discovery of plateau-shaped waves that produced paradoxical symptoms previously designated as “decerebrate” and “tonic fits” or “acute coning.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intracranial pressure (ICP) can generally only be measured by means of a parenchymal probe, ventriculostomy, lumbar puncture, or lumbar drain. Human studies of ICP and their variations have been best described by Nils Lundberg from the University of Lund (Sweden), who monitored cerebrospinal fluid (CSF) pressure with continuous ventricular pressure in over 400 patients, both non-traumatic cases and traumatic brain injury. Lundberg continuously recorded patients for a week on average. The new, highly informative data that appeared made a strong argument for recording over time.
Nils Lundberg, a neurosurgeon, published his major contributions to the literature in the late 1950s and early 1960s [1, 2]. He established that visual review of the waves yielded additional value beyond a pressure number. His studies also definitively established that symptoms of abnormal brainstem function resulted from abnormal ICP, and moreover, even variations in ICP could produce clinical symptoms.
Early Clinical Observations
A number of experimental studies preceded Lundberg’s ventricular catheter studies. Most recognizable among these were Cushing’s animal studies published in several manuscripts [3]. His work led to an easy clinical acceptance that expanding lesions lead to increased ICP, hypertension, bradycardia, and respiratory irregularities including apnea. However, equally important work was done by Ryder’s group, who looked at the mechanism of changes in osmotic pressure, changes in CSF pressure with volume change, and the effect of changes in systemic venous pressure on cerebrovascular pressure. In their experiments in Rhesus monkeys, they found that the rate of intracranial blood flow and back pressure from the great veins controlled ICP [4].
Ryder’s group was among the first to question the Cushing’s claim that significantly elevated ICP could occur “without the slightest immediate effect on the pulse, respiration, or blood pressure or without the subjects’ effect on any sensation.” These experiments also found that changes in cerebral blood flow caused changes in cerebral fluid pressure and that changes in cerebral blood flow and arterial and jugular venous pressure do not correlate closely [4, 5]. How these experiments could be translated to a clinical situation was important because not many clinicians saw these changes in patients with presumed increased ICP and wondered.
Continuous Ventricular Fluid Pressure (VFP) Recordings
Lundberg’s technique exposed patients to little risks (Fig. 1) but provided a treasure trove of observations. Lundberg described it as follows:
Monitoring of patient with ventriculostomy and use of water level to position the transducer (Lundberg appreciated that a horizontal plane through the approximate center of the cranial cavity would be more accurate but chose this landmark for convenience), used with permission of Wiley and Company [1])
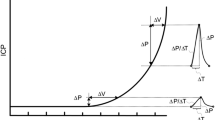
The (VFP) tracing may reveal a number of different spontaneous pressure variations. Among these, one type is of particular interest, because it is closely related to acute cerebral symptoms in patients with intracranial hypertension. This type of pressure variation is characterized by a sudden rapid rise, continuation on a high level for some time, and finally a rapid fall. Because of the typical shape which these pressure variations give to the VFP curve, I call them “plateau waves [1].”
Lundberg found the clinical signs during the plateau waves were largely a combination of “headache, nausea, vomiting, facial flush, air-hunger, forced or irregular or periodic breathing, changes in pulse frequency, rise in blood pressure, involuntary micturition, restlessness, confusion, motor agitation, changes in the level of consciousness, and various motor phenomena such as clonic movements and tonic rigidity of the limbs” [1]. He concluded that transient elevations of the ICP of the plateau wave type signaled impending deterioration (Fig. 2).
Plateau wave (and rhythmic oscillations), used with permission of the Journal of Neurosurgery [2])
The most significant result of continuous monitoring was the discovery of plateau-shaped waves that produced paradoxical symptoms previously designated as decerebrate and tonic fits or acute coning. In addition, there were rhythmic oscillations related to Cheyne–Stokes breathing (1 per minute) or Traube–Hering–Mayer waves of blood pressure (6 per minute). These ICP waves were characterized further as follows.
-
“Plateau waves” had the most substantial increase due to their steep up-slope static increase at 50–110 mm Hg for at least 5 min; they decreased to nearly normal levels only to re-appear quickly. Patients had headache and nausea followed by stupor and tonic posturing in limbs often with clonic convulsive movements.
-
A second form of waves was the so-called 1-per-minute wave. These were 50-mm Hg pressure increases with hyperpnoea and restlessness.
-
A third ICP-rising wave was the 4-minute wave (called 6-per), which was often seen with markedly increased ICP and changes in brainstem function.
Clinical Consequences
Recording the ICP waves led Lundberg’s group to the clinical decision “to induce hypothermia and to postpone craniotomy until the intracranial pressures were under control [1].” These wave configurations also demonstrated a condition that became known as “decompensated intracranial hypertension.” Hypotension would reduce cerebral blood flow, ischemia, and edema, with increased ICP becoming fatal. Follow-up studies showed hyperventilation (change in respiratory minute volume with decreased PCO2) to cause very rapid changes in ICP but with an rebound increase after 1 or 2 h. Plateau waves were not responsive. This relationship between respiration and cerebral blood flow was known [6], but its effect on ICP was hitherto undemonstrated.
Confirmatory studies on CSF pressure waves came from several groups, most notably from Institute of Neurological Sciences in Glasgow. The group defined “relatively normal” ICP as < 20 mm Hg but also emphasized cerebral perfusion related to systemic arterial pressure [7], arguing that perfusion pressure < 30 to 40 mm Hg substantially reduced cerebral blood flow [8, 9].
Acceptance and Criticism
Lundberg’s study of pressures waves did not lead to increased use of continuous ICP monitoring through ventriculostomy. Neurosurgeons and intensivists preferred the seemingly less complicated ICP bolts. Also contributing to their reluctance to adopt this technique was the fear of increased infection in the wards and ICU as well as equipment maintenance costs [10,11,12]. Lundberg’s group had a very low infection rate, but the incidence in the initial trials performed at other sites varied greatly and fostered continuing concerns (9–27% in Wyler and Kelly, and 5% in Smith [11, 12]), although later studies found the risks to be low [13]. Lundberg claimed small dural openings to keep tubing fitting snugly would reduce infection. Smith additionally suggested antibiotics during tube placement and several days after removal [11]. Gradually, in the 1970s and 1980s, ventriculostomies monitored ICP in subarachnoid hemorrhage and traumatic head injury, but patients with traumatic brain injury were placed on intraparenchymal monitors. Invasive monitoring continues to be the gold standard for diagnosis of elevated ICP. Prolonged duration of measured ICP ≥ 20 mmHg has been associated with worse outcomes [14].
Findings on physical examination that classically correlate with signs of elevated ICP include mydriasis, motor posturing, and decreased levels of consciousness. Many neurosurgeons advocate for early ICP management in patients with these symptoms. However, none of these classically described physical examination findings are, in themselves, sensitive or specific enough to predict elevated ICP adequately.
Lundberg (but also others) felt that ICP of 10 mmHg was normal, slightly elevated if sustained above 15 mmHg, moderately elevated at 25 mmHg, and severely elevated above 40 mmHg. Moreover, the cutoff value of increased ICP is not established and may vary significantly in different patient groups. Most clinical trials have looked at ICP > 25 mmHg without much attention to wave morphology. Lundberg’s plateau waves are rarely mentioned in clinical trials evaluating treatment of increased ICP. It remains a bedside clinical observation with the opportunity in some centers to analyze it further [15]. Recommendations for ICP monitoring are in flux, largely as a result of failure to find a change in patient outcome after aggressive treatment with standard medical and surgical measures.
References
Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36:1–193.
Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. A preliminary report. J Neurosurg. 1965;22:581–90.
Cushing H. Concerning a regulating mechanism of the vasomotor center which controls blood pressure during cerebral compression. Bull John Hopkins Hosp. 1901;12:290–2.
Ryder HW, Espey FF, Kimbell FD, Penka EJ, Rosenauer A, Podolsky B, Evans JP. Modification of effect of cerebral blood flow on cerebrospinal fluid pressure by variations in craniospinal blood volume. AMA Arch Neurol Psychiatry. 1952;68:170–4.
Evans JP, Espey FF, Kristoff FV, Kimbell FD, Ryder HW, Lamb DH, Barnes EB, Young DJ. Experimental and clinical observations on rising intracranial pressure. AMA Arch Surg. 1951;63:107–14.
Gibbs EL, Gibbs FA, Lennox WG, Nims LF. Regulation of cerebral carbon dioxide. Arch Neurol Psychiat. 1942;47:879–89.
Johnston IH, Johnston JA, Jennett B. Intracranial-pressure changes following head injury. Lancet. 1970;2:433–6.
Brierley JB, Brown AW, Excell BJ, Meldrum BS. Brain damage in the rhesus monkey resulting from profound arterial hypotension I Its nature, distribution and general physiological correlates. Brain Res. 1969;13:68–100.
Rowan JO, Harper AM, Miller JD, Tedeschi GM, Jennett WB. Relationship between volume flow and velocity in the cerebral circulation. J Neurol Neurosurg Psychiatry. 1970;33:733–8.
Shapiro HM, Wyte SR, Harris AB, Galindo A. Disposable system for intraventricular pressure measurement and CSF drainage. Technical note. J Neurosurg. 1972;36:798–801.
Smith RW, Alksne JF. Infections complicating the use of external ventriculostomy. J Neurosurg. 1976;44:567–70.
Wyler AR, Kelly WA. Use of antibiotics with external ventriculostomies. J Neurosurg. 1972;37:185–7.
Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, Jane JA, Ward JD, Young HF, Marmarou A. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419–24.
Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370:2121–30.
Shaw M, Piper I, Hawthorne C. Multi-resolution convolution methodology for ICP waveform morphology analysis. Acta Neurochir Suppl. 2016;122:41–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contribution
EFMW researched and wrote the manuscript.
Source of Support
None.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the collection “Neurocritical Care Through History”.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wijdicks, E.F.M. Lundberg and his Waves. Neurocrit Care 31, 546–549 (2019). https://doi.org/10.1007/s12028-019-00689-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00689-5